Back to Journals » Infection and Drug Resistance » Volume 16
Chlamydia psittaci Pneumonia Complicated with Lower Extremity Atherosclerotic Occlusive Disease
Authors He Y , Wang S, Deng J, Pu Q, Chen H, Huang L
Received 1 February 2023
Accepted for publication 29 March 2023
Published 12 April 2023 Volume 2023:16 Pages 2141—2145
DOI https://doi.org/10.2147/IDR.S393256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
YongHong He,1 SongPing Wang,1 Jun Deng,1 Qian Pu,2 Hong Chen,3 Lan Huang3
1Department of Respiratory Medicine and Critical Care Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou City, Sichuan Province, 646000, People’s Republic of China; 2Department of Intensive Care Medicine, Chengdu Second People’s Hospital, ChengDu City, Sichuan Province, 610000, People’s Republic of China; 3Department of Respiratory Medicine, Chengdu Second People’s Hospital, ChengDu City, Sichuan Province, 610000, People’s Republic of China
Correspondence: SongPing Wang, Department of Respiratory Medicine and Critical Care Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou City, Sichuan Province, 646000, People’s Republic of China, Email [email protected]
Abstract: Chlamydia is a zoonotic pathogen that mainly infects poultry and pet birds. This Gram-negative obligate intracellular parasite also causes human psittacosis, the severity of which varies from mild flu-like symptoms to life-threatening severe pneumonia, including sepsis, acute respiratory distress syndrome, and multiple organ failure. Inhalation of aerosols from contaminated bird excreta through the respiratory tract is the main route of transmission to humans. Here, we present a case of Chlamydia psittaci pneumonia accompanied by lower extremity atherosclerotic occlusive disease. A 48-year-old man was admitted to the emergency department with a four-day history of cough and dyspnea. A detailed history revealed his contact with domestic pigeons. The results of metagenomic next-generation sequencing of bronchoalveolar lavage fluid suggested C. psittaci infection. Antibacterial agents were switched to targeted doxycycline, but in the next week, skin examination revealed acrocyanosis of both lower extremities, and the remarkable palpable purpura progressively worsened. Re-examination of the lower extremity vascular ultrasound suggested left dorsalis pedis artery occlusion and right peroneal vein thrombosis, which resulted in the amputation of both legs. This case is the first report of C. psittaci pneumonia combined with arterioocclusive sclerosis of both lower extremities
Keywords: Chlamydia psittaci, lower extremity atherosclerotic occlusive disease
Introduction
Chlamydia psittaci is an intracellular Gram-negative bacterium that obligately parasitizes eukaryotic cells, mainly infecting birds, poultry, and humans.1 It cause severe respiratory and reproductive disease, pneumonia, and even death in some cases. In recent years, the increasing application of next-generation sequencing has enabled cases of human psittacosis and the related mortality to be reported more frequently, especially in those having close contact with pet birds and poultry.2
Case Presentation
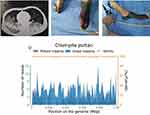
A 48-year-old man was admitted to the emergency department with a four-day history of cough and dyspnea. The patient had had a fever for two days before admission, with the highest temperature of 39.3°C, accompanied by small amounts of yellowish sputum, chills, and sweats. He denied hemoptysis and had taken “oral cefixime and diclofenac sodium”, which failed to have an effect, prior to admission. He had previously been in good health, with no history of hypertension, diabetes, stroke, or myocardial infarction. Also, he had had no prior illnesses or hospitalizations before the present illness, and had no known drug allergies. He had been living in Chengdu for a long time, and denied any previous tobacco, alcohol, or drug abuse. He was married and had three healthy daughters. His family history was negative for genetic disease. According to the physical examination, his vital signs included body temperature 40.0°C, heart rate 130 beats/min, breathing rate 41 breaths/min, blood pressure 111/59 mmHg, and oxygen saturation (SpO2) of 93%. Chest computed tomography (CT) revealed diffuse, bilateral, and interstitial infiltrates (Figure 1A).
A routine blood examination revealed a white blood cell (WBC) count of 6.88×109/L; the neutrophil percentage was 85.4%, lymphocyte percentage 11.0%, erythrocyte sedimentation rate (ESR) 23 mm/h, and concentration of procalcitonin (PCT) 0.12 ng/mL. Coagulation indices were: prothrombin time (PT) 12.5 s, international normalized ratio (INR) 1.16, fibrinogen (Fbg) 7.01 g/L, thrombin time (TT) 148 s, D-dimer (D2) 1.66 µg/mL, and fibrin degradation products (FDP) 6.80 µg/mL. Blood gas analysis indicated pH 7.492, PCO2 36 mmHg, PO2 60.4 mmHg, and an oxygenation index of 234 mmHg. Electrolytes, renal function, myocardial injury markers, tumor markers, infection markers, Chlamydia pneumoniae, and Mycoplasma pneumoniae were not significantly abnormal. On the first day after admission, the patient developed respiratory failure, and we transferred him to the intensive care unit, where he received mechanical ventilation and supportive care. Pleural effusion and subsegmental pulmonary embolisms were not present, and there was no history of cardiac vegetation. Urine tests indicated the presence of glucose, and re-examination showed no pathogenic bacteria. He was treated with moxifloxacin (0.4 g intravenously qd), piperacillin tazobactam (3 g intravenously q6h), and oseltamivir (75 mg orally q12h) for 3 days. The body temperature reached 40.0°C, fever continued, and the pulmonary infection on the repeat CT scan suggested progression. Moxifloxacin was then replaced with linezolid (600 mg intravenously q12h) and meropenem (1 g intravenously q8h). On day 4, after combined use of linezolid, meropenem, and oseltamivir, although the fever subsided, other symptoms and signs persisted, and the WBC count and PCT indicated inflammatory activity. Physical examination revealed inspiratory crackles in both lungs. On day 6, the laboratory test results showed a WBC total count of 10.11×109/L, neutrophil count 6.38×109/L, 81.8% neutrophils, 32.5% lymphocytes, and lymphocytes 1.51×109/L. C-reactive protein (CRP) was 20 mg/L, ESR was 51 mm/h, and PCT was 15.4 ng/mL (<0.5 ng/mL). All of the tests for viruses were negative, including influenza A and B virus, cytomegalovirus, Toxoplasma, Epstein–Barr virus, rubella virus, and herpes simplex virus. Cryptococcal antigen, galactomannan test, and β-D-glucan test were also negative. Routine laboratory screening revealed all the indicators, with no abnormal findings for anti-nuclear antibody, anti-extractable nuclear antigen antibody, or anti-neutrophilic cytoplasmic antibody. The results of four sets of peripheral blood cultures during his fever episodes showed no abnormalities.
To obtain more information on etiology, we further communicated with the patient’s family and obtained a detailed history that revealed his contact with domestic pigeons. Unbiased metagenomic next-generation sequencing (mNGS) of the bronchoalveolar lavage fluid (BALF) identified 571 of 70,732 sequence reads corresponding to C. psittaci. The total number of bases in the genome of this species was 1,179,220 bp and the total length of the sequence coverage of this species was 52,940 bp, with a coverage of 4.4894% and an average depth of 1.08×, and there was no sequence read corresponding to other pathogens, including sequence reads of bacteria or viruses (Figure 1D). The results of mNGS of BALF suggested C. psittaci infection. Thus, antibacterial agents were switched to targeted doxycycline (0.2 g on day 7, followed by 0.1 g bid on days 8–15) combined with moxifloxacin (0.4 g qd intravenous drop infusion, days 7–13) and meropenem (1 g intravenously q8h, days 7–13), and the empirical antiviral agents were ceased. From the 7th day, the PCT and WBC count continued to decline, and his fever finally returned to normal on day 15. On the 1st day, there were no obvious abnormalities in lower limb vascular ultrasonography, but from the 3rd day, skin examination revealed acrocyanosis of both lower extremities, and the remarkable palpable purpura progressively worsened. Re-examination of the lower extremity vascular ultrasound suggested left dorsalis pedis artery occlusion and right peroneal vein thrombosis (Figure 1B and C). The pulse of the left dorsal pedal artery was not palpable. During hospitalization, no pathogens were detected in sputum, BALF, or blood culture, indicating that mNGS was more sensitive than conventional detection methods. Despite his critical condition, which resulted in amputation of both legs, the patient survived multiple organ failure and was repatriated for further rehabilitation. At one-year follow-up, he was doing well, with no further symptoms.
Discussion
Chlamydia psittaci can be classified into 10 genotypes with varying preference for host species.3 Genotypes A and E can be transmitted to humans through exposure to infectious avian feces, inhalation of aerosols, nasal secretions, and even dust from the feathers of infected birds such as parrots. Psittacosis, a disease caused by C. psittaci infection, is considered a rare cause of pneumonia;4 exposure to birds is the main risk factor for psittacosis. It has been reported that of approximately 1200 patients, 70% had had contact with birds in a domestic environment, 5% had been exposed to wild birds, 13% were poultry workers, and 12% had no relevant contact history.5 Patients are infected by inhalation of C. psittaci when exposed to excreta or feather dust from various birds, especially psittacines. Chlamydia psittaci pneumonia accounts for 1.5% of community-acquired pneumonia cases, with symptoms mimicking those of influenza, typically including fever, fatigue, headache, and coughing,6 owing to a lack of routine detection of C. psittaci as well as the limited sensitivity of common diagnostic methods. The accurate determination of the incidence and prevalence of C. psittaci is difficult, and it is necessary to discuss psittacosis to strengthen clinical awareness and management of the disease.
Patients with psittacosis commonly present with flu-like symptoms of fever, dyspnea, dry cough, and headache. In this case, the first symptoms of the patient were fever, muscle aches accompanied by limb weakness, and paroxysmal cough, followed by sepsis and multiple organ failure. The patient’s lung CT findings revealed pneumonia, the WBC count was normal in routine blood tests, and the neutrophil percentage was 85.4%. However, the high CRP and PCT indicated serious pneumonia, and the patient was treated with meropenem initially. During hospitalization, all of the tests of the alveolar lavage fluid, sputum, and blood cultures were negative. But the patient got worse, and his temperature and signs of infection continued to rise. To obtain an accurate diagnosis, mNGS of BALF was performed. Four days after admission, the results from the BALF confirmed the presence of C. psittaci.
Effective drugs against C. psittaci include tetracyclines, macrolides, and quinolones. We used doxycycline combined with meropenem for anti-infective therapy, while from the 3rd day the patient presented with arterial occlusion. The disease progressed rapidly and eventually required amputation.7 To our knowledge, multiple organ failure together with atherosclerotic occlusive disease leading to amputation has not been previously reported in cases of psittacosis uncomplicated by endocarditis. After multidisciplinary discussion, we speculated that it may have been due to the severe infection caused by C. psittaci pneumonia, which resulted in the destruction of the endothelial cell layer of the lower limb artery vessels, combined with serious hemodynamic disorder, and finally led to arterioocclusive sclerosis of both lower extremities. The mechanism still needs further investigation. This case was the first report of C. psittaci pneumonia combined with arterioocclusive sclerosis of both lower extremities.
Because of a relatively low level of awareness, C. psittaci infection has been overlooked by physicians. Laboratory testing for C. psittaci includes culture, serological assay, and PCR, which require complex facilities.8,9 Serological tests are only appropriate for retrospective diagnosis because sera from both the acute and convalescent phases of the illness are required. Only in the acute phase can PCR provide a specific, fast method, and mNGS is recommended to diagnose C. psittaci infection. According to a set of universally accepted standards, mNGS could even provide semiquantitative information regarding the load of C. psittaci via sequence reads. The information is important in determining whether a specific microbe is the causative pathogen in the samples, and host background sequences are a must. mNGS can provide a high detection rate and help in initiating treatment early, because it can also simultaneously examine the nucleic acid sequences of multiple pathogenic microorganisms. Hence, patient outcomes can be improved, while minimizing the use of non-targeted antibiotics.
Tetracycline antibiotics are the first choice for the treatment of human psittacosis.10 Mild psittacosis can be treated with oral doxycycline, and severe disease with intravenous doxycycline. After treatment with tetracycline antibiotics, there is a response within 48 h, with a decline in infection indicators and body temperature. The course of medication should last for at least 14–21 days; otherwise, insufficient treatment may cause relapse.11 For patients with contraindications to tetracyclines, macrolide antibiotics, such as azithromycin, provide the best alternative. Fluoroquinolones, such as moxifloxacin or levofloxacin, have been proven to be effective against C. psittaci.12 Treatment using a combination of tetracyclines with macrolides or quinolones can be effective in severely infected patients, especially those with a life-threatening condition.
Some previous research used mNGS to identify the presence of C. psittaci from the BALF or from biopsy of lung tissue.13,14 A critical review showed that there have been limited studies on the simultaneous confirmation of the presence of the pathogen in the blood and BALF using mNGS. This case of severe pneumonia in our department, which was complicated by sepsis and multiple organ failure due to C. psittaci, was diagnosed through mNGS of BALF. The review suggested that mNGS is a novel test method for accurately diagnosing pathogens, especially in rare pathogen infections.15 It is not appropriate for this case report to explore psittacosis and the role of mNGS in the diagnosis in more depth, but the accumulation of such reports is the need of the hour.
Conclusion
In summary, this is the first case of C. psittaci pneumonia complicated with lower extremity atherosclerotic occlusive disease in China. Using mNGS and serology, we have proven that multiple organ failure in this patient, following direct contact with birds, was caused by C. psittaci. This case provides a reminder that clinicians should expect the unexpected in terms of infectious agents, and highlights the importance of the microbiological diagnostic procedures. Given its superiority in simultaneously examining the nucleic acid sequences of multiple pathogenic microorganisms, mNGS can provide a high detection rate and help clinicians to initiate early treatment, thus improving patient outcomes and minimizing the use of non-targeted antibiotics.
Ethical Approval and Consent to Participate
The patient signed the informed consent form, and agreed to the publication of the case details and any accompanying images.
It was not necessary to obtain ethical approval because this article is based on a case report, and does not involve any new studies of human or animal subjects performed by any of the authors, and therefore does not need institutional approval.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Veletzky L, Huebl L, Schulze Zur Wiesch J, Schmiedel S. Psittacosis in a traveller. J Travel Med. 2021;28(6). doi:10.1093/jtm/taab062
2. Li N, Li S, Tan W, Wang H, Xu H, Wang D. Metagenomic next-generation sequencing in the family outbreak of psittacosis: the first reported family outbreak of psittacosis in China under COVID-19. Emerg Microbes Infect. 2021;10(1):1418–1428. doi:10.1080/22221751.2021.1948358
3. Radomski N, Einenkel R, Müller A, Knittler MR. Chlamydia-host cell interaction not only from a bird’s eye view: some lessons from Chlamydia psittaci. FEBS Lett. 2016;590(21):3920–3940. doi:10.1002/1873-3468.12295
4. Rybarczyk J, Versteele C, Lernout T, Vanrompay D. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. 2020;75(1):42–48. doi:10.1080/17843286.2019.1590889
5. Hulin V, Bernard P, Vorimore F, et al. Assessment of chlamydia psittaci shedding and environmental contamination as potential sources of worker exposure throughout the mule duck breeding process. Appl Environ Microbiol. 2015;82(5):1504–1518. doi:10.1128/AEM.03179-15
6. Hogerwerf L, Gier DE, Baan B, Van Der Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105. doi:10.1017/S0950268817002060
7. Yung AP, Grayson ML. Psittacosis--a review of 135 cases. Med J Aust. 1988;148(5):228–233. doi:10.5694/j.1326-5377.1988.tb99430.x
8. Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control chlamydia psittaci infection among humans (Psittacosis) and pet birds (Avian Chlamydiosis), 2017. J Avian Med Surg. 2017;31(3):262–282. doi:10.1647/217-265
9. Kohlhoff SA, Hammerschlag MR. Treatment of Chlamydial infections: 2014 update. Expert Opin Pharmacother. 2015;16(2):205–212. doi:10.1517/14656566.2015.999041
10. Stewardson AJ, Grayson ML. Psittacosis. Infect Dis Clin North Am. 2010;24(1):7–25. doi:10.1016/j.idc.2009.10.003
11. Hyde SR, Benirschke K. Gestational psittacosis: case report and literature review. Mod Pathol. 1997;10(6):602–607.
12. Donati M, Rodrìguez Fermepin M, Olmo A, D’Apote L, Cevenini R. Comparative in-vitro activity of moxifloxacin, minocycline and azithromycin against Chlamydia spp. J Antimicrob Chemother. 1999;43(6):825–827. doi:10.1093/jac/43.6.825
13. Simner PJ, Miller S, Carroll KC. Understanding the promises and hurdles of metagenomic next-generation sequencing as a diagnostic tool for infectious diseases. Clin Infect Dis. 2018;66(5):778–788. doi:10.1093/cid/cix881
14. Gu L, Liu W, Ru M, et al. The application of metagenomic next-generation sequencing in diagnosing Chlamydia psittaci pneumonia: a report of five cases. BMC Pulm Med. 2020;20(1):65. doi:10.1186/s12890-020-1098-x
15. Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. doi:10.1056/NEJMoa1401268
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.