Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Chemical Constituents and Antidiabetic Activity of Dichloromethane Extract from Ficus carica Leaves
Received 18 January 2023
Accepted for publication 25 March 2023
Published 5 April 2023 Volume 2023:16 Pages 979—991
DOI https://doi.org/10.2147/DMSO.S405150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Limei Lin, Yin Zhang
Department of Pharmacy, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian, People’s Republic of China
Correspondence: Yin Zhang, Department of Pharmacy, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian Province, 362000, People’s Republic of China, Tel +86 13328579972, Email [email protected]
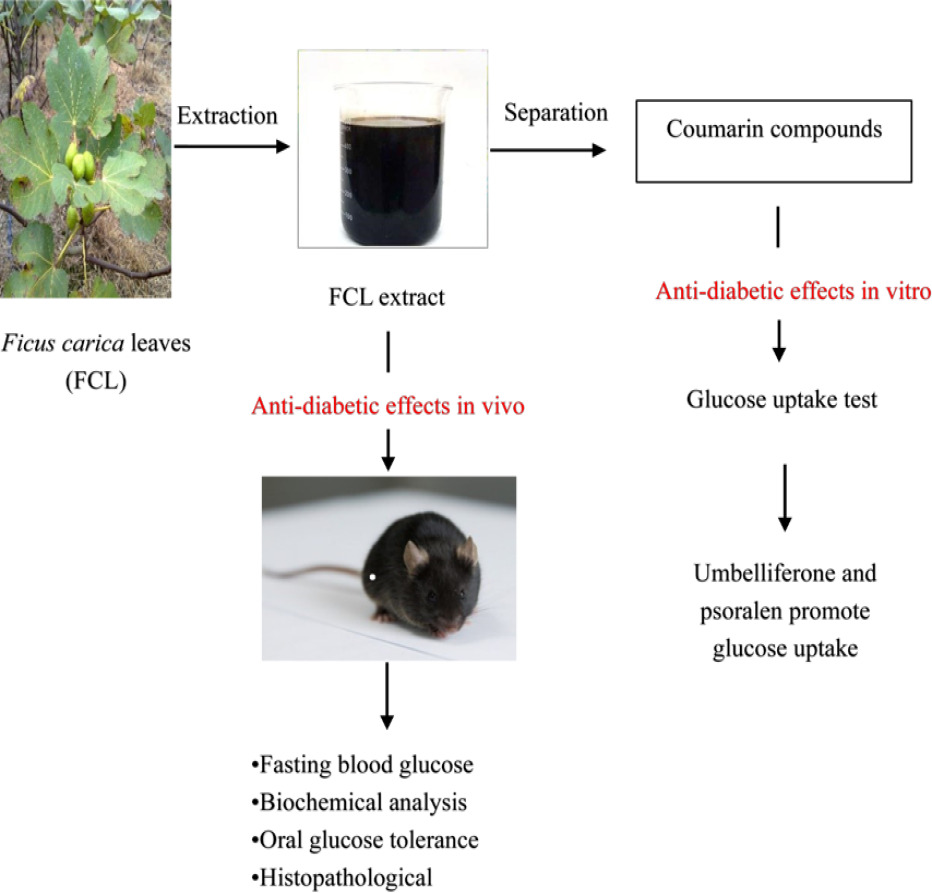
Purpose: To evaluate the dichloromethane extract of Ficus carica leaves (FCL) had a hypoglycemic impact in diabetic mice, as well as to identify the bioactive components in the extract and analyze their anti-hyperglycemia potential in HepG2 cells.
Material and Methods: The antidiabetic activity of dichloromethane extract of Ficus carica leaves was evaluated in diabetic mice induced by streptozotocin (STZ,100 mg/kg) combined with high-fat diet. The fasting blood glucose (FBG), blood lipids, oral glucose tolerance, glycated hemoglobin (HbA1c), and pathological change effects of the extract were measured after administering two doses of the extract (500 and 1000 mg/kg). On the other hand, we used column chromatography to isolate the dichloromethane extract, and we structurally identified the compounds based on 1H NMR and 3C NMR spectra. The hypoglycemic activity of isolated compounds was investigated in palmitic acid (PA)-induced HepG2 cells.
Results: FCL extract lowers blood glucose and improves blood lipids and the pancreatic β-cell also tend to recover whether the psoralen is removed or not. Meanwhile, three coumarins except psoralen were isolated from dichloromethane extract: 3,4-dihydropsoralen, umbelliferone and 7-hydroxyl-6-methylcoumarin. Psoralen and umbelliferone promoted glucose uptake in HepG2 cells.
Discussion and Conclusion: In vivo experiments, dichloromethane extract of FCL has potential antidiabetic activity, mainly by lowering blood glucose, improving blood lipids, glucose tolerance and repairing pancreatic islet damage, which justifies its use in the treatment of diabetes in Spanish folklore. Additionally, in vitro experiments, psoralen and umbelliferone demonstrated substantial glucose-lowering activity.
Keywords: FCL, diabetes, dichloromethane extract, psoralen, umbelliferone
Graphical Abstract:
Introduction
Diabetes is a group of metabolic disease characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. According to the International Diabetes Federation, there are roughly 463 million diabetics worldwide in 2019, with that number anticipated to rise to 700 million by 2045.1 Diabetes has become much more common in recent decades, with type 2 diabetes accounting for more than 90% of cases.2 Chronic hyperglycemia has already shown to increase the risk of cardiovascular disease and organ failure.3 As a result, effective blood glucose control is a critical step preventing or correcting diabetic complications and enhancing the quality of life of diabetic patients.
Nowadays, the agents used for diabetes treatment mainly are synthetic drugs such as biguanides, sulfonylureas, thiazolidinediones, SGLT2 inhibitors and GLP-1.4,5 Synthetic drugs are effective and can improve glucose concentration to varying degrees. However, all of these medications have specific toxic side effects. Long-term use of insulin causes decreased insulin receptor sensitivity, resulting in insulin resistance and eventually leading to worsening of control conditions.6 Biguanides are often associated with gastrointestinal adverse reactions. Major concerns related to the use of sulfonylureas are hypoglycemia and weight gain. The use of pioglitazone has been associated with an increased risk of edema, heart failure and weight gain.7 Genital and urinary tract infections are common side effects of modern anti-diabetic medicines such SGLT2 inhibitors.8 Therefore, searching for high efficiency and low toxicity hypoglycemic agents is a hot topic. Plant materials are considered promising sources of novel medications to counteract numerous diseases, including diabetes mellitus.9 Traditional Chinese medicine offers the advantages of moderate action and multi-component-multi-target therapy. Many plant-derived substances, such as astragalus polysaccharide,10 resveratrol11 and ginsenoside Rb112 have anti-diabetic properties. Ficus carica leaves (FCL) is another example.
Ficus carica belongs to Moraceae family that has therapeutic potential. Its leaves, fruits and seeds have long been utilized traditional medicine to treat various ailments, including antioxidant, hepatoprotective and hypoglycemic.13,14 Experiments have proven that FCL decoction has a hypoglycemic effect. In Spain, decoction of FCL was used in the folklore treatment of diabetes.15 A study reported that water decoction from FCL has been shown to possess hypoglycemic effects in diabetic rats and diabetic patients.16 Researchers discovered that FCL aqueous extract has the ability to lower glucose levels and may also have the power to minimize insulin dosage.17 Research has also shown that aqueous extract of FCL lowered blood glucose in diabetic rats in a dose dependent way, and speculated that the extract contained active components that protect pancreatic cells.18 The results of Ramadan et al19 also showed that MeOH extracts of FCL attenuated pancreatic β-cell damage. Furthermore, data reported that FCL water and MeOH extracts inhibited α-glucosidase and α-amylase significantly, with the water extract having stronger inhibitory activity than the MeOH extract.20 Subsequently, the study by Mopuri et al21 also confirmed that FCL extract has different inhibitory activities on α-glucosidase and α-amylase. In streptozotocin (STZ)-induced diabetic mice, Irudayaraj et al22 discovered that psoralen extracted from FLC dramatically lowered blood glucose levels. FCL dichloromethane extract dramatically lowered blood glucose in diabetic mice, according to previous research in our lab.23
To our knowledge, the systematic isolation of the chemical composition of FCL and the hypoglycemic studies of the isolated compounds have not been reported. Hypoglycemic research on FCL is still at crude extract level, and the primary active components are unclear. As a result, we investigated the chemical elements of FCL as well as its hypoglycemic activity in order to identify the most effective molecules and develop novel anti-diabetic drugs. Despite the fact that psoralen in FCL has been shown to have hypoglycemic properties,22 the chemical composition of FCL is complex, and more research is needed in addition to psoralen.
Materials and Methods
Plant Material
The FCL were collected from Lvyang Agriculture Development Co., Ltd. (119.00° Latitude and 25.43° Longitude) in Putian city, Fujian province, in May 2018 and were identified by Professor Cui-Lan Ma, School of Horticulture, Fujian Agriculture and Forestry University. The FCL planted in greenhouse planting base, where the average greenhouse temperature and relative humidity were kept at roughly 35°C and 60%, respectively. A voucher specimen (NO. FMU, 20180510) has been deposited at the central laboratory of Second Affiliated Hospital of Fujian Medical University, Quanzhou City, Fujian Province, China.
Preparation of Dichloromethane Extract
The dried FCL (1.0 kg) were soaked in 95% EtOH. After that, the filtrate was evaporated in a rotary evaporator, yielding extract. With distilled water, the whole extract was dispersed and deposited in a separatory funnel. Petroleum ether was used to defeat the filtrate, which was then followed by an equivalent amount of dichloromethane (4 extractions of each solvent). To obtain the dichloromethane extract, the filtrate was evaporated in a rotary evaporator. The dichloromethane extract was dissolved with 2% sodium hydroxide, and the pre-treated D101 macroporous adsorption resin column is placed on the dissolving solution. The macroporous adsorption resin was rinsed with water for about 3 column volumes to remove the psoralen, and the substance on the macroporous adsorption resin was completely eluted with 95% MeOH and the dichloromethane extract containing almost no psoralen was obtained after concentration.
Animals and Preparation of Diabetic Mellitus Model
SPF C57BL/6J male mice (6–7 weeks old, weighing 18-20 g) were purchased from Shanghai Silaike Experimental Animal Co., Ltd. All animals were kept under standard environmental conditions of temperature (25±2°C), relative humidity (40–70%), and 12/12-h dark/light cycles. All animals had free access to pellet food and water ad libitum. All animals (including the mice euthanasia procedure) were approved by the animal ethics committee of the Second affiliated hospital of Fujian Medical University (NO. 2019 [175]) and according to the AAALAC and the IACUC guidelines. In addition, maximum efforts were made to minimize pain and suffering of the animals.
In the study, all mice were divided into normal control (NC) group and experimental group after 2 weeks of adaptive feeding. NC group was fed normal pellet diet and experimental group was high fat diet (HFD: 17.9% of energy from fat, 12.6% of energy from sugar, 17.5% of energy from protein, 2.5% of energy from cholesterol, 1% of energy from sodium cholate, and 48.5% of energy from carbohydrate, purchased from Huafukang Biotechnology Co., Ltd., Beijing). After 4 weeks of dietary manipulation, the HFD feeding mice received an intraperitoneal injection of 100 mg/kg STZ (Sigma, USA).24,25 The NC group was injected with equal volumes of citrate buffer. The fasting blood glucose (FBG) levels were assessed 3 days after STZ injection using a glucometer (Roche Diagnostic, Basel, Switzerland), and only mice with FBG levels over 11.1 mM were considered to be diabetic mice26–28 and used for future study. All animals, with the exception of NC group, are still fed HFD.
Drug Administration
The anti-hyperglycemia activity of dichloromethane extract was tested using Arafa’s method.29 The animals were randomly divided into 7 groups (n = 7 per group). NC group: normal mice were treated with vehicle alone. Diabetic model (DM) group: diabetic mice were treated with vehicle alone. Pioglitazone (Pio) group: diabetic mice were treated with pioglitazone (20 mg/kg). Ficus carica leaves dichloromethane extract (FCLE-500) group: diabetic mice were treated with FCLE (500 mg/kg). Ficus carica leaves dichloromethane extract (FCLE-1000) group: diabetic mice were treated with FCLE (1000 mg/kg). Ficus carica leaves dichloromethane extract removed the psoralen (FCLP-500) group: diabetic mice were treated with FCLP (500 mg/kg). Ficus carica leaves dichloromethane extract removed the psoralen (FCLP-1000) group: diabetic mice were treated with FCLP (1000 mg/kg). Oral gavage was used to administer the drug to all animals once a day for 6 weeks.
Biochemistry
On days of 0, 7, 14, 21, 28, 35, and 42, the FBG levels were measured. The oral glucose tolerance test (OGTT) was performed on day of 41 of treatment. After an overnight (12h) fasting, all animals were gavaged with 2% glucose (2.0 g/kg). After loading glucose, blood was drawn from the tail vein at 0,30,60 and 120 min. Glucose contents were calculated as the areas under the curve (AUC). The animals were anesthetized with 1% pentobarbital and euthanized on day 42, after a 12-hour fast. Blood was collected from mice orbits before euthanized. The blood was centrifuged for 10 minutes at 4°C and 3500 r/min. Serum was tested for triglycerides (TG) and total cholesterol (TC) using commercial assay kits (Beckman, Coulter, Brea, CA, USA) according to the manufacturer’s recommendations. The whole blood was tested for glycosylated hemoglobin (HbA1c) using HbA1c kits (Beckman, Coulter, Brea, CA, USA) according to the manufacturer’s recommendations.
Hematoxylin and Eosin (H & E) Staining of Mice
The pancreatic tissue samples were preserved in 10% formalin. The pancreas was dehydrated and embedded in paraffin, and cut into 3–5 μm thickness. To observe the structure of pancreatic tissue, sections were stained with hematoxylin and eosin (H&E). The sections were visualized using light microscopy (OLYMPUS BX53),30 and digital images were captured and analyzed.
Extraction and Purification of Dichloromethane Extract
The dried FCL (15 kg) were extracted 4 times with 95% EtOH for 7 days. The EtOH filtrate was concentrated under vacuum using a rotary evaporator (Yigong Yuhua Instrument Co., Ltd, Henan, China) to obtain the residue. The EtOH extract was suspended in distilled water (6 L) and extracted with petroleum ether (6 L × 3) and dichloromethane (6 L × 3), successively (Guangdong Guanghua Technology Co., Ltd, Guangdong, China). The fraction was concentrated in vacuo to get the dichloromethane fraction (700 g). Then, the dichloromethane fraction was chromatographed on D-101 macroreticular resin (Qingdao Haiyang Chemical Co., Ltd, Qingdao, China) eluting with a gradient of MeOH (60%→90%) to afford three fractions (Fr.1-3). The fraction 1 (427 g) was subjected on a silica gel column (Qingdao Haiyang Chemical Co., Ltd, Qingdao, China) chromatography eluting with a gradient of CH2Cl2-MeOH (20:1→10:1) to afford 4 subfractions (Fr.1-1~1-4). The subfraction 1-4 (6.2 g) was recrystallized to give compound 1 (3 g). Subfraction 1-2 (4.2 g) was further chromatographed on a silica gel column chromatography eluting with a gradient of CH2Cl2-MeOH (80:1→10:1) to afford 6 subfractions (1-2-1~1-2-6). Then, fraction 1-2-1 (500 mg) was subjected to silica gel column chromatography eluting with a gradient of CH2Cl2-MeOH (30:1→5:1) to give compound 2 (19 mg). The subfraction 1-2-2 (200 mg) was subjected to silica gel column chromatography eluting with a gradient of petroleum- CH2Cl2 (9:1→2:1) and CH2Cl2- MeOH (20:1→5:1) to get compound 3 (20.6 mg) and compound 4 (6.9 mg).
Cell Culture and Cell Viability Assessment
The HepG2 cell was bought from the Chinese Academy of Sciences’ Shanghai Institute of Cell Science. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; GIBCO, Invitrogen Inc., Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS; Hyclone, Thermo Fisher Scientific, Waltham, MA, USA). The cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
HepG2 cells (1 × 104) were seeded in 96-well plates. When the confluence reaches 80%, DMEM with a concentration of glucose (4.5 g/L) is changed, and different concentrations (25, 50, 100, 200 μM) of compounds isolated from FCL extracts are added to each well for 24h, the culture medium was removed and 10 μL of Cell Counting Kit-8 (CCK8, Dalian Meilun Biotechnology Co., Ltd) was added to each well. After 30 minutes of incubation at 37°C, the absorbance was measured at 450 nm. Each group has 3 replicate wells and repeated 3 times.
Glucose Uptake Assay
HepG2 cells (1×104) were plated in 96-well plates. The experiment was divided into normal control group, palmitic acid (PA) group, pioglitazone group and different treatment groups. Except for the normal control group, all of the other groups were given 0.25 mM PA31 for 16 hours on HepG2 cells to create an insulin resistance model. After 16 hours of PA induction, the medium was discarded and then the treatment groups were treated for 24 hours in FBS-free DMEM supplemented with 0.25% bovine serum albumin with various doses of isolated compounds. The concentration of glucose in the medium was determined by the glucose oxidase-peroxidase assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The glucose uptake (GU) was calculated by the glucose concentration of blank group subtracting the remaining glucose in cell plated wells. After the glucose uptake experiment, the CCK8 was performed to evaluate the effect of compounds on cell viability. The result of CCK8 was used to normalize the glucose utilization results. Glucose uptake due to the cell proliferation can be deducted by calculating the ratio of the GU and CCK8.
Statistical Analysis
The results were presented as means ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Student-Newman-Keuls test or Dunnett’s T3 test. All data was performed by using the SPSS 19.0 software (IBM, Armonk, NY, USA). Differences between groups were considered statistically significant when P < 0.05.
Results
The Antihyperglycemic of Dichloromethane Extract in Diabetic Mice
The mice’s FBG levels were measured once a week during the 6-week treatment period, as indicated in Figure 1. The diabetic mice had a significant (P < 0.01) rise in FBG levels. After treatment with FCLE-500 and FCLE-1000, blood glucose levels were lowered significantly (35.75% and 44.17%, respectively) compared to DM group. The blood glucose levels were significantly lowered (39.04% and 27.20%, respectively) when psoralen was removed from dichloromethane extract (FCLP-500 and FCLP-1000). The results reveal that after removing psoralen, it still includes additional hypoglycemic active components.
Effect of Dichloromethane Extract on OGTT
In OGTT, the blood glucose levels in each group peaked at 30 min after the glucose load (Figure 2A). Compared to the DM group, NC group and the other treatment group showed a decline within 30 to 120 min. As seen from the AUC (Figure 2B), compared to the DM group, the FCLE-1000 and FCLP-1000 groups significantly reduced (P < 0.01). When compared to the extract following psoralen removal, the dichloromethane extract was not statistically significant. The above data show that FCLP also contains active ingredients for hypoglycemia.
Effect of Dichloromethane Extract on TG, TC and HbA1c
T2DM is often associated with dyslipidemia. Untreated diabetic mice had considerably increased serum TG and TC levels. FCLE-1000 and FCLP-1000 can significantly (P < 0.01) reduce TG levels (Figure 3A). FCLE-1000 and FCLP-500 significantly (P < 0.05) reduced TC levels (Figure 3B). The change trend of HbA1c was the same as that of TC (Figure 3C).
Protective Effects of Dichloromethane Extract on Pancreas
To investigate the effect of dichloromethane extract on pancreas in T2DM mice, HE staining was performed. HE staining showed that the pancreatic morphology of mice in the NC group was complete and regular, the pancreatic volume was large, and the pancreatic cells were evenly distributed (Figure 4A). In the DM group, the pancreatic structure was incomplete, pancreatic volume was significant smaller and atrophic, the number of pancreatic cells was small and the arrangement was loose. There is also a large area of densification in the nucleus (Figure 4B). Pioglitazone has a positive effect on pancreatic cell preservation. The islets have clear borders and a regular oval shape, and the cells in the islets are evenly distributed (Figure 4C). Dichloromethane extract had a protective effect on the pancreas of diabetic mice. The islets of the FCLE-500 group were more regular than those of the DM group, with a higher number of islet cells and a little more even distribution, but still lower than those of the NC group (Figure 4D). Compared with the DM group, the islets in the FCLE-1000 group also showed a regular oval shape, with clear boundaries and a slightly larger volume. The number of cells in the islets increased and the distribution was uniform. The degree of islet cell degeneration and necrosis was lighter than that in the FCLE-500 group (Figure 4E). While most of psoralen was removed from dichloromethane extract, it remained protective of the pancreas in diabetic mice (Figure 4F and G). The FCLP-500 group’s islets were still irregularly shaped, and their cells were not equally distributed. The islets in the FCLP-1000 group, on the other hand, were oval in shape and had a very uniform cell distribution. These results also suggest that besides psoralen, dichloromethane extract still has other active compounds that can inhibit the apoptosis of pancreatic cells in diabetic mice.
Structures of Isolated Compounds
Compound 1, colorless needles. 1H-NMR (500 MHz, CDCl3) δ 7.82 (d, J = 9.6 Hz, 1H, H-4), 7.70 (s, 1H, H-5), 7.48 (d, J = 0.9 Hz, 1H, H-2’), 7.28 (s,1H, H-8), 6.85 (dd, J = 2.2, 1.0 Hz, 1H, H-3’), 6.39 (d, J = 9.6 Hz, 1H, H-3).13C NMR (126 MHz, CDCl3) δ 161.02 (s, C-2), 156.41 (s, C-7), 152.03 (s, C-9), 146.91 (s, C-2’), 144.08 (s, C-4), 124.88 (s, C-6), 119.84 (s, C-10), 115.42 (s, C-5), 114.65 (s, C-3), 106.38 (s, C-3’), 99.87 (s, C-8). The intersection of these data with those from the literature32 strongly suggested that compound 1 referred to psoralen (Figure 5).
 |
Figure 5 Structures of isolated compounds. |
Compound 2, whitish amorphous powder. 1H-NMR (500 MHz, CDCl3) δ 7.62 (d, J = 2.2 Hz, 1H, H-2’), 7.41 (d, J = 11.1 Hz, 1H, H-5), 7.32–7.16 (m, 1H, H-8), 6.81–6.64 (m, 1H, H-3’), 3.25–3.00 (m, 2H, H-4), 2.82 (dd, J = 8.1, 6.3 Hz, 2H, H-3). The intersection of these data with those from the literature33 strongly suggested that compound 2 referred to 3,4-dihydropsoralen (Figure 5).
Compound 3, whitish needle crystals. 1H-NMR (500 MHz, DMSO) δ 7.92 (dd, J = 9.5, 0.6 Hz, 1H, H-4), 7.52 (d, J = 8.5 Hz, 1H, H-5), 6.79 (dd, J = 8.5, 2.3 Hz, 1H, H-6), 6.71 (dd, J = 2.3, 0.6 Hz, 1H, H-8), 6.20 (d, J = 9.5 Hz, 1H, H-3). The intersection of these data with those from the literature32 strongly suggested that compound 3 referred to umbelliferone (Figure 5).
Compound 4, yellowish solid. 1H-NMR (500 MHz, DMSO) δ 7.93 (s, 1H, H-4), 7.48 (s, 1H, H-5), 6.77 (s, 1H, H-8), 6.21 (d, J = 9.5 Hz, 1H, H-3), 2.51 (s, 3H, CH3-H). The intersection of these data with those from the literature34 strongly suggested that compound 4 referred to 7-hydroxy-6-methylcoumarin (Figure 5).
Effects of Isolated Compounds 1, 2, 3 and 4 on HepG2 Cell Viability
The cytotoxicity of the compounds was examined using HepG2 hepatocytes. HepG2 hepatocytes were exposed to various concentrations of compounds (25,50,100 and 200 μm) and the cytotoxicity was determined using the CCK8 assay. Under basic conditions, cell viability was unaffected by compounds with all the used concentrations for 24 hours treatment (Figure 6). In this experiment, 50 μM and 100 μM were selected as the low and high-dose, respectively.
 |
Figure 6 Effects of the isolated compounds on HepG2 cells viability. |
Effects of Psoralen and Umbelliferone on Glucose Uptake in HepG2 Cell
To further investigate the effects of compounds on insulin resistance, the glucose uptake was studied in human HepG2 cells. As shown in Figure 7, glucose uptake was significantly up-regulated in PA-stimulated HepG2 cells following psoralen (100 μM) or umbelliferone (100 μM) treatment. However, the compound low-dose (50 μM) group failed to promote glucose uptake when compared with the PA group. The glucose uptake was significantly increased in PA-stimulated HepG2 cells following psoralen (100 μM) or umbelliferone (100 μM) treatment (Figure 7).
 |
Figure 7 Effects of compounds on glucose consumption of HepG2 cells. Notes: ##P < 0.05 vs the NC group; *P < 0.05 vs the PA group; **P < 0.01 vs the PA group. |
Discussion
Our research showed that dichloromethane extract of FCL can significantly reduce the FBG and oral glucose tolerance levels in diabetic mice (P<0.01). It also showed a significant improvement in blood lipids in diabetic mice. Four compounds were isolated from the dichloromethane extract: psoralen, 3,4-dihydropsoralen, umbelliferone and 7-hydroxy-6-methylcoumarin, which psoralen and umbelliferone promoted glucose uptake of insulin resistance in HepG2 cells. Diabetes mellitus is a common chronic metabolic disease of the endocrine system characterized by hyperglycemia. Due to the long-term presence of hyperglycemia, it is often accompanied by a variety of diabetic complications, such as diabetic cardiomyopathy,35 diabetic ketoacidosis,36 which not only has a tremendous impact on the patient’s body and mind, but also brings great pressure to the society. Therefore, drugs designed for a single target sometimes fail to produce effects, while traditional Chinese medicine and its active ingredients have the characteristics of multiple targets, multiple pathways and multiple effects in the treatment of diabetes, which have their unique advantages in clinical applications. Ficus carica belongs to the family of Ficus mulberry. In the past few decades, many studies have reported that crude extracts and isolated compounds from various Ficus species (F. benghalensis, F. religiosa, F. glumosa, and F. carica) have significant anti-diabetic potential in vitro and in vivo.37 These Ficus plants have hypoglycemic effects mainly by inducing insulin secretion, inhibiting glucose absorption, increasing glucose uptake and enhancing insulin sensitivity.38 Mahmoudi et al39 isolated psoralen, bergapten, ferulic acid and quercetin from FCL. Zhang et al40 showed that Ficus carica mainly reduce blood glucose through antioxidant mechanism, the antioxidant substances in FCL contribute to hypoglycemic effect are quercetin41 and psoralen.22 The FCL extract exhibited a hypoglycemic effect in STZ-induced diabetic mice. A similar hypoglycemic activity of FCL extract on STZ-induced diabetic mice has been demonstrated.42
All of the above studies suggested the presence of antihyperglycemic active components in FCL, but the specific hypoglycemic active components have rarely been studied, and this experiment mainly focuses on the study of its hypoglycemic active components. The data of this study showed that whether psoralen is removed or not, dichloromethane extract can significantly reduce the FBG levels of diabetic mice, indicating that there are other effective components in FCL besides psoralen. The hypoglycemic effect was still achieved after the removal of psoralen, indicating that there are other hypoglycemic active ingredients in addition to psoralen. In vitro tests also confirmed that umbelliferone can stimulate glucose consumption. It was found that STZ-induced diabetic rats significantly enhanced PPAR γ and GLUT4 expression in adipose tissue after psoralen treatment.22 Additionally, the most prevalent glucose transporter in muscle and fat cells that are sensitive to insulin is GLUT4. Under insulin resistance or insulin shortage, GLUT4 cannot be efficiently transferred from an intracellular pool to the plasma membrane.43 Therefore, we dared to hypothesize that FCL might produce a hypoglycemic impact via promoting the expression of insulin in adipose tissue. Whether psoralen and umbelliferone have a synergistic effect depends on their similar chemical structures.
It is argued that postprandial blood glucose level is more reflective of glucose control than FBG.44 The normal body causes blood glucose concentration to increase after glucose load, and high blood glucose concentration stimulates pancreatic islet cells to secrete insulin, thereby lowering blood glucose concentration. It can be seen that dichloromethane extract has the effect of improving OGTT in diabetic mice. The protective effect of dichloromethane extract on pancreatic β-cell was also observed from pathological histology. The previous experiment of our group showed that FCL could inhibit pancreatic β-cell apoptosis by inhibiting the AMPK/JNK/caspase-3 signaling pathway and by antioxidant properties.45 We speculate that one of the hypoglycemic mechanisms of action of dichloromethane extract of FCL may be the protective effect on pancreatic islet cells, while the psoralen and umbelliferone isolated in this study can promote glucose uptake in vitro. Whether psoralen and umbelliferone are the main active components of pancreatic islet β-cell protection remains to be confirmed by further study.
In diabetic patients, hyperglycemia is often accompanied by disorders of lipid metabolism. According to the results of the experiment, the serum TG and TC were significantly reduced. The presence of bioactive metabolites in Ficus carica, such as triterpenoids, coumarins and flavonoids have been reported to have hypoglycemic and lipid-regulating effects.37 In this experiment, four coumarins were isolated from FCL. FCL may promote fat storage in adipose tissue, lower circulating TG, and block the absorption of dietary cholesterol from the intestine to explain its reduced effect on hyperlipidemia. It is hypothesized that the coumarin compounds contained in dichloromethane extract may lower blood glucose levels by promoting the release of insulin from adipose tissue.
Our study is the first time to describe the hypoglycemic active components of FCL from the perspective of systematic separation, and isolated three other coumarin compounds except psoralen: 3,4-dihydropsoralen, umbelliferone and 7-hydroxy-6-methylcoumarin. Although psoralen has been reported to have hypoglycemic effect, we found that another coumarin, umbelliferone also has hypoglycemic effect, which is another hypoglycemic compound isolated from FCL for the first time. It is assumed that coumarin compounds in FCL are the main hypoglycemic active components. The results of this experiment enriched the research on the chemical constituents of coumarin in FCL and provided an experimental basis for the in-depth exploration of the medicinal value of FCL.
Conclusion
In this study, animal experiments showed that the dichloromethane extract of FCL had anti-diabetic activity. It is mainly reflected in lowering blood glucose, improving blood lipids, glucose tolerance and pancreatic pathology in diabetic mice. Other than psoralen, the following coumarins were isolated from dichloromethane extracts: 3,4-dihydropsoralen, umbelliferone, and 7-hydroxy-6-methylcoumarin. In vitro experiments, psoralen and umbelliferone could promote glucose uptake in HepG2 cells.
Abbreviations
FCL, Ficus carica leaves; HFD, high-fat diet; FCLE, Ficus carica leaves dichloromethane extract; FCLP, Ficus carica leaves dichloromethane extract removed the psoralen; FBG, fasting blood glucose; OGTT, oral glucose tolerance test; HbA1c, glycated hemoglobin; TG, triglycerides; TC, total cholesterol; PA, palmitic acid; STZ, streptozotocin; NC, normal control group; DM, diabetic model group; Pio, pioglitazone group; AUC, area under the curve; GU, glucose uptake; FBS, fetal bovine serum; DMEM, Dulbecco’s Modified Eagle’s Medium; CCK8, cell counting kit-8; BSA; bovine serum albumin.
Ethical Approval and Consent Participate
The animal experimental procedures were approved by the animal ethics committee of the Second affiliated hospital of Fujian Medical University (NO. 2019 [175]) and according to the AAALAC and the IACUC guidelines. In addition, maximum efforts were made to minimize pain and suffering of the animals.
Acknowledgments
This work was supported by the Natural Science Foundation of Fujian Province (2020 J01213). The authors would like to extend their gratitude for its funding of this research. I would also like to thank Mr. Jie-qing Liu from the School of Biology, Huaqiao University, Quanzhou, Fujian Province, China, for his great help in this experiment.
Funding
This work was supported by the Natural Science Foundation of Fujian Province (2020 J01213).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
2. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021;17(9):534–548. doi:10.1038/s41574-021-00512-2
3. Sunil B, Ashraf AP. Dyslipidemia in pediatric type 2 diabetes mellitus. Curr Diab Rep. 2020;20(10):53. doi:10.1007/s11892-020-01336-6
4. He JH, Chen LX, Li H. Progress in the discovery of naturally occurring anti-diabetic drugs and in the identification of their molecular targets. Fitoterapia. 2019;134:270–289. doi:10.1016/j.fitote.2019.02.033
5. Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398(10296):262–276. doi:10.1016/S0140-6736(21)00536-5
6. Bai L, Li X, He L, et al. Antidiabetic potential of flavonoids from traditional Chinese medicine: a review. Am J Chin Med. 2019;47(5):933–957. doi:10.1142/S0192415X19500496
7. Valeron PF, de Pablos-Velasco PL. [Limitations of insulin-dependent drugs in the treatment of type 2 diabetes mellitus]. Med Clin. 2013;141(Suppl 2):20–25. Spanish. doi:10.1016/S0025-7753(13)70059-9
8. Singh M, Kumar A. Risks associated with SGLT2 inhibitors: an overview. Curr Drug Saf. 2018;13(2):84–91. doi:10.2174/1574886313666180226103408
9. Doan HV, Riyajan S, Iyara R, Chudapongse N. Antidiabetic activity, glucose uptake stimulation and alpha-glucosidase inhibitory effect of Chrysophyllum cainito L. stem bark extract. BMC Complement Altern Med. 2018;18(1):267. doi:10.1186/s12906-018-2328-0
10. Zhang Z, Zhang L, Xu H. Effect of Astragalus polysaccharide in treatment of diabetes mellitus: a narrative review. J Tradit Chin Med. 2019;39(1):133–138.
11. Huang DD, Shi G, Jiang Y, Yao C, Zhu C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed Pharmacother. 2020;125:109767. doi:10.1016/j.biopha.2019.109767
12. Yang X, Dong B, An L, et al. Ginsenoside Rb1 ameliorates glycemic disorder in mice with high fat diet-induced obesity via regulating gut microbiota and amino acid metabolism. Front Pharmacol. 2021;12:756491. doi:10.3389/fphar.2021.756491
13. Salehi B, Prakash Mishra A, Nigam M, et al. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res. 2021;35(3):1187–1217. doi:10.1002/ptr.6884
14. Kim JW, Kim TB, Kim HW, Park SW, Kim HP, Sung SH. Hepatoprotective flavonoids in opuntia ficus-indica fruits by reducing oxidative stress in primary rat hepatocytes. Pharmacogn Mag. 2017;13(51):472–476. doi:10.4103/pm.pm_232_16
15. Pérez C, Domínguez E, Ramiro JM, Romero A, Torres M, Torres MD. A study on the glycaemic balance in streptozotocin-diabetic rats treated with an aqueous extract of Ficus carica (fig tree) leaves. Phytother Res. 1996;10(1):82–83. doi:10.1002/(SICI)1099-1573(199602)10:1<82::AID-PTR776>3.0.CO;2-R
16. Serraclara A, Hawkins F, Perez C, Dominguez E, Campillo JE, Torres MD. Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract. 1998;39(1):19–22. doi:10.1016/S0168-8227(97)00112-5
17. Ibrahim KM, Al-Juboury RA, Alshawi N. A study on the hypoglycemic effect of ficus carica L. Leaves aqueous extract against alloxan-induced diabetes in rabbits. Med J Babylon. 2009;6:3–4.
18. Rashidi AA, Noureddini M. Hypoglycemic effect of the aromatic water of leaves of ficus carica in normal and streptozotocin induced diabetic rats. Pharmacologyonline. 2011;1:372–379.
19. Ramadan S, Hegab AM, Al-Awthan YS, Al-Duais MA, Tayel AA, Al-Saman MA. Comparison of the efficiency of Lepidium sativum, Ficus carica, and Punica granatum methanolic extracts in relieving hyperglycemia and hyperlipidemia of streptozotocin-induced diabetic rats. J Diabetes Res. 2021;2021:6018835. doi:10.1155/2021/6018835
20. Ergul M, Ergul M, Eruygur N, Atas M, Ucar E. In vitro evaluation of the chemical composition and various biological activities of ficus carica leaf extracts. Turk J Pharm Sci. 2019;16(4):401–409. doi:10.4274/tjps.galenos.2018.70037
21. Mopuri R, Ganjayi M, Meriga B, Koorbanally NA, Islam MS. The effects of Ficus carica on the activity of enzymes related to metabolic syndrome. J Food Drug Anal. 2018;26(1):201–210. doi:10.1016/j.jfda.2017.03.001
22. Irudayaraj SS, Stalin A, Sunil C, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S. Antioxidant, antilipidemic and antidiabetic effects of ficusin with their effects on GLUT4 translocation and PPARgamma expression in type 2 diabetic rats. Chem Biol Interact. 2016;256:85–93. doi:10.1016/j.cbi.2016.06.023
23. Zhang Y, Chen J, Zeng Y, Huang D, Xu Q. Involvement of AMPK activation in the inhibition of hepatic gluconeogenesis by Ficus carica leaf extract in diabetic mice and HepG2 cells. Biomed Pharmacother. 2019;109:188–194. doi:10.1016/j.biopha.2018.10.077
24. Li Y, Hou JG, Liu Z, et al. Alleviative effects of 20(R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-kappaB signaling pathways in C57BL/6 mice. J Ethnopharmacol. 2021;267:113500. doi:10.1016/j.jep.2020.113500
25. Liu Y, Deng J, Fan D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 2019;10(5):2538–2551. doi:10.1039/C9FO00095J
26. Zhang J, Qiu H, Huang J, et al. Establishment of a diabetic myocardial hypertrophy model in Mus musculus castaneus mouse. Int J Exp Pathol. 2018;99(6):295–303. doi:10.1111/iep.12296
27. Li S, Huang Q, Zhang L, et al. Effect of CAPE-pNO2 against type 2 diabetes mellitus via the AMPK/GLUT4/ GSK3beta/PPARalpha pathway in HFD/STZ-induced diabetic mice. Eur J Pharmacol. 2019;853:1–10. doi:10.1016/j.ejphar.2019.03.027
28. Sheng L, Chen Q, Di L, Li N. Evaluation of anti-diabetic potential of corn silk in high-fat diet/ streptozotocin-induced type 2 diabetes mice model. Endocr Metab Immune Disord Drug Targets. 2021;21(1):131–138. doi:10.2174/1871530320666200606224708
29. Arafa EA, Hassan W, Murtaza G, Buabeid MA, Cigarran S. Ficus carica and sizigium cumini regulate glucose and lipid parameters in high-fat diet and streptozocin-induced rats. J Diabetes Res. 2020;2020:6745873. doi:10.1155/2020/6745873
30. Liu P, Zhang Z, Wang J, Zhang X, Yu X, Li Y. Empagliflozin protects diabetic pancreatic tissue from damage by inhibiting the activation of the NLRP3/caspase-1/GSDMD pathway in pancreatic beta cells: in vitro and in vivo studies. Bioengineered. 2021;12(2):9356–9366. doi:10.1080/21655979.2021.2001240
31. Ren T, Yang WS, Lin Y, et al. A novel PPARalpha/gamma agonist, propane-2-sulfonic acid octadec-9-enyl-amide, ameliorates insulin resistance and gluconeogenesis in vivo and vitro. Eur J Pharmacol. 2018;826:1–8.
32. Kim JS, Kim JC, Shim SH, et al. Chemical constituents of the root of dystaenia takeshimana and their anti-inflammatory activity. Arch Pharm Res. 2006;29(8):617–623. doi:10.1007/BF02968244
33. Hayakawa K, Yodo M, Ohsuki S, Kanematsu K. Novel bicycloannulation via tandem vinylation and intramolecular Diels-Alder reaction of five-membered heterocycles: a new approach to construction of psoralen and azapsoralen. J Am Chem Soc. 1984;106(22):230–235. doi:10.1021/ja00334a044
34. Xie L, Takeuchi Y, Cosentino LM, Lee KH. Anti-AIDS agents. 37. Synthesis and structure-activity relationships of (3’R,4’R)-(+)-cis-khellactone derivatives as novel potent anti-HIV agents. J Med Chem. 1999;42(14):2662–2672.
35. Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11(1):31–39.
36. Farmer AD, Kadirkamanathan SS, Aziz Q. Diabetic gastroparesis: pathophysiology, evaluation and management. Br J Hosp Med. 2012;73(8):451–456. doi:10.12968/hmed.2012.73.8.451
37. Deepa P, Sowndhararajan K, Kim S, Park SJ. A role of Ficus species in the management of diabetes mellitus: a review. J Ethnopharmacol. 2018;215:210–232.
38. Farsi E, Ahmad M, Hor SY, et al. Correction to: standardized extract of Ficus deltoidea stimulates insulin secretion and blocks hepatic glucose production by regulating the expression of glucose-metabolic genes in streptozitocin-induced diabetic rats. BMC Complement Altern Med. 2018;18(1):262. doi:10.1186/s12906-018-2333-3
39. Mahmoudi S, Khali M, Benkhaled A, et al. Phenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varieties. Asian Pac J Trop Biomed. 2016;6(3):82–83. doi:10.1016/j.apjtb.2015.12.010
40. Zhang YJ, Gan RY, Li S, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–21156. doi:10.3390/molecules201219753
41. Boukhalfa F, Kadri N, Bouchemel S, et al. Antioxidant activity and Hypolipidemic effect of Ficus carica leaf and twig extracts in Triton WR-1339-induced hyperlipidemic mice. Med J Nutrition Metab. 2018;11(1):37–50. doi:10.3233/MNM-17180
42. Stephen Irudayaraj S, Christudas S, Antony S, Duraipandiyan V, Naif Abdullah AD, Ignacimuthu S. Protective effects of Ficus carica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and beta-cells in type 2 diabetic rats. Pharm Biol. 2017;55(1):1074–1081. doi:10.1080/13880209.2017.1279671
43. Naowaboot J, Somparn N, Saentaweesuk S, Pannangpetch P. Umbelliferone improves an impaired glucose and lipid metabolism in high-fat diet/streptozotocin-induced type 2 diabetic rats. Phytother Res. 2015;29(9):1388–1395. doi:10.1002/ptr.5392
44. Haile T, Cardoso SM, de Oliveira Raphaelli C, et al. Chemical composition, antioxidant potential, and blood glucose lowering effect of aqueous extract and essential oil of Thymus Serrulatus Hochst. Ex Benth. Front Pharmacol. 2021;12:621536. doi:10.3389/fphar.2021.621536
45. Zhang Y, Li Y, Ma P, Chen J, Xie W. Ficus carica leaves extract inhibited pancreatic beta-cell apoptosis by inhibiting AMPK/JNK/caspase-3 signaling pathway and antioxidation. Biomed Pharmacother. 2020;122:109689. doi:10.1016/j.biopha.2019.109689
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.