Back to Journals » Infection and Drug Resistance » Volume 16
Chlamydia Psittaci Pneumonia-Induced Myocarditis: A Case Report
Authors Yang X, Liu Z, Liu X, Li Q, Huang H, Li R , He M
Received 28 April 2023
Accepted for publication 21 June 2023
Published 29 June 2023 Volume 2023:16 Pages 4259—4264
DOI https://doi.org/10.2147/IDR.S417241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xiaoming Yang,1,* Zhongda Liu,1,* Xiaojing Liu,1 Quan Li,1 Hui Huang,1 Rui Li,2 Meiyan He1
1Department of Respiratory and Critical Care Medicine, Lishui Hospital of Traditional Chinese Medicine Affiliated to Zhejiang University of Traditional Chinese Medicine, Lishui, 323000, People’s Republic of China; 2Department of Cardiology, Lishui Hospital of Traditional Chinese Medicine Affiliated to Zhejiang University for Traditional Chinese Medicine, Lishui, 323000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Meiyan He, Department of Respiratory and Critical Care Medicine, Lishui Hospital of Traditional Chinese Medicine Affiliated to Zhejiang University of Traditional Chinese Medicine, No. 800 Zhongshan Road, Liandu District, Lishui, Zhejiang Province, People’s Republic of China, Tel +8618767861290, Email [email protected]
Abstract: The incidence of Chlamydia psittaci infection has been reported to increase in recent years. The presentation of psittacosis infection varied widely, from none to severe symptoms. Mainly, psittacosis infection presents with pulmonary manifestations. Here, we report the case of a 60-year-old female patient with Chlamydia psittaci pneumonia complicated by myocarditis. After administering antibiotics, the patient recovered from severe atypical pneumonia and myocarditis. In general, Chlamydia psittaci rarely induces myocarditis. Moreover, the optimal therapeutic strategies remain unclear for such cases, especially with a high troponin T level. Metagenomic Next-Generation Sequencing (mNGS) can provide a quick and effective diagnosis of Chlamydia psittaci pneumonia; early intervention (antibiotic therapy and nutritional supplements for myocarditis) favors a good outcome, although complications may worsen the condition. Therefore, more studies are required to help improve understanding of the disease.
Keywords: Chlamydia psittaci, pneumonia, myocarditis, metagenomic next-generation sequencing, case report
Introduction
Psittacosis is a zoonotic infectious disease caused by Chlamydia psittaci (C. psittaci) and is usually due to its transmission from birds to humans. Psittacosis is often overlooked in practice because C. psittaci pneumonia has a low incidence, which causes approximately 1% of community-acquired pneumonia worldwide.1 C. psittacosis has atypical clinical manifestations, such as fever, cough, and fatigue.2,3 The formal identification of psittacosis depends on elevated chlamydia-specific IgG antibodies in serological assays increasing four-fold.4 This usually leads to a misdiagnosis in clinical practice.5 However, next-generation metagenomic sequencing (mNGS) was recently developed and is considered the primary method for diagnosing C. psittaci infection.6
Although more and more cases of C. psittaci pneumonia have been reported with the development of mNGS, the characteristics of C. psittaci pneumonia remain unclear, especially when complicated by myocarditis. To our knowledge, few cases of C. psittaci pneumonia complicated by myocarditis have been reported previously. This study describes a case of C. psittaci pneumonia diagnosed by bronchoalveolar lavage fluid (BALF) and mNGS, complicated by myocarditis and cured after early intervention.
Case Presentation
On February 12, 2022, a 60-year-old female patient was admitted to the respiratory clinic due to a fever for five days. She had no history of hypertension, diabetes, or cardiovascular and cerebrovascular disease. Although she had no history of alcohol consumption and cigarette smoking, she had a history of breeding pigeons. At the onset of her illness, she had a fever of 39 °C, headache, dizziness, chills, and a slight dry cough. No other symptoms were reported, such as chest tightness and chest pain. Before admission, nimesulide was self-administered, and her body temperature returned to normal. The chest computed tomography (CT) examination suggested pneumonia with mild right lower lobe atelectasis (Figure 1A).
At admission, the vital signs were: body temperature 35.7 °C, pulse 87/min, blood pressure 125/88 mmHg, and respiratory rate 20/min. The breath sounds were harsh, but no dry or wet rales were heard, and no other abnormal findings were found during the physical examination. The abnormal laboratory results were: white blood cell 22.6×109/L, lymphocyte (95.3%), C-reactive protein 96.6mg/L, procalcitonin 0.27ng/mL, D-dimer 1.75mg/L, blood gas analysis (pH, 7.466), pCO2 32.5mmHg, pO281.8mmHg, urine analysis (proteinuria and hematuria), increased CK, CK-MB, AST, ALT, Creatinine, Ferritin, Blood urea nitrogen, and PLT, normal troponin T (Table 1), normal electrocardiogram and echocardiogram.
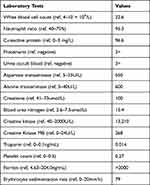 |
Table 1 The Patient’s Laboratory Test Results on Admission |
Subsequently, the patient was treated with ceftazidime (2.0g q12h), moxifloxacin (0.4g daily) for infection, and polyene phosphatidylcholine and Silibinin for liver injuries. A recurrent fever (39.9 °C) was observed. On the third day after admission (February 14, 2022), as she had been breeding pigeons and her fever had not subsided, antibiotic treatment was changed to intravenous meropenem (1.0g, 8 hours) and doxycycline (0.1g, 12 hours). Bronchoalveolar lavage fluid (BALF) was collected for mNGS, and the mNGS result supported the diagnosis of infection with C. psittaci (sequence number 333). Subsequently, meropenem was removed, but doxycycline was continued. On the sixth day after admission (February 17, 2022), her temperature had subsided to normal (36.2°C). However, her chest CT findings supported a diagnosis of adverse lung pathology, revealed by more lesions in the right lower lobe and new lesions in the left upper lobes (Figure 1B). Further physical examination revealed a normal body temperature and relief from dry cough and fatigue.
The patient had chest pain on day 13 after admission (February 24, 2022). Examination supported a diagnosis of myocarditis, with troponin 0.508ng/mL (ref, 0–0.1ng/mL), CK-MB 18.14ng/mL (ref, 0–2.88ng/mL), myoglobin <21ng/mL (ref, 25–58ng/mL), and abnormal ECG findings (V1-V3, slightly elevated; prolonged QT interval, maximum of 511ms). There was no obvious abnormality on the CTA examination. Several treatment modalities were used, such as antiplatelet aggregation therapy (aspirin enteric coated tablets, 100 mg, QN, PO; clopidogrel bisulfate tablets, 75 mg, QD, PO), anticoagulant therapy (low molecular weight heparin calcium, 4100AXaIU), atorvastatin calcium (20mg, QD, PO) for reducing lipid levels and nutrient supplements (Trimetazidine dihydrochloride tablets, 20mg, TID, PO) for myocarditis. Due to increased troponin levels (Figure 2), the patient was transferred to the cardiovascular department for further management, including antiplatelet and anticoagulant therapy (March 1, 2022). Meanwhile, the doxycycline treatment was discontinued, and after discharge from the hospital, she was treated with aspirin enteric coated tablets (antiplatelet) and atorvastatin calcium (lipid reduction) for two months. The patient was followed up three months later, and her chest CT imaging and troponin level had returned to normal (Figure 1C).
 |
Figure 2 Dynamic changes in the level of troponin in patients during hospitalization. |
Discussion
We described a case of psittacosis, a zoonotic disease caused by C. psittaci (a known Gram-negative intracellular parasitic bacterium). C. psittaci was first isolated from parrots, with birds being the primary host. Aerosols from sick birds mainly infect humans.1 This patient had a history of exposure to pigeons. Psittacosis can affect multiple organs; the symptoms are nonspecific and similar to pneumonia. Most patients have pneumonia, but infected patients could have secondary systemic damage,7 such as endocarditis. It is known to enter the body through the respiratory tract, increase within the reticuloendothelial cells of the liver and spleen, and then enter the pulmonary or other organs.8
C. psittaci may rarely affect the heart as the only manifestation of infection.9 In this case, it was reported with liver, kidney, and heart involvement. A previous report showed that cardiac injury caused by C. psittaci occurred in 11.5% of patients,10 indicating that myocardial damage may be rare in clinical settings. Recent studies have shown that increased myocardial enzymes, such as myoglobin, troponin, and creatine kinase, are the main risk factors for severe pneumonia and death from infection with C. psittaci.11 Early identification and intervention may be vital in improving outcomes for our case. The possible explanation for the myocarditis may be that: 1) C. psittaci infects myocytes and causes direct damage to cardiac tissues (such as membrane permeability), which usually presents with lactate dehydrogenase (LDH) release, superoxide production, and a reduced ATP level;12 2) myocarditis may be due to autoimmunity.9 Myocarditis is a predominantly induced inflammatory cardiac disorder that presents broad pathological and immunological changes. Monocytes and macrophages are the primary inflammatory cell subsets found in human and experimental myocarditis; the number and function of lymphocyte subsets and monocytes change, and antibody-mediated injury is produced during acute and chronic infection. Treatment strategies for asymptomatic myocarditis with increased cTnT and abnormal ECG findings remain unclear. However, patients with such conditions are not uncommon in practice. The short-term prognosis of acute myocarditis is generally good, but the long-term consequences are unknown.13 In addition to antibiotic treatment, our patient had several timely interventions, such as nutritional supplements, antiplatelet aggregation, and anticoagulant therapy. Finally, the patient recovered from the myocarditis, indicating that suspicion of myocarditis complicating C. psittaci pneumonia may result in better management of such a severe disease.
mNGS is designed using high-throughput sequencing technology, analyzing the existing microbial nucleic acid sequence in clinical samples and detecting the pathogenic microorganism by comparing the sequencing results with existing sequence databases. It has been reported that mNGS is a sensitive detection method with a detection rate of almost 89%, much higher than the standard pathogen detection rate (25.73%).14 Theoretically, this tool can detect all pathogens in practice, especially rare microorganisms. In addition, antibiotic use has a less prominent role in mNGS results.15 Furthermore, mNGS is hypothesized, without culture, and can produce results quickly (approximately 48–72 hours).
The traditional culture of blood and sputum pathogens takes time and has a low detection rate.16 In addition, C. psittaci is not cultivable in usual media but only in specific cell cultures. In most reports, if repeated sputum and blood cultures were unable to detect Chlamydia, mNGS may be a valuable tool for the diagnosis of C. psittaci infection.17 Hence, BALF mNGS could be considered the first choice to ensure timely and effective treatment.18 Tetracycline antibiotics are the drug of choice for C. psittaci infection in humans. Mild to moderate conditions can be treated with oral doxycycline or tetracycline hydrochloride.19 In the study, the patient recovered from fever after doxycycline administration. There are some limitations to this case report. Firstly, due to its retrospective nature and without sequence evidence of C. avium, further discrimination between C. psittaci and C. avium was not performed for the case. Second, diagnosing myocarditis may require more evidence, such as cardiac imaging and biopsy.
Conclusion
In conclusion, although C. psittaci pneumonia complicated by myocarditis may be infrequent, it is considered a severe underlying condition. mNGS is a tool for rapidly diagnosing C. psittaci infection and can minimize diagnostic delays. Besides treating C. psittaci pneumonia complicated by myocarditis with antibiotics, better managing the myocarditis is the first step toward successful recovery. Nutritional supplements, antiplatelet aggregation, and anticoagulant therapy can significantly improve the symptoms and outcomes. These findings provide fresh evidence for improving the current management of C. psittaci pneumonia complicated by myocarditis. Furthermore, troponin levels should be routinely measured when C. psittaci infection is suspected.
Ethics Approval and Informed Consent
The Ethics Committees for the Lishui Hospital of Traditional Chinese Medicine affiliated with the Zhejiang University for Traditional Chinese Medicine ((2023) LW-021) approved this study. Informed consent was obtained from patients and guardians.
Consent for Publication
The patient has provided written informed consent to have the case details and accompanying images published.
Funding
The study was supported by the Science and Technology Project of Lishui (No.2022GYX53).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hogerwerf L, Baan B. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105.
2. Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis. 2015;73(1):1–15. doi:10.1093/femspd/ftu007
3. Dai J, Lian X, Mo J, et al. Case report: a clinical case study of six patients with Chlamydia psittaci pneumonia. Front Cell Infect Microbiol. 2023;13:1084882. doi:10.3389/fcimb.2023.1084882
4. You W, Chen B, Li J, et al. Pulmonary migratory infiltrates due to mycoplasma infection: case report and review of the literature. J Thorac Dis. 2016;8(6):E393–398. doi:10.21037/jtd.2016.03.85
5. Branley JM, Weston KM, England J, Dwyer DE, Sorrell TC. Clinical features of endemic community-acquired psittacosis. New Microbes New Infect. 2014;2(1):7–12. doi:10.1002/2052-2975.29
6. Wang K, Liu X, Liu H, et al. Metagenomic diagnosis of severe psittacosis using multiple sequencing platforms. BMC Genomics. 2021;22(1):406. doi:10.1186/s12864-021-07725-9
7. Ojeda Rodriguez JA, Modi P, Brady MF. Psittacosis Pneumonia. In: StatPearls. Treasure Island (FL): StatPearls; 2022.
8. Bartlett JG. Johns Hopkins ABX Guide 2012. Jones & Bartlett Publishers; 2011.
9. Diaz F, Collazos J. Myopericarditis due to Chlamydia psittaci. The role of autoimmunity. Scand J Infect Dis. 1997;29(1):93–94. doi:10.3109/00365549709008673
10. Li Y, Lin F, Li W, et al. Comparison of clinical, laboratory and radiological characteristics between Chlamydia psittaci and adenovirus pneumonias: a multicenter retrospective study. Int J Infect Dis. 2023;126:114–124. doi:10.1016/j.ijid.2022.11.029
11. Yang F, Li J, Qi B, et al. Clinical symptoms and outcomes of severe pneumonia caused by chlamydia psittaci in Southwest China. Front Cell Infect Microbiol. 2021;11:727594. doi:10.3389/fcimb.2021.727594
12. Wang G, Burczynski F, Hasinoff B, Zhong G. Infection of myocytes with chlamydiae. Microbiology. 2002;148(Pt 12):3955–3959. doi:10.1099/00221287-148-12-3955
13. Sagar S, Liu PP, Cooper LT
14. Huang J, Jiang E, Yang D, et al. Metagenomic next-generation sequencing versus traditional pathogen detection in the diagnosis of peripheral pulmonary infectious lesions. Infect Drug Resist. 2020;13:567–576. doi:10.2147/IDR.S235182
15. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi:10.1093/cid/ciy693
16. Nieuwenhuizen AA, Dijkstra F, Notermans DW, van der Hoek W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis. 2018;18(1):442. doi:10.1186/s12879-018-3317-0
17. Teng XQ, Gong WC, Qi TT, et al. Clinical analysis of metagenomic next-generation sequencing confirmed chlamydia psittaci pneumonia: a case series and literature review. Infect Drug Resist. 2021;14:1481–1492. doi:10.2147/IDR.S305790
18. Wu HH, Feng LF, Fang SY. Application of metagenomic next-generation sequencing in the diagnosis of severe pneumonia caused by Chlamydia psittaci. BMC Pulm Med. 2021;21(1):300. doi:10.1186/s12890-021-01673-6
19. Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control chlamydia psittaci infection among humans (Psittacosis) and Pet Birds (Avian Chlamydiosis), 2017. J Avian Med Surg. 2017;31(3):262–282. doi:10.1647/217-265
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

