Back to Journals » International Journal of Nanomedicine » Volume 15
Chlorhexidine Nanoemulsion: A New Antiseptic Formulation
Authors Horstmann Risso N , Ottonelli Stopiglia CD , Oliveira MT , Haas SE , Ramos Maciel T , Reginatto Lazzari N , Kelmer EL, Pinto Vilela JA, Beckmann DV
Received 22 April 2020
Accepted for publication 30 July 2020
Published 22 September 2020 Volume 2020:15 Pages 6935—6944
DOI https://doi.org/10.2147/IJN.S228280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Natalia Horstmann Risso,1 Cheila Denise Ottonelli Stopiglia,2 Marília Teresa Oliveira,1 Sandra Elisa Haas,2 Tamara Ramos Maciel,2 Natália Reginatto Lazzari,3 Edilson Luis Kelmer,3 Jorge Abrão Pinto Vilela,3 Diego Vilibaldo Beckmann1
1Postgraduate Program in Animal Sciences, Federal University of Pampa (UNIPAMPA), Uruguaiana, Brazil; 2Postgraduate Program in Pharmaceutical Sciences, Federal University of Pampa (UNIPAMPA), Uruguaiana, RS, Brazil; 3Veterinary Medicine Course, Federal University of Pampa (UNIPAMPA), Uruguaiana, Brazil
Correspondence: Diego Vilibaldo Beckmann
BR 472 - Km 585, Campus UNIPAMPA-Uruguaiana, Uruguaiana, RS 97501-970, Brazil
Tel +55 55999725509
Fax +55 5539110204
Email [email protected]
Introduction: Nanoparticle solutions have been studied to improve antimicrobial effect. The aim of this study was to develop, characterize, and evaluate the in vitro and in vivo antiseptic efficacy of 0.25% aqueous-based chlorhexidine nanoemulsion (NM-Cl 0.25% w/v).
Methods: The NM-Cl 0.25% w/v (2.5mg/mL) and free chlorhexidine nanoemulsion (FCN; same composition of NM-Cl without the molecule of chlorhexidine) were synthetized by the spontaneous emulsification method. Characterization analyses of physical and chemical properties were performed. The NM-Cl 0.25% w/v was compared with chlorhexidine 0.5% alcohol base (CS-Cl 0.5%) in vitro studies (microdilution study and kill curve study), and in vivo study (antisepsis of rats dorsum). Kruskal–Wallis test was used between groups and inside the same group, at different sample times and the Mann–Whitney test was performed when difference was detected.
Results: The NM-Cl 0.25% w/v presented adequate physicochemical characteristics for a nanoemulsion, revealing a more basic pH than FCN and difference between zeta potential of NM-Cl 0.25% w/v and FCN. The NM-Cl 0.25% w/v and CS-Cl 0.5% solutions were more effective on Gram-positive than on Gram-negative bacteria (p≤ 0.05). NM-Cl 0.25% w/v presented upper antiseptic effect in the microdilution study and residual antiseptic effect was maintained for a longer time when compared to CS-Cl 0.5% (kill curve study). The four-fold (minimal inhibitory concentration) of NM-Cl 0.25% were the formulations with most durable effect within those tested, presenting residual effect until T6 for both bacteria. In the in vivo study, both formulations (NM-Cl 0.25% w/v and CS-Cl 0.5%) had a reduction of the microorganisms in the skin of the rats (p< 0.0001) not revealing any difference between the formulations at different times, showing the antiseptic effect of NM-Cl (p≤ 0.05).
Conclusion: Both in vitro and in vivo experiments demonstrated that NM-Cl showed promising future as an antiseptic for cutaneous microbiota.
Keywords: antisepsis, cutaneous microbiota, nanoformulation, nanotechnology
Introduction
The cutaneous microbiota is constituted of microorganisms that colonize the skin, generally living in symbiosis with the host and can be differentiated in resident and transient.1,2 The population of resident bacteria is generally not pathogenic and is located in the epidermis and hair follicles of the host.2 The transient microbiota has pathogenic potential and is composed of agents acquired by contact, located in superficial layers of the skin.2,3
Antisepsis is an important element of asepsis and consists of total removal of transient microbiota and partial removal of resident microbiota.3 The practice is indicated before performing invasive transcutaneous procedures owing the prevention of infections caused by bacteria translocation from the cutaneous microbiota.3–5 Although antisepsis is routine in veterinary procedures, infections in surgical wounds are frequent, producing 0.8% to 18.1% of postoperative complications.6
Antiseptics are chemical composts used to prevent sepsis either by inhibition of microorganism growth or by bactericidal effect.3 Formulations with iodophors, chlorhexidine and alcohol in their composition are widely used.7,8 Chlorhexidine is recommended as a topical antiseptic for surgery and for insertion of intravascular devices.9 Its residual effect can be explained by the attachment of the active molecule with the stratum corneum,10 working as antiseptic reservoir, prolonging its action and effect.11
The wide use of antiseptics in hospitals may result in residual levels facilitating the intrinsic and adaptive resistance establishment.9,12,13 Researchers have addressed the issue in a study which shows that the development of different strategies to control microorganisms is required to maximize formulation effect and also for the development of new formulations with the purpose of opposing bacterial mechanisms of acquired resistance.14
Increasing reports of resistance to chlorhexidine formulation are the main reason to the current demand of the investigation of antiseptic alternatives. Nanostructured formulations are believed to be an alternative for hospital antiseptics,7,14,15 and among them, the nanoemulsions are heterogeneous dispersions of immiscible liquids in droplet form, stabilized by interfacial surfactant film usually in combination with surfactant molecules.17–29
Nanoparticles have been widely studied as potential carriers for antimicrobials, and some advantages compared to conventional antibacterials are: the contour to the resistance mechanisms, mainly due to the size, as it results in a greater interaction due to the surface area; impediment of biofilm formation; targeting to the site of infection and consequently, minimizing side effects; controlled and sustained release and association of antimicrobials in a single nanoparticulate system.30 Organic nanoparticles bring promising results, highlighting nanoemulsions that may contain antimicrobial drugs, essential oils with antimicrobial activity, or even the combination of both.31–37
Numerous studies bring results when it comes to the use of inorganic nanoparticles using different plant extracts with antimicrobial and healing activities, which include silver nanoparticles,38–42 titanium nanoparticles,43,44 gold nanoparticles,45–47 copper nanoparticles,48,49 zinc nanoparticles,50 iron nanoparticles51 and manganese nanoparticles.52
The aim of this study was to evaluate and validate an aqueous-based chlorhexidine nanoemulsion at 0.25% (NM-Cl). This paper describes the development and characterization of the formulation and compares it with alcohol-based chlorhexidine commercial solution at 0.5% (CS-Cl), evaluating the antiseptic effect in vitro and in vivo.
Materials and Methods
Materials
The antimicrobial agents used were the chlorhexidine gluconate (Sigma Aldrich Laboratory, São Paulo, Brazil) used to NM-Cl synthesis, and the solution of chlorhexidine, Riohex® 0.5% alcohol base (Rioquímica Laboratory, São Paulo, Brazil) used as the CS-Cl. The broths and agars utilized in the assays were manufactured by HiMedia Laboratories (HiMedia Laboratories, Mumbai, India), and the defibrinated sheep blood used was from Newprov Laboratory (Newprov Laboratory, Paraná, Brazil).
Development and Characterization of Aqueous-based Chlorhexidine Nanoemulsion
The formulations NM-Cl 0.25% w/v (2.5 mg/mL) and free chlorhexidine nanoemulsion (FCN— same composition of NM-Cl without the molecule of chlorhexidine) were synthesized by the spontaneous emulsification method.18 The organic phase was composed of capric/caprylic triglycerides, Lipoid S45®, and chlorhexidine (2.5 mg/mL) dissolved in acetone (45±1°C). After solubilization, the organic phase was poured under the aqueous phase, which was composed of polysorbate 80 and distilled water, then stirred for 10 min, and the organic solvent was evaporated in a rotary evaporator under reduced pressure. All formulations were prepared in triplicate.
The physicochemical characterization of the formulations was carried out immediately after preparation:
The average diameter and particle size distribution of the NMs were determined using the laser diffractometry technique, using the Mastersizer 2000 (Malvern Instruments, Malvern, UK) equipment, where 100 μL of the sample was diluted in 100 mL of distilled water in the equipment sampler. The analyses were performed in triplicate and the average diameter based on volume (d4,3) was used as a parameter for particle size distribution. To determine the polydispersity index (SPAN), the accumulated distribution parameters of 10%, 50%, and 90% (d0.1, d0.5, d0.9, respectively) were used.53
The zeta potential of NMs was determined by the electrophoretic migration technique using the NanoBrook 90Plus PALS equipment (Brookhaven Instruments, Holtsville, NY, USA), where 10 μL of the formulation was diluted in 10 mL of 1 mM NaCl solution, previously filtered solution in 0.22 μm filter (EMD Millipore, Billerica, MA, USA), in a specific equipment cell. The analyses were performed in triplicate.53
The pH of NMs was determined through readings, in triplicate, of the formulations using a potentiometer (HANNA Instruments) previously calibrated.53
For the quantification of chlorhexidine by high performance liquid chromatography (HPLC), a method described by Lboutounne et al,54 in which a C18 chromatographic column (150⨰3.9 mm) was used, with a mobile phase composed of acetonitrile: 0.1% phosphoric acid (5:95, v/v) and a flow of 1 mL/min. The wavelength for the detection of chlorhexidine was 260 nm.
For the measurement of chlorhexidine (2.5 mg/mL), 10 μL of the NC suspension was transferred to a 1 mL volumetric flask, diluted in acetonitrile, obtaining a final concentration of 25 μg/mL. The dilution was maintained in an ultrasound bath for 30 min to disrupt the nanoparticulate systems and consequently release the drug for quantification. The solutions were filtered through a 0.45 μm membrane filter (EMD Millipore) before injection into the equipment.53,55–57 Subsequently, all samples were quantified by HPLC.
For the encapsulation rate, the ultrafiltration-centrifugation method of the suspensions (300 μL) Ultrafree®-MC (Millipore) was used for five minutes at 10000 revolutions per minute.20 The concentration of nonassociated chlorhexidine was quantified in the ultrafiltrate, using the same conditions for determining the concentration. The encapsulation rate was calculated by the difference between the concentration of chlorhexidine (described in the dosage) in the formulation and the amount present in the aqueous phase of the suspension (ultrafiltrate).53,55–57
In Vitro Assay
The susceptibility profile of the Staphylococcus aureus ATCC 6531, Staphylococcus epidermidis ATCC 1228, Escherichia coli ATCC 10536 and Klebsiella pneumoniae carbapenemase (clinical isolate) strains against the chlorhexidine formulations (NM-Cl and CS-Cl) was evaluated by the broth microdilution method according to the Clinical and Laboratory Standards Institute (CLSI), protocol M100-S25.21 Serial dilutions of chlorhexidine formulations with free molecule in the concentration range of 0.0005 μg/mL to 250 μg/mL and chlorhexidine nanoemulsion (from 0.00025 μg/mL to 125 μg/mL) were evaluated. All tests were performed in triplicate including the blank formulation, positive control (drug free) and negative control (sterility control). The blank formulation presented no inhibitory activity of the bacteria. Minimal inhibitory concentration (MIC) was considered as the lowest concentration capable of inhibiting 100% of microbial growth.
The residual effect of the formulations was evaluated in 24 h against the S. epidermidis (ATCC 1228) and K. pneumoniae carbapenemase (clinical isolate) by the kill curve method.22 Growth control of the isolates and sterility control were performed as the method describes.22 Two and fourfold MIC concentrations were tested for each microorganism (2⨰MIC and 4⨰MIC, respectively). The samples were collected at the following times after inoculation of the formulation: T0, before test; T1, immediately; T2, two hours; T3, four hours; T4, six hours; T5, eight hours; T6, 12 hours; T7, 24 hours.
In Vivo Assay
The in vivo study was performed with 20 male rats (Rattus norvegicus albinus—Wistar lineage). The research was approved by the Committee on Ethics in the Use of Animals of the Federal University of Pampa (UNIPAMPA) by the protocol number 44/2017. All animal experiments followed the normative resolutions of National Animal Experimentation Control Council of Brazil (CONCEA), specifically the Brazilian Federal Law on Animal Experimentation no. 11794–2008 and Normative Resolution CONCEA no. 33–2016 (Chapter “Procedures—Rodents and Lagomorphs kept in facilities of educational institutions or scientific research”). Normative Resolutions CONCEA are in accordance with international guidelines of laboratory animal welfare.
The in vivo study was performed in the surgical room of the veterinary hospital, respecting surgical asepsis. The animals were randomly distributed into control groups (G1 and G2 in the left and right side, respectively) and chlorhexidine groups (G3 and G4 in the left and right side, respectively): G1, antisepsis out; G2, free chlorhexidine nanoemulsion; G3, antisepsis with CS-Cl at 0.5% alcohol-based; and G4, antisepsis with NM-Cl at 0.25% aqueous-based (test formulation). The animals underwent anesthesia with isoflurane and wide trichotomy was performed on their dorsum, observing the scapula border as the cranial limit and the ischiatic tuberosity as the caudal limit. The antisepsis of each antimere was performed in cranial-caudal direction by two gauzes soaked in 7.0 mL according to the tested solution (G1, G2, G3, and G4). The gauze size was the same as the antimere and each gauze side was used once, totalizing four movements.
All samples of cutaneous microbiota were carefully collected before (T0) and after the antisepsis: T1, immediately after antisepsis; T2, 60 min; T3, 120 min; and T4, 180 min) with a soaked swab in 0.9% saline solution. The biological material sampling was done from different positions of animals skin by the division of the area with trichotomia, in order to avoid swabbing the same site. Swab content was inoculated in plates containing blood agar and incubated at 37°C for 24 h in aerobiosis. Manual counting of colony-forming units (CFUs) was performed according to established methodology, using 300 CFU as the maximum limit for counting.23 After performing antisepsis and the sampling times, the presence or absence of skin irritation was visually evaluated by verification of presence of erythema.
Statistical Analysis
The in vitro results were expressed as mean ±SD and one-way analysis of variance (ANOVA) followed by Tukey’s test, which was used to compare mean values. The difference of antiseptic action of in vivo study was verified by Kruskal–Wallis test, followed by Mann–Whitney test when a difference was detected. The statistical analysis was performed with SPSS Software (IBM Corporation, Armonk, NY, USA). Differences among mean and median values were considered statistically significant when p≤0.05.
Results and Discussion
Development and Characterization of Aqueous-based Chlorhexidine Nanoemulsion
The NM-Cl 0.25% w/v was characterized by physicochemical parameters such as diameter (nm), polydispersity, pH, zeta potential (mV), drug loading (%) and encapsulation rate (%). The FCN (unloaded nanoparticle) was prepared for comparison. All analyses were performed in triplicate and the results are shown in Table 1.
 |
Table 1 Physicochemical Characterization of NM-Cl 0.25% and FCN |
The mean diameters of FCN and NM-Cl 0.25% w/v were 165 nm and 343 nm, respectively. Nanoemulsions with diameter between 200 nm and 500 nm trend to be physically more steady.16,58 Both formulations presented SPAN below two with a low standard deviation, indicating homogeneity of the particles in nanosuspension.55,57 The mean diameter of NM-Cl 0.25% was significantly greater than FCN, probably by incorporation of chlorhexidine in the oily phase.59
The pH of NM-Cl 0.25% w/v was more basic than FCN (Table 1), and there was difference between the zeta potential of NM-Cl 0.25% (positive) and FCN (negative). The chemical composition of NM-Cl 0.25% w/v and FCN is the same, with the exception of chlorhexidine. The pH and zeta potential differences can be explained by the presence of chlorhexidine. The occurrence of positive zeta potential including chlorhexidine in nanoparticulate systems has been previously described in literature.19,54,60,61
In an assay of a developed nanoemulsion formula containing chlorhexidine using Tween 85, authors observed that by adding chlorhexidine, the zeta potential of all developed formulations was positive. In addition, they also observed that by incorporating chlorhexidine the pH of the formulations become more basic, compared to formulations without the drug.60
Furthermore, in other formulations containing chlorhexidine (the particle diameter was 278±6nm) the authors also observed the positive zeta potential (32.4±0.1 mV) for nanocapsules containing chlorhexidine and negative zeta potential (−20.9 ± 0.6 mV) for nanocapsules without the drug,19 corroborating our study. The authors attributed the increase in the surface charge of nanocapsules containing chlorhexidine to two hypotheses: chlorhexidine is adsorbed at the interface between the polymer and the surrounding environment, and/or its association in the polymer. The cationic zeta potential allows a greater interaction with the cell membrane due to the difference in charges, enabling greater affinity between the particle-membrane and, consequently, greater biological performance.55,61
In Vitro Assay
The values resulting from in vitro susceptibility of different bacterial strains against NM-Cl and CS-Cl are exhibited in Table 2. The NM-Cl presented better inhibitory activity in comparison to CS-Cl when analyzing the geometric mean of Gram-positive and Gram-negative bacteria in this study (p≤0.05). The MIC was smaller against the formulation of NM-Cl, evidencing better action of the nanoformulation.
 |
Table 2 In Vitro Susceptibility of Different Bacterial Strains Against Nanoemulsion of Chlorhexidine and Commercial Solution of Chlorhexidine |
The superior action of nanostructured formulations containing chlorhexidine when compared to the free drug solution could be justified by the difference in the physicochemical characteristics of the formulation.25 Chlorhexidine mechanism of action is on the plasmatic membrane25,28 and the cationic zeta potential of the NM-CL 0.25% allows a greater interaction with cell membrane, consequently generating a better performance of the formula.55,60 The reduction in the size of the molecule may potentiate mechanisms of passive cellular absorption and facilitate the drug to enter the microorganism cell, resulting in an increase in antimicrobial activity and in a better therapeutic index.16,25 This mechanism could justify the best action of NM-Cl in the study, presenting smaller MIC than those of CS-Cl and bacteriostatic effect at lower concentrations.
The NM-Cl and CS-Cl solutions were more effective on Gram-positive than on Gram-negative bacterial (p≤0.05). The chlorhexidine has better action on Gram-positive bacteria, because of intrinsic mechanisms of resistance present in Gram-negative bacteria such as the presence of an external membrane that limits the action of drugs.3,12
The higher sensitivity of Gram-positive bacteria to chlorhexidine was expected,3 however S. aureus was the Gram-positive bacteria with higher MIC against the tested formulations, and when tested against the nanostructured formulation it presented higher resistance than E. coli, which is a Gram-negative bacteria. S. aureus plays an important role in the establishment of infectious processes postoperatively, since it is one of the main bacteria involved2 due to its capacity to pump the chlorhexidine out.24 This mechanism of resistance could explain the results found in this study.
Other studies had already found better action from formulation of nanocapsules with chlorhexidine against the S. epidermidis when compared to the commercial solution of chlorhexidine.19,54 S. epidermidis is an important pathogen in postoperative complications, because it is one of the most common bacterium of the resident microbiota–bacteria translocation.3–6 The present study found that the nanoemulsion had a better effect than the formulation with free molecules against Gram-positive bacteria and corroborates previous studies,19,54 even in different formulations.
The results found for Gram-negative bacteria in nanoformulation (Table 2) can be explained because E. coli was as susceptible to chlorhexidine as Gram-positive bacteria,12 besides E. coli showed higher sensitivity than K. pneumoniae carbapenemase (KPC). Although chlorhexidine is an effective antimicrobial, it has limitations in some Gram-negative bacteria.62 Recent studies found that clinical isolates of K. pneumoniae can show difference in susceptibility to chlorhexidine.9 KPC was the most resistant bacterium against the formulations, which can be explained by mechanisms of resistance.13,62 The mechanisms of resistance are related to cross-resistance to colistin, and are associated with the upregulation of the proteins that are involved in the assembly of the lipopolysaccharide for outer membrane biogenesis and virulence factors.62
The kill curve method was done in accordance with the Verma protocol,22 suggesting that several concentration ranges can be tested (usually they are multiples of the MIC). The kill curve presents limitations: selection of concentrations to be tested (could be adapted to the assay observing the recommendations of multiples of the MIC), limitations of the preparation of materials and the technique itself. A recent study demonstrated that low concentrations of chlorhexidine, such as the MIC, had a relatively weak effect on membrane breaking of bacteria.63 In accordance with the protocol we selected 2⨰MIC and 4⨰MIC expecting that higher concentrations were needed for the maintenance of the effect in time (residual effect). The residual effect of chlorhexidine is well known64 but the aim was to compare it with the nanostructured formulation.
The kill curve results are exhibited in Figures 1 and 2. The different NM-Cl concentrations presented slower action, however, that was maintained for a longer time when compared to CS-Cl action for the bacterial strains of S. epidermidis (Figure 1) and K. pneumoniae carbapenemase (Figure 2). The result demonstrates the residual effect of the CS-Cl,37 and also of the NM-Cl. The delay in the onset of action could occur because of the connection of nanoemulsion droplets on the surface, forming a dense film that remains for a longer time in prolonged action.26 The 2⨰MIC concentrations were more effective to reduce the CFUs at first evaluation, and the 4⨰MIC concentrations were more effective to maintain lower CFUs counting over time. Furthermore, the 4⨰MIC NM-Cl presented a prolonged residual effect (T6, 18 h) compared to the other concentrations.
 |
Figure 1 Action of CS-Cl and NM-Cl solutions against Staphylococcus epidermidis twice (2⨰MIC) and four (4⨰MIC) times MIC concentrations in different sample times. |
 |
Figure 2 Action of CS-Cl and NM-Cl solutions against Klebsiella pneumoniae Carbapenemase twice (2⨰MIC) and four (4⨰MIC) time MIC concentrations in different sample times. |
In Vivo Assay
The counting of CFU chlorhexidine groups (NM-Cl and CS-Cl) is exhibited in Table 3. Two animals were removed from the study because there was a breach in antisepsis, resulting in contamination of the antimeres and disabling the inclusion of the sample results. The counting of the CFU control group (antisepsis out and free chlorhexidine nanoemulsion) was over 300 CFU, a result that was expected because of the normal colonization of skin. The presence of erythema was not visualized after use of formulations (antisepsis out, free chlorhexidine nanoemulsion, CS-Cl at 0.5% alcohol-based and NM-Cl at 0.25% aqueous-based) during the in vivo assay.
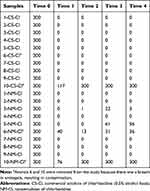 |
Table 3 In Vivo Antiseptic Actions of CS-Cl and NM-Cl Solutions in Wistar Rats Skin at Different Times by the CFU Manual Counting |
The satisfactory antiseptic effect of NM-Cl and CS-Cl formulations can be demonstrated by the nongrowth of bacteria after rat skin antisepsis (T1) in the CFU counting. One hour after antisepsis (T2), an animal of the NM-Cl group presented only one CFU, evidencing similar reduction (p<0.0001). Two animals of the NM-Cl group presented higher CFU than the CS-Cl group in T3 and T4 (Table 3), but there was no difference between them (p≤0.05). The presence of alcohol in CS-Cl according to Davids et al23 could have potentiated the antiseptic effect of the solution in the present study, however the potentiation was not verified and the use of CS-Cl did not present advantage over NM-Cl (p≤0.05).
The association of chlorhexidine and alcohol, as CS-Cl at 0.5% alcohol-based, is used for complementary presurgical antisepsis and alone as a presurgical antiseptic,27 which is the reason why it was compared with NM-Cl aqueous-based. The antiseptics association is usual,7 due to broad spectrum and fast action of alcohol.3,7 In nanoemulsions there is no presence of alcohol in the composition, since they are composed of both an aqueous and an oily phase.17 An alternative comparison could be the aqueous solution of chlorhexidine. The main recommendation is to use aqueous solution in mucous membranes and eventually as a complementary antiseptic for presurgical of skin,27 a way in which an alcohol-based product is more used for skin antisepsis than the aqueous-based one.
The similar antiseptic effect in vivo of NM-Cl when compared to CS-Cl encourages further research in health routine, once chlorhexidine is an antiseptic agent recommended worldwide for topical use in preoperative preparation and in insertion of intravascular devices.9 One of the possible benefits of using nanostructures is the decrease of the concentration in the formulation, presenting equal or greater effect than the usual formulation, as previously demonstrated with chlorhexidine nanocapsules19,54 and vegetable oil.15 Consequently, our group decided to compare a low-concentrated nanostructured formulation to a consolidated formulation, resulting in similar effects both for NM-Cl and for CS-Cl, demonstrating that the use of nanoformulations could decrease chlorhexidine concentrations.
The antimicrobial resistance to chlorhexidine, one of the most widely used antiseptic products,9,13,28 has generated considerable efforts for the development of studies that evaluate antiseptic action of new formulations. These formulations can be used for establishing alternatives to resistance induction through indiscriminate use.2,28 Considering this problem and the in vitro and in vivo results from this study, our group highlights and encourages further studies with chlorhexidine nanoemulsion in the hospital routine.
Conclusion
The NM-Cl formulation showed efficacy at in vitro and in vivo assays with lower concentrations of chlorhexidine than the commercial product that is recommended for presurgical antisepsis. In this paper we have showed that NM-Cl presented adequate physicochemical characteristics, as well as antiseptic and residual effects. Taken together, the results suggest that NM-Cl is a promising potential alternative formulation. Results so far have been very positive, encouraging the development of future pharmacological studies for determining the mechanisms of action of the nanostructured formulation, for evaluating different concentrations of chlorhexidine in different nanostructured formulations, and for evaluating penetration mechanisms and potential toxicity of formulations in cutaneous cells when used topically.
Disclosure
SE Haas is a recipient of CNPq fellowship. The authors report no other conflicts of interest in this work.
References
1. Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244. doi:10.1038/nrmicro2537
2. Echols K, Graves M, LeBlanc KG, et al. Role of antiseptics in the prevention of surgical site infections. Dermatol Surg. 2015;41(6):667–676. doi:10.1097/DSS.0000000000000375
3. Fossum TW. Preparation of the preoperative field. In: Fossum TW, editor. Surgery of Small Animals.
4. Schulz KS. Principles of surgical asepsis. In: Fossum TW, editor. Surgery of Small Animals.
5. Sidhwa F, Itani KMF. Skin preparation before surgery: options and evidence. Surg Infect. 2015;16(1):14–23. doi:10.1089/sur.2015.010
6. Freeman KD, Southwood LL, Lane J, et al. Post-operative infection, pyrexia and perioperative antimicrobial drug use in surgical colic patients. Equine Vet J. 2012;44(4):476–481. doi:10.1111/j.2042-3306.2011.00515.x
7. Macias JH, Arreguin V, Munoz JM, et al. Chlorhexidine is a better antiseptic than povidone iodine and sodium hypochlorite because of its substantive effect. Am J Infect Control. 2013;41(7):634–637. doi:10.1016/j.ajic.2012.10.002
8. Dumville JC, McFarlane E, Edwards P, et al. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2013;28(3):52.
9. Bock LJ, Wand ME, Sutton JM. Varying activity of chlorhexidine-based disinfectants against Klebsiella pneumoniae clinical isolates and adapted strains. J Hosp Infect. 2016;93(1):42–48. doi:10.1016/j.jhin.2015.12.019
10. Larson EL. APIC guidelines for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251–269. doi:10.1016/0196-6553(95)90070-5
11. Sogawa Y, Kobayashi H, Kajiura T, et al. Comparison of residual antimicrobial activity of chlorhexidine-containing antiseptics: an express report. J Health Associated Infect. 2010;3(2):74–78.
12. Mcdonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147–179.
13. Abuzaid A, Hamouda A, Amyes SG. Klebsiella pneumoniae susceptibility to biocides and its association with cepA, qacDeltaE and qacE efflux pump genes and antibiotic resistance. J Hosp Infect. 2012;81(2):87–91. doi:10.1016/j.jhin.2012.03.003
14. Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomedicine. 2012;7:2767. doi:10.2147/IJN.S30631
15. Sagave L, Gressler LT, Flores FC, et al. Activity of nanoformulations of Melaleuca alternifolia and terpinen-4-ol in Rhodococcus equi isolates. Arquivo Brasileiro De Medicina Veterinária e Zootecnia. 2015;67(1):221–226. doi:10.1590/1678-7454
16. Soppimath KS, Aminabhavi TM, Kulkarni AR, et al. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70(1–2):1–20. doi:10.1016/S0168-3659(00)00339-4
17. Shakeel F, Shafiq S, Haq N, et al. Nanoemulsions as potential vehicles for transdermal and dermal delivery of hydrophobic compounds: an overview. Expert Opin Drug Deliv. 2012;9(8):953–974. doi:10.1517/17425247.2012.696605
18. Bouchemal K, Briançon S, Perrier E, et al. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280(1–2):241–251. doi:10.1016/j.ijpharm.2004.05.016
19. Lboutounne H, Chaulet JF, Ploton C, et al. Sustained ex vivo skin antiseptic activity of chlorhexidine in poly (ϵ-caprolactone) nanocapsule encapsulated form and as a digluconate. J Control Release. 2002;82(2):319–334. doi:10.1016/S0168-3659(02)00142-6
20. Guterres SS, Fessi H, Barratt G, et al. Poly (DL-lactide) nanocapsules containing diclofenac: I. Formulation and stability study. Int J Pharm. 1995;113(1):57–63. doi:10.1016/0378-5173(94)00177-7
21. Clinical Lab Standart Institute. Performace Standards for Antimicrobial Susceptibility Testing. Twenty-Fifthin formation Suplement. Pennsylvania; 2016:240p.
22. Verma P. Methods for determining bactericidal activity and antimicrobial interactions: synergy testing, time-kill curves, and population analysis. In: Schwalbe R, Steele-moore L, Goodwin AC, editors. Antimicrobial Susceptibility Testing Protocols.
23. Davids BI, Davidson MJ, TenBroeck SH, et al. Efficacy of mechanical versus non-mechanical sterile preoperative skin preparation with chlorhexidine gluconate 4% solution. Vet Surg. 2015;44(5):648–652. doi:10.1111/vsu.12335
24. DeMarco CE, Cushing LA, Frempong-Manso E, et al. Efflux-related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(9):3235–3239. doi:10.1128/AAC.00430-07
25. Donsì F, Annunziata M, Vincensi M, et al. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol. 2012;159(4):342–350. doi:10.1016/j.jbiotec.2011.07.001
26. Zhou H, Yue Y, Liu G, et al. Preparation and characterization of a lecithin nanoemulsion as a topical delivery system. Nanoscale Res Lett. 2010;5(1):224. doi:10.1007/s11671-009-9469-5
27. Brazilian Company of hospital services (EBSERH). Standardized protocol/antiseptics – health surveillance and hospital quality unit/patient safety and health sector of HC-UFTM. Uberaba; 2017:15.
28. Kampf G. Acquired resistance to chlorhexidine–is it time to establish an ‘antiseptic stewardship’ initiative? J Hosp Infect. 2016;94(3):213–227. doi:10.1016/j.jhin.2016.08.018
29. Kumar M, Bishnoi RS, Shukla AK, et al. Techniques for formulation of nanoemulsion drug delivery system: a review. Prev Nutr Food Sci. 2019;24(3):225–234. doi:10.3746/pnf.2019.24.3.225
30. Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine. 2017;12:1227–1249. doi:10.2147/IJN.S121956
31. Zahi MR, El Hattab M, Liang H, Yuan Q. Enhancing the antimicrobial activity of d-limonene nanoemulsion with the inclusion of ε-polylysine. Food Chem. 2017;221:18–23. doi:10.1016/j.foodchem.2016.10.037
32. Hakemi-Vala M, Rafati H, Aliahmadi A, Ardalan A. Nanoemulsions: a novel antimicrobial delivery system. In: Grumezescu Nano-and Microscale Drug Delivery Systems.
33. Ryu V, McClements DJ, Corradini MG, McLandsborough L. Effect of ripening inhibitor type on formation, stability, and antimicrobial activity of thyme oil nanoemulsion. Food Chem. 2018;245:104–111. doi:10.1016/j.foodchem.2017.10.084
34. Krishnamoorthy R, Athinarayanan J, Periasamy VS, et al. Antimicrobial activity of nanoemulsion on drug-resistant bacterial pathogens. Microb Pathog. 2018;120:85–96. doi:10.1016/j.micpath.2018.04.035
35. Sonu KS, Mann B, Sharma R, Kumar R, Singh R. Physico-chemical and antimicrobial properties of d-limonene oil nanoemulsion stabilized by whey protein–maltodextrin conjugates. J Food Sci Technol. 2018;55(7):2749–2757. doi:10.1007/s13197-018-3198-7
36. Hasssanzadeh H, Alizadeh M, Bari MR. Formulation of garlic oil-in-water nanoemulsion: antimicrobial and physicochemical aspects. IET Nanobiotechnol. 2018;12(5):647–652. doi:10.1049/iet-nbt.2017.0104
37. Seibert JB, Bautista-Silva JP, Amparo TR, et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2018;287:61–67. doi:10.1016/j.foodchem.2019.02.078
38. Zangeneh MM, Bovandi S, Gharehyakheh S, Zangeneh A, Irani P. Green synthesis and chemical characterization of silver nanoparticles obtained using Allium saralicum aqueous extract and survey of in vitro antioxidant, cytotoxic, antibacterial and antifungal properties. Appl Organomet Chem. 2019;33(7):e4961.
39. Zangeneh MM, Joshani Z, Zangeneh A, Miri E. Green synthesis of silver nanoparticles using aqueous extract of Stachys lavandulifolia flower, and their cytotoxicity, antioxidant, antibacterial and cutaneous wound-healing properties. Appl Organomet Chem. 2019;33(9):e5016.
40. Hemmati S, Rashtiani A, Zangeneh MM, et al. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron. 2019;158:8–14. doi:10.1016/j.poly.2018.10.049
41. Hamelian M, Zangeneh MM, Shahmohammadi A, Varmira K, Veisi H. Pistacia atlantica leaf extract mediated synthesis of silver nanoparticles and their antioxidant, cytotoxicity, and antibacterial effects under in vitro condition. Appl Organomet Chem. 2020;34(1):e5278. doi:10.1002/aoc.5278
42. Zangeneh MM. Green synthesis and chemical characterization of silver nanoparticles from aqueous extract of Falcaria vulgaris leaves and assessment of their cytotoxicity and antioxidant, antibacterial, antifungal and cutaneous wound healing properties. Appl Organomet Chem. 2019;33(9):e4963.
43. Seydi N, Mahdavi B, Paydarfard S, et al. Preparation, characterization, and assessment of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of titanium nanoparticles using aqueous extract of Ziziphora clinopodioides Lam leaves. Appl Organomet Chem. 2019;33(9):e5009. doi:10.1002/aoc.5009
44. Seydi N, Saneei S, Jalalvand AR, et al. Synthesis of titanium nanoparticles using Allium eriophyllum Boiss aqueous extract by green synthesis method and evaluation of their remedial properties. Appl Organomet Chem. 2019;33(11):e5191. doi:10.1002/aoc.5191
45. Zhaleh M, Zangeneh A, Goorani S, et al. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using Gundelia tournefortii L. as a capping and reducing agent. Appl Organomet Chem. 2019;33(9):e5015. doi:10.1002/aoc.5015
46. Shahriari M, Hemmati S, Zangeneh A, Zangeneh MM. Biosynthesis of gold nanoparticles using Allium noeanum Reut. ex Regel leaves aqueous extract; characterization and analysis of their cytotoxicity, antioxidant, and antibacterial properties. Appl Organomet Chem. 2019;33(11):e5189. doi:10.1002/aoc.5189
47. Zangeneh MM, Saneei S, Zangeneh A, et al. Preparation, characterization, and evaluation of cytotoxicity, antioxidant, cutaneous wound healing, antibacterial, and antifungal effects of gold nanoparticles using the aqueous extract of Falcaria vulgaris leaves. Appl Organomet Chem. 2019;33(11):e5216. doi:10.1002/aoc.5216
48. Zangeneh MM, Ghaneialvar H, Akbaribazm M, et al. Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J Photochem Photobiol. 2019;197:111556. doi:10.1016/j.jphotobiol.2019.111556
49. Tahvilian R, Zangeneh MM, Falahi H, Sadrjavadi K, Jalalvand AR, Zangeneh A. Green synthesis and chemical characterization of copper nanoparticles using Allium saralicum leaves and assessment of their cytotoxicity, antioxidant, antimicrobial, and cutaneous wound healing properties. Appl Organomet Chem. 2019;33(12):e5234. doi:10.1002/aoc.5234
50. Mahdavi B, Saneei S, Qorbani M. Ziziphora clinopodioides Lam leaves aqueous extract mediated synthesis of zinc nanoparticles and their antibacterial, antifungal, cytotoxicity, antioxidant, and cutaneous wound healing properties under in vitro and in vivo conditions. Appl Organomet Chem. 2019;33(11):e5164. doi:10.1002/aoc.5164
51. Zangeneh MM, Zangeneh A, Pirabbasi E, Moradi R, Almasi M. Falcaria vulgaris leaf aqueous extract mediated synthesis of iron nanoparticles and their therapeutic potentials under in vitro and in vivo condition. Appl Organomet Chem. 2019;33(12):e5246. doi:10.1002/aoc.5246
52. Mahdavi B, Paydarfard S, Zangeneh MM, Goorani S, Seydi N, Zangeneh A. Assessment of antioxidant, cytotoxicity, antibacterial, antifungal, and cutaneous wound healing activities of green synthesized manganese nanoparticles using Ziziphora clinopodioides Lam leaves under in vitro and in vivo condition. Appl Organomet Chem. 2020;34(1):e5248. doi:10.1002/aoc.5248
53. Velasques K, Maciel TR, Dal Forno AHDC, et al. Co-nanoencapsulation of antimalarial drugs increases their in vitro efficacy against Plasmodium falciparum and decreases their toxicity to Caenorhabditis elegans. Eur J Pharm Sci. 2108;118:1–12. doi:10.1016/j.ejps.2018.03.014
54. Lboutounne H, Faivre V, Falson F, et al. Characterization of transport of chlorhexidine-loaded nanocapsules through hairless and wistar rat skin. Skin Pharmacol Physiol. 2004;17(4):176–182. doi:10.1159/000078820
55. Michels LR, Maciel TR, Nakama KA, et al. Effects of surface characteristics of polymeric nanocapsules on the pharmacokinetics and efficacy of antimalarial quinine. Int J Nanomedicine. 2019;14:10165–10178. doi:10.2147/IJN.S227914
56. Bajerski L, Maciel TR, Haas SE. Simultaneous determination of curcumin and quinine co-encapsulated in nanoemulsion by stability-indicating LC method. Curr Pharm Analy. 2018;14(3):255–261. doi:10.2174/1573412913666170330151347
57. Gomes GS, Maciel TR, Piegas EM, et al. Optimization of curcuma oil/quinine-loaded nanocapsules for malaria treatment. AAPS Pharm Sci Tech. 2018;19(2):551–564. doi:10.1208/s12249-017-0854-6
58. Driscoll DF. Lipid injectable emulsions: pharmacopeial and safety issues. Pharm Res. 2006;23(9):1959–1969. doi:10.1007/s11095-006-9092-4
59. Bruxel F, Laux M, Bartmann L, et al. Nanoemulsions as parenteral drug delivery systems. Quim Nova. 2012;35(9):1827–1840. doi:10.1590/S0100-40422012000900023
60. Zadymova NM, Tao M, Poteshnova MV. Tween 85 oil-in-water nanoemulsions with incorporated chlorhexidine base. Colloid J. 2018;80(2):158–166. doi:10.1134/S1061933X18020138
61. Durán-Lobato M, Muñoz-Rubio I, Holgad M, et al. Enhanced cellular uptake and biodistribution of a synthetic cannabinoid loaded in surface-modified poly (lactic-co-glycolic acid) nanoparticles. J Biomed Nanotech. 2014;10(6):1068–1079. doi:10.1166/jbn.2014.1806
62. Hashemi MM, Holden BS, Coburn J, et al. Proteomic analysis of resistance of Gram-negative bacteria to chlorhexidine and impacts on susceptibility to colistin, antimicrobial peptides, and ceragenins. Front Microbiol. 2019;10:210. doi:10.3389/fmicb.2019.00210
63. Zhu J, Huang Y, Chen M, Hu C, Chen Y. Functional synergy of antimicrobial peptides and chlorhexidine acetate against gram-negative/gram-positive bacteria and a fungus in vitro and in vivo. Infect Drug Resist. 2019;12:3227. doi:10.2147/IDR.S218778
64. Faoagali J, Fong J, George N, Mahoney P, O’Rourke V. Comparison of the immediate, residual, and cumulative antibacterial effects of Novaderm R, Novascrub R, Betadine Surgical Scrub, Hibiclens, and liquid soap. Am J Infect Control. 1995;23(6):337–343. doi:10.1016/0196-6553(95)90263-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
