Back to Journals » OncoTargets and Therapy » Volume 11
Characterization of luteinizing hormone-releasing hormone receptor type I (LH-RH-I) as a potential molecular target in OCM-1 and OCM-3 human uveal melanoma cell lines
Authors Sipos E, Dobos N, Rozsa D, Fodor K, Olah G, Szabo Z , Szekvolgyi L, Schally AV , Halmos G
Received 3 August 2017
Accepted for publication 25 November 2017
Published 22 February 2018 Volume 2018:11 Pages 933—941
DOI https://doi.org/10.2147/OTT.S148174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Eva Sipos,1 Nikoletta Dobos,1 David Rozsa,1 Klara Fodor,1 Gabor Olah,1 Zsuzsanna Szabo,1 Lorant Szekvolgyi,2,3 Andrew V Schally,4–7 Gabor Halmos1,4
1Department of Biopharmacy, School of Pharmacy, University of Debrecen, Debrecen, Hungary; 2MTA-DE Momentum, Genome Architecture and Recombination Research Group, Debrecen, Hungary; 3Research Centre for Molecular Medicine; Department of Biochemistry and Molecular Biology, Debrecen, Hungary; 4Endocrine, Polypeptide and Cancer Institute, Veterans Affairs Medical Center, Miami, FL, USA; 5Department of Pathology, Miller School of Medicine, University of Miami, Miami, FL, USA; 6Department of Medicine, Divisions of Hematology–Oncology and Endocrinology, Miller School of Medicine, University of Miami, Miami, FL, USA; 7Sylvester Comprehensive Cancer Center, University of Miami, Miami, FL, USA
Introduction: Uveal melanoma (UM) is the most common primary intraocular malignancy with very poor prognosis. Conventional chemotherapy only rarely prolongs the survival, therefore patients require novel treatment modalities. The discovery of specific receptors for hypothalamic hormones on cancer cells has led to the development of radiolabeled and cytotoxic hormone analogs.
Materials and methods: In the present study, our aim was to investigate the expression of mRNA for receptors of luteinizing hormone-releasing hormone type I (LH-RH-I) and LH-RH ligand in OCM-1 and OCM-3 human uveal melanoma cell lines. The presence and binding characteristics of LH-RH-I receptor protein was further studied by Western blot, immunocytochemistry and ligand competition assay. The expression of mRNA and protein for LH-RH-I receptors has been also studied using tumor samples originating from nude mice xenografted with OCM-1 or OCM-3 cells.
Results: The mRNA for LH-RH-I receptor has been detected in OCM-1 and OCM-3 cell lines and was found markedly higher in OCM-3 cells. The mRNA for LH-RH-I receptors was also observed in both UM xenograft models in vivo with higher levels in OCM-3. The presence of LH-RH-I receptor protein was found in both cell lines in vitro by immunocytochemistry and Western blot, and also in tumor tissue samples grown in nude mice by Western blot. Both human uveal melanoma models investigated showed specific high affinity receptors for LH-RH-I using ligand competition assay. The mRNA for LH-RH ligand has also been detected in OCM-1 and OCM-3 cell lines and cancer tissues.
Conclusion: The demonstration of the expression of LH-RH-I receptors in OCM-1 and OCM-3 human UM cell lines suggests that they could serve as potential molecular target for therapy. Our findings support the development of new therapeutic approaches based on cytotoxic LH-RH analogs or modern powerful antagonistic analogs of LH-RH targeting LH-RH-I receptors in UM.
Keywords: human uveal melanoma, LH-RH receptor, LH-RH ligand, targeted cancer therapy
Introduction
Uveal melanoma (UM) is an intraocular melanoma arising from melanocytes of the uveal tract, which is composed of the choroidea, ciliary body (CB), and iris.1 UM is the most common intraocular tumor and its prognosis depends on the size of the primary tumor, the time of diagnosis, and the presence of metastases.2 A number of clinical and histological risk factors have been defined over the last three decades, among others – clinicopathological factors like location, extraocular growth, involvement of the CB, and the epitheloid cell type of the tumor.3 Larger tumors are associated with a mortality rate of ~50% shortly after the diagnosis, while patients with medium-sized tumors show 50% survival rate over 15 years measured from primary tumor treatment.2 Nearly 50% of patients suffering from UM develop metastatic disease, that usually involves the liver and is almost inevitably fatal.4 When metastases develop, the median survival of the patients is only 5–7 months.5
Primary tumors are treated either by brachytherapy using radioactive plaques to preserve the tissues of the eye or by enucleation.6 Treatment by chemotherapy only rarely prolongs survival and new treatment modalities are needed.7 Two distinct classes of UMs have been identified by its gene expression profile.8 Class I tumors exhibit low aneuploidy, and patients rarely have metastases, whereas class II tumors have a higher chance of aneuploidy and patients have a high risk to develop metastases.9 Monosomy 3 strongly relates to several clinical and histopathological parameters such as epithelioid cells, closed vascular patterns, large tumor diameter, and CB involvement.10 Several groups have already shown that there is a strong correlation between monosomy 3 and the development of metastatic diseases.10,11 Lack of chromosome 3 has been demonstrated in 5%–10% of all the patients and the remaining copy is duplicated.12 Monosomy 3 is present in 50%–60% of UM tumors, and is associated with isochromosome 8q and high level of 8q gain.9 In our previous study, we demonstrated aneuploidy of chromosome 4 in 70% of human UM specimens.13 Furthermore, a correlation was found between the copy number of chromosome 3 and 4 and the survival of patients.13 BRCA1-associated protein 1 (BAP1) is located on chromosome 3p21.1 and is thought to be a tumor suppressor gene.14 Inactivating somatic mutations were found in 84% of the metastasizing UMs, implicating that BAP1 mutations occur late in the UM progression.14 Recently, several candidate genes have been proposed in UM; LZTS1, GNAQ, DDEF1, NBS1, HDM2, BCL-2, and CCND1, among others. For most of these genes, a definite role in tumorigenesis or progression toward metastasis is still yet to be validated.15,16
The discovery of specific receptors for hypothalamic hormones on cancer cells has led to the development of radiolabeled and cytotoxic hormone analogs. These analogs are more selective in wiping out cancer cells and less toxic than conventional chemotherapeutic agents.17–20 Hypothalamic luteinizing hormone-releasing hormone (LH-RH) is the primary regulator of gonadal function and plays a pivotal role in vertebrate reproduction.18–20 The actions of LH-RH are mediated by specific G protein-coupled receptors (GPCRs) for LH-RH present on the plasma membranes of the pituitary gonadotrophs.19,20 Those specific membrane receptors for LH-RH have been found in various animal and human cancers and can mediate direct effects of LH-RH agonists, antagonists, and cytotoxic LH-RH conjugates.18–21 Receptors for LH-RH have been demonstrated on breast, prostate, ovarian, endometrial cancers, melanomas, and renal cell and colorectal carcinomas.18–26 Previously, we have also demonstrated the expression of LH-RH ligand and LH-RH type I (LH-RH-I) receptors in human UM specimens.27
In the present study, our aim was to investigate the mRNA expression of LH-RH-I receptor and LH-RH ligand in ocular choroidal melanoma (OCM)-1 and OCM-3 human UM cell lines. The presence and binding characteristics of the LH-RH-I receptor protein have been also examined by Western blot, immunocytochemistry, and ligand competition assays. In addition, we have studied the expression of mRNA and protein of LH-RH-I receptors in tumor tissue samples from nude mice xenografted with OCM-1 and OCM-3 cell lines.
Materials and methods
Cell lines and culture conditions
OCM-1 and OCM-3 human primary UM cell lines were kindly provided by the Department of Biophysics and Cell Biology, University of Debrecen and this research had approval from the Institutional Ethics Committee of the University of Debrecen. The cells were cultured in RPMI 1640 medium supplemented with L-glutamine, 10% fetal bovine serum (FBS), and 1% penicillin/streptomycin in a humidified chamber in 5% CO2 at 37°C. Cells were subcultured every 3 days using a standard trypsinization procedure.
Animals
Athymic (nude) mice (Ncr nu/nu) were obtained from Charles River Laboratories (Germany). Mice were housed in sterile, individually ventilated cages in an air-conditioned (21°C±2°C), humidity-controlled room (≈50%) with a 12/12 hour light/dark cycle. Animals were fed with autoclaved chow and water ad libitum. All experiments were conducted in accordance with the institutional guidelines for the welfare of experimental animals and regulations of the European Union. The experimental protocol was approved by the Laboratory Animal Care and Use Committee of the University of Debrecen. Six million tumor cells were subcutaneously injected into the femoral region of the mice. Four weeks after the initiation of donor animals, when tumors had developed in donor animals, tumors were aseptically dissected and mechanically minced. Approximately 3 mm3 tumor tissue was transplanted subcutaneously into nude mice by a trocar needle. At the end of each experiment, mice were sacrificed under 3% isoflurane anesthesia using a small animal anesthetic device. Tumors were excised and weighed and necropsy was done. Tumor specimens were snap-frozen and stored at −70°C until further experiments.
RNA isolation, reverse transcription and quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA was isolated using NucleoSpin RNA and Protein Purification Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. RNA from each sample (2,000 ng) was reverse transcribed to cDNA using a Tetro cDNA Synthesis Kit (Bioline, London, UK) in a final volume of 20 μL. In order to evaluate the expression of type I LH-RH receptors and LH-RH ligand, primer sets were designed. Gene-specific primers for LH-RH-I receptor: sense 5′-GACCTTGTCTGGAAAGATCC-3′ (EXON 1 1,844–1,863), antisense 5′-CAGGCTGATCACCACCATCA-3′ (EXON 1 1,844–1,863), for LH-RH ligand: sense 5′-GGCCTTATTCTACTGACTTGG-3′, antisense 5′-TCTTCTGCCCAGTTTCCTCT-3′. Hypoxanthine phosphoribosyltransferase 1 (HPRT1) was used as an internal reference gene (sense 5′-GTATTCATTATAGTCAAGGGCATATCC-3′, antisense 5′-AGATGGTCAAGGTCGCAAG-3′). mRNA levels of LH-RH-R-I, LH-RH, and HPRT1 have been assessed by iQ™ SYBR® Green Supermix (Bio-Rad Laboatories Inc, Hercules, CA, USA). Reactions were conducted according to the manufacturer’s protocol using MyiQ2 two-color real-time PCR detection system (Bio-Rad Laboratories Inc). All real-time amplifications were measured in triplicates. Results were evaluated with Bio-Rad iQ5 software (Bio-Rad Laboatories Inc) and changes in mRNA levels were calculated using the 240-Ct method.
Immunocytochemistry
Immunoperoxidase staining
To detect LH-RH-I receptors, OCM-1 and OCM-3 cells were fixed in ice-cold methanol (10 minutes). Endogenous peroxidase activity was blocked in 3% hydrogen peroxide (10 minutes). Samples were permeabilized with 0.1% Triton X-100 and blocked with 1% bovine serum albumin (BSA) – 1% FBS solution in 0.1% Triton X-100 (room temperature, 1 hour). Samples were incubated with primary anti-LH-RH-R antibody (sc-13944 rabbit polyclonal; Santa Cruz Biotechnology Inc, Dallas, TX, USA; 1:50) (overnight, 4°C) and EnVision Flex, horseradish peroxidase (Agilent Technologies, Santa Clara, CA, USA) (room temperature, 1 hour). Signals were detected using a ready-to-use 3,3′-diaminobenzidine substrate kit (Agilent Technologies). Samples were rinsed with tap water, dehydrated through a graded series of alcohol, and mounted with ProLong® Diamond Antifade Mountant (Molecular Probes, Eugene, OR, USA).
Immunofluorescent labeling
To investigate LH-RH-I receptors, OCM-1 and OCM-3 cells were fixed in 4% paraformaldehyde at room temperature for 10 minutes, permeabilized with 0.1% Triton X-100 at room temperature for 1 hour, and blocked with 5% BSA in 0.1% Triton X-100 solution at room temperature for 1 hour. Samples were incubated with primary anti-LH-RH-R antibody (sc-13944 rabbit polyclonal; Santa Cruz Biotechnology Inc; 1:50) (overnight, 4°C) and anti-rabbit fluorescein isothiocyanate secondary antibody (Thermo Fisher Scientific, Waltham, MA, USA; 1:1,000). Samples were rinsed and mounted with ProLong® Diamond Antifade Mountant with DAPI (Molecular Probes). Staining was evaluated using an Olympus FV-1000 confocal microscope (Olympus Corporation, Tokyo, Japan).
Western blot
Total protein was isolated using NucleoSpin RNA and Protein Purification Kit (Macherey-Nagel) according to the manufacturer’s instructions. Total protein amount of the supernatant was determined by a Nanodrop ND-1000 UV-Vis Spectrophotometer (ThermoFisher Scientific). Equal amounts of proteins (20 μg) were separated in 10% sodium dodecyl sulfate-polyacrylamide gels and then transferred to polyvinylidene fluoride membrane using standard procedures.28 Upon blocking with 5% BSA, membranes were incubated with primary antibodies (overnight, 4°C): anti-LH-RH-R, 1:200 dilution (sc-13944 rabbit polyclonal; Santa Cruz Biotechnology Inc), anti-GAPDH, 1:1,000 dilution (D16H11 rabbit monoclonal; Cell Signaling Technology, Danvers, MA, USA). Proteins were detected with anti-rabbit horseradish peroxidase conjugated antibody (mouse sc-2357; Santa Cruz Biotechnology Inc) and Luminata Forte Western horseradish peroxidase Substrate (Merck Millipore, Billerica, MA, USA). The protein bands were quantified using Image Lab software (Bio-Rad Laboratories Inc).
Preparation of membranes and radioligand binding studies
Preparation of membranes for receptor studies was performed as described previously.23,24,27 Receptor binding was characterized using sensitive in vitro ligand competition assay based on binding of [125I][D-Trp6]LH-RH as radioligand to membrane homogenates.23,24,27 The binding characteristics of receptors for LH-RH-I were determined in the membrane fraction of OCM-1 and OCM-3 human UM cell lines (1.8–2.4×108 cells each) and in OCM-1 and OCM-3 tumors grown in nude mice. Radioiodinated derivatives of [D-Trp6]LH-RH were prepared by the chloramine-T method and purified by reverse-phase high-performance liquid chromatography as described.23,24 This radioligand has been well-characterized previously and showed high affinity binding to LH-RH-I receptors expressed in human and rat pituitaries and human breast, prostate, and other cancers.19,20,23,24,29 In brief, membrane homogenates containing 50–160 μg protein were incubated in duplicate or triplicate with 60–80,000 cpm [125I][D-Trp6]LH-RH and increasing concentrations (10−12–10−6 M) of nonradioactive peptides as competitors in a total volume of 150 μL of binding buffer. At the end of incubation, 125 μL aliquots of the suspension were transferred onto the top of 1 mL of ice-cold binding buffer containing 1.5% BSA in siliconized polypropylene microcentrifuge tubes (Sigma-Aldrich Co, St Louis, MO, USA). The tubes were centrifuged at 12,000× g for 3 minutes at 4°C. Supernatants were aspirated and the bottoms of the tubes containing the pellet were cut off and counted in a gamma counter. Protein concentration was determined by the method of Bradford using a protein assay kit (Bio-Rad Laboratories Inc). The LIGAND-PC computerized curve-fitting program of Munson and Rodbard was used to determine the type of receptor binding, dissociation constant (Kd), and the maximal binding capacity of the receptors (Bmax).23,24,27
Statistical analysis
Correlation analysis was carried out between the expression of mRNA for LH-RH-I receptor and expression of mRNA for LH-RH ligand with the use of GraphPad Prism 7 (GraphPad Software Inc, La Jolla, CA, USA). The two data sets were evaluated using Kolmogorov–Smirnov normality test, and then Pearson correlation analysis was performed.
Results
Expression of type I LH-RH receptors in human UM cell lines in vitro
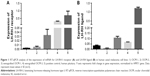
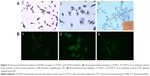
mRNA expression for LH-RH-I receptors was analyzed in OCM-1 and OCM-3 cell lines by RT-qPCR. LH-RH-I receptors were detected in both these human UM cell lines, with a slightly higher expression of LH-RH-I receptor observed in OCM-3 cells (Figure 1A). Western blot analysis confirmed the presence of LH-RH-I receptors in OCM-1 and OCM-3 cells and revealed a signal corresponding to a protein of ~68 kDa, which is the molecular mass of the LH-RH-I receptor reported earlier30 (Figure 2). In accordance with the receptor mRNA data, a slightly higher protein expression of LH-RH-I receptors was observed in OCM-3 cells by Western blot and immunocytochemical analysis (Figure 3).
Expression of type I LH-RH receptors in vivo in tumor xenograft models
The mRNA expression for receptors for LH-RH-I in OCM-1 and OCM-3 tumors grown in nude mice was analyzed by RT-qPCR. mRNA for LH-RH-I receptors could be detected in all tumor xenografts (Figure 1A). In accordance with our in vitro results, the level of the LH-RH-I receptor transcript in our OCM-3 model was considerably higher than in the OCM-1 tumor samples in vivo. Western blot analysis also confirmed the presence of LH-RH-I receptor protein in OCM-1 and OCM-3 tumor tissues. Similar to our in vitro finding, higher expression level of LH-RH-I receptor was found in OCM-3 tumor xenografts, also detected by Western blots (Figure 2).
Radioligand binding studies
The presence of specific LH-RH binding sites and characteristics of binding of [125I][D-Trp6]LH-RH to membrane receptors on OCM-1 and OCM-3 human UM models were determined using ligand competition assays. Analyses of the typical displacement of radiolabeled [D-Trp6]LH-RH by the same unlabeled peptide revealed that the one-site model provided the best fit, indicating the presence of one class of high affinity LH-RH-I receptors in crude membranes derived from human UM cells. In cell membranes of OCM-1 and OCM-3 human UM cell lines, ligand competition studies also revealed a single class of high affinity binding sites for LH-RH-I with mean dissociation constants (Kd) of 4.11±0.3 and 4.26±0.6 nM, respectively (Table 1). The concentration of LH-RH-I receptors was 233.6±21.7 fmol/mg membrane protein in OCM-1 cells while OCM-3 cells showed a markedly higher receptor level (1,029.1±68.5 fmol/mg membrane protein) (Table 1). Receptors for LH-RH-I were also found in membranes of OCM-1 and OCM-3 tumor tissue samples. Radiolabeled [D-Trp6]LH-RH was bound to a single class of specific, high affinity binding sites in both human UM models investigated. Mean Kd values were 5.85±0.7 nM for OCM-1 tumors and 6.18±0.8 nM for OCM-3 tumors (Table 2). Mean Bmax values were 267.3±38.5 fmol/mg membrane protein in OCM-1 tumors and about 2.7 times higher (713.0±29.4 fmol/mg membrane protein) for OCM-3 xenografts (Table 2). Biochemical parameters essential to establish the identity of specific binding sites were also determined. Thus, the binding of [125I][D-Trp6]LH-RH was found to be reversible, time- and temperature-dependent, and linear with protein concentration in human UM samples. The specificity of LH-RH binding was demonstrated by competitive binding experiments using several peptides structurally related or unrelated to LH-RH. The binding of radiolabeled [D-Trp6]LH-RH was completely displaced by increasing concentrations (10−12–10−6 M) of LH-RH agonist buserelin and LH-RH antagonist cetrorelix (data not shown).
Expression of LH-RH mRNA in human UM cell lines and tumor xenografts
In addition to LH-RH receptor studies, the expression of LH-RH ligand in OCM-1 and OCM-3 models was also investigated by RT-qPCR. Presence of mRNA for LH-RH ligand was detected in both cell lines and tumors grown in nude mice (Figure 1B). Although the expression of LH-RH-R was markedly higher in the OCM-3 UM model, the mRNA expression of LH-RH ligand was only slightly higher in the OCM-1 UM cell line (Figure 1B).
Correlation between type I LH-RH receptor and LH-RH ligand
According to our statistical analysis, there is a significant correlation between the expression of LH-RH-I receptor and LH-RH ligand in OCM-1 cell line and in OCM-1 tumor xenografts in vivo (Pearson r=0.8380; p=0.0373, CI =0.95%). Moreover, we also observed a significant correlation between the expressions of LH-RH-R-I and LH-RH ligand in OCM-3 cells and OCM-3 tumors grown in nude mice (Pearson r=0.9878; p=0.0002, CI =0.95%) (Figure 4).
Discussion
UM is the most common primary intraocular cancer of the eye. Risk factors for the development of UM are, among others, Caucasian ethnicity, light eye color, ocular melanocytosis and dysplastic naevus syndrome.31 Therefore, a better understanding of the molecular background of UM and the development of new therapeutic approaches are urgently needed.
A growing body of evidence shows that the LH-RH receptor can serve as a potential therapeutic target.17–24,29 LH-RH regulates the reproductive system through a specific GPCR in pituitary gonadotropes.19,20,32 The presence of various forms of LH-RH is connected with the existence of LH-RH receptor subtypes. In vertebrates, three subtypes of LH-RH receptors have been identified.32–37 Principally, type I receptors are localized in the pituitary and mediate the regulation of gonadotropin secretion.32–36 Type II LH-RH receptors are well preserved in many vertebrates.32,34 In humans, type II receptors have become nonfunctional.32,34 Their function – being the target for LH-RH-II – has been taken up by type I receptors.32,34 However, LH-RH-II activates type I receptors differently than LH-RH-I.35 Interestingly, it has been shown that type II LH-RH receptors might play a role in tumor cell growth.33 Type III LH-RH receptors seem to be related to type II receptors; thus, they may have common genetic roots.34
Receptors for LH-RH-I are mainly localized in the pituitary and LH-RH ligand in the hypothalamus. However, there are several studies showing LH-RH and LH-RH-I receptor expression in extrapituitary reproductive tissues: not only in the ovary, placenta, testis, and prostate, but also in nonreproductive organs including the liver, heart, kidney, skeletal muscle, blood mononuclear cells, and incisor teeth.20,21,38–44 In UM research, cell lines have been widely used in order to identify and characterize the disease in vitro.45 In the present study, our aim was to investigate the mRNA expression for LH-RH ligand and LH-RH-I receptors in OCM-1 and OCM-3 human UM cell lines. Those cultures were derived from primary UMs.46 The presence and binding characteristics of LH-RH-I receptor protein were also determined by Western blot, immunocytochemistry, and ligand competition assays. Furthermore, we demonstrated the expression of LH-RH-I receptors at mRNA as well as protein levels in OCM-1 and OCM-3 models transplanted into nude mice.
The expression of LH-RH-I receptors lends support to the therapeutic use of LH-RH analogs linked to cytotoxic drugs in human UM as well as many other hormone-dependent tumors.19–23,47,48 Targeted tumor therapy reduces peripheral toxicity and adverse reactions compared to systemic chemotherapeutic agents, and increases selective damage to cancer cells.17,19–22,47,48 For instance, AN-152 (AEZS-108), a cytotoxic LH-RH analog widely used in targeted therapy, consists of doxorubicin linked covalently to LH-RH agonist [D-Lys6]LH-RH. AN-152 binds with high affinity to LH-RH receptors on the membrane of various cancer cells.19–23,30,47–50 Moreover, therapy with cytotoxic LH-RH analogs does not inflict permanent damage to pituitary function and does not induce any cardiotoxicity.49,51,52 AN-152 has been tested in Phase I/II trials in castration-resistant prostate cancer and in Phase II and III clinical trials in endometrial and ovarian cancers.47,49,53,54 Modern LH-RH antagonists such as degarelix could be also tried.
In this study, we provide evidence for the existence of LH-RH-I receptors in two human UM cell lines and demonstrate that OCM-3 cells express LH-RH-I receptors at a higher level than OCM-1 cells. The same expression pattern has been observed in our in vivo models. Moreover, a remarkable expression of mRNA for LH-RH ligand was detected in both cell lines and cancer tissues grown in nude mice. Significant correlation was found between the LH-RH-I receptor and LH-RH ligand expression in OCM-1 and OCM-3 cell lines. The presence of LH-RH-I receptor protein was confirmed in both cell lines cultured in vitro and tissue samples from nude mice using Western blot. In addition, using ligand competition assay we examined the binding of [125I][D-Trp6]LH-RH to membrane preparations of OCM-1 and OCM-3 human UM models. In both human UM models investigated, specific high affinity LH-RH-I receptors were found. The expression of LH-RH ligand and co-expression of LH-RH-I receptors support the idea that they might play a role in an autocrine and/or paracrine regulatory system in human UM; however, further studies are required to confirm this.
Our results provide further support to the hypothesis that locally produced LH-RH may participate in the regulation of tumor growth.19,20,25,50,55,56
Conclusion
In this study we demonstrated the expression of LH-RH-I receptor as a potential therapeutic target and LH-RH ligand in two human UM cell lines and tumor xenografts grown in nude mice. Our findings support the development of new therapeutic approaches based on LH-RH antagonists or cytotoxic analogs of LH-RH targeting LH-RH receptors in UM.
Acknowledgments
This work was supported by Hungarian Scientific Research Fund (OTKA) K 81596 (G.H.), TAMOP 4.2.2.A- 11/1/KONV-2012-0025 project (G.H.), TAMOP-4.2.2/B-10/1-2010-0024 (E.S), the Gedeon Richter’s Talentum Foundation (E.S.), the ÚNKP-17-3 New National Excellence Program of the Ministry of Human Capacities (E.S.) and EFOP-3.6.1-16-2016-00022 (E.S). The publication is also supported by the GINOP-2.3.2-15-2016-00043 (G.H.), −00024 (L.S.) project. The project is co-financed by the European Union and the European Regional Development Fund. This work is dedicated to the late Andrea Treszl, PhD, who died of metastatic breast cancer. Her intellectual, spiritual and personal contributions provided a great inspiration to our work in uveal melanoma. We thank Rudolf Gesztelyi M.D., Ph.D. for his excellent assistance in the statistical part of this work and Gyorgy Trencsenyi Ph.D. and Janos Gardi Ph.D for their help in preparation of radioligand and animal studies.
Disclosure
The authors report no conflicts of interests in this work.
References
Nichols EE, Richmond A, Daniels AB. Tumor characteristics, genetics, management, and the risk of metastasis in uveal melanoma. Semin Ophthalmol. 2016;31(4):304–309. | ||
Abildgaard SKO, Vorum H. Proteomics of uveal melanoma: a minireview. J Oncol. 2013;2013:820953. | ||
Petrausch U, Martus P, Tönnies H, et al. Significance of gene expression analysis in uveal melanoma in comparison to standard risk factors for risk assessment of subsequent metastases. Eye (Lond). 2008;22(8):997–1007. | ||
Dopierala J, Damato BE, Lake SL, Taktak AFG, Coupland SE. Genetic heterogeneity in uveal melanoma assessed by multiplex ligation-dependent probe amplification. Invest Ophthalmol Vis Sci. 2010;51(10):4898–4905. | ||
Yang H, Cao J, Grossniklaus HE. Uveal melanoma metastasis models. Ocul Oncol Pathol. 2015;1(3):151–160. | ||
Singh AD, Kivelä T. The collaborative ocular melanoma study. Ophthalmol Clin North Am. 2005;18(1):129–142. | ||
Augsburger JJ, Corrêa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148(1):119–127. | ||
Mensink HW, Vaarwater J, Kiliç E, et al. Chromosome 3 intratumor heterogeneity in uveal melanoma. Invest Ophthalmol Vis Sci. 2009;50(2):500–504. | ||
Ehlers JP, Worley L, Onken MD, Harbour JW. Integrative genomic analysis of aneuploidy in uveal melanoma. Clin Cancer Res. 2008;14(1):115–122. | ||
Scholes AGM, Damato BE, Nunn J, Hiscott P, Grierson I, Field JK. Monosomy 3 in uveal melanoma: correlation with clinical and histologic predictors of survival. Invest Ophthalmol Vis Sci. 2003;44(3):1008–1011. | ||
Sisley K, Parsons MA, Garnham J, et al. Association of specific chromosome alterations with tumour phenotype in posterior uveal melanoma. Br J Cancer. 2000;82(2):330–338. | ||
Hughes S, Damato BE, Giddings I, Hiscott PS, Humphreys J, Houlston RS. Microarray comparative genomic hybridisation analysis of intraocular uveal melanomas identifies distinctive imbalances associated with loss of chromosome 3. Br J Cancer. 2005;93(10):1191–1196. | ||
Sipos E, Hegyi K, Treszl A, et al. Concurrence of chromosome 3 and 4 aberrations in human uveal melanoma. Oncol Rep. 2017;37(4):1927–1934. | ||
Harbour JW, Onken MD, Roberson EDO, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. | ||
van den Bosch T, Kilic E, Paridaens D, de Klein A. Genetics of uveal melanoma and cutaneous melanoma: two of a kind? Dermatol Res Pract. 2010;2010(1):360136. | ||
Onken MD, Worley LA, Harbour JW. A metastasis modifier locus on human chromosome 8p in uveal melanoma identified by integrative genomic analysis. Clin Cancer Res. 2008;14(12):3737–3745. | ||
Schally AV, Nagy A. Cancer chemotherapy based on targeting of cytotoxic peptide conjugates to their receptors on tumors. Eur J Endocrinol. 1999;141(1):1–14. | ||
Schally AV. Luteinizing hormone-releasing hormone analogs: their impact on the control of tumorigenesis. Peptides. 1999;20(10):1247–1262. | ||
Schally AV, Halmos G. Targeting to peptide receptors. In: Kratz F, Senter Peter SH, editors. Drug Delivery in Oncology. Weinheim: Wiley-VCH; 2012:1219–1261. | ||
Schally AV, Comaru-Schally AM, Nagy A, et al. Hypothalamic hormones and cancer. Front Neuroendocrinol. 2001;22(4):248–291. | ||
Schally AV, Nagy A. Chemotherapy targeted to cancers through tumoral hormone receptors. Trends Endocrinol Metab. 2004;15(7):300–310. | ||
Nagy A, Schally AV. Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers. Biol Reprod. 2005;73(5):851–859. | ||
Szepeshazi K, Schally AV, Halmos G. LH-RH receptors in human colorectal cancers: unexpected molecular targets for experimental therapy. Int J Oncol. 2007;30(6):1485–1492. | ||
Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol. 2000;163(2):623–629. | ||
Moretti RM, Marelli MM, Van Groeninghen JC, Limonta P. Locally expressed LHRH receptors mediate the oncostatic and antimetastatic activity of LHRH agonists on melanoma cells. J Clin Endocrinol Metab. 2002;87(8):3791–3797. | ||
Liu SV, Schally AV, Hawes D, et al. Expression of receptors for luteinizing hormone-releasing hormone (LH-RH) in prostate cancers following therapy with LH-RH agonists. Clin Cancer Res. 2010;16(18):4675–4680. | ||
Treszl A, Steiber Z, Schally AV, et al. Substantial expression of luteinizing hormone-releasing hormone (LHRH) receptor type I in human uveal melanoma. Oncotarget. 2013;4(10):1721–1728. | ||
Liu N, Sun Q, Chen J, et al. MicroRNA-9 suppresses uveal melanoma cell migration and invasion through the NF-κB1 pathway. Oncol Rep. 2012;28(3):961–968. | ||
Schally AV, Szepeshazi K, Nagy A, Comaru-Schally AM, Halmos G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell Mol Life Sci. 2004;61(9):1042–1068. | ||
Jaszberenyi M, Schally AV, Block NL, et al. Inhibition of U-87 MG glioblastoma by AN-152 (AEZS-108), a targeted cytotoxic analog of luteinizing hormone-releasing hormone. Oncotarget. 2013;4(3):422–432. | ||
Shields CL, Kaliki S, Livesey M, et al. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: analysis of 7872 consecutive eyes. JAMA Ophthalmol. 2013;131(8):993–1003. | ||
Millar R, Conklin D, Lofton-Day C, et al. A novel human GnRH receptor homolog gene: abundant and wide tissue distribution of the antisense transcript. J Endocrinol. 1999;162(1):117–126. | ||
Neill JD. Mammalian gonadotropin-releasing hormone (GnRH) receptor subtypes. Arch Physiol Biochem. 2002;110(1–2):129–136. | ||
Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25(2):235–275. | ||
Millar R. Gnrh II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14(1):35–43. | ||
Harrison GS, Wierman ME, Nett TM, Glode LM. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocr Relat Cancer. 2004;11(4):725–748. | ||
Tolkach Y, Joniau S, Van Poppel H. Luteinizing hormone-releasing hormone (LHRH) receptor agonists vs antagonists: a matter of the receptors? BJU Int. 2013;111(7):1021–1030. | ||
Fraser HM, Sellar RE, Illingworth PJ, Eidne KA. GnRH receptor mRNA expression by in-situ hybridization in the primate pituitary and ovary. Mol Hum Reprod. 1996;2(2):117–121. | ||
Wolfahrt S, Kleine B, Jarry H, Rossmanith WG. Endogenous regulation of the GnRH receptor by GnRH in the human placenta. Mol Hum Reprod. 2001;7(1):89–95. | ||
Botté MC, Chamagne AM, Carré MC, Counis R, Kottler ML. Fetal expression of GnRH and GnRH receptor genes in rat testis and ovary. J Endocrinol. 1998;159(1):179–189. | ||
Finch AR, Sedgley KR, Caunt CJ, McArdle CA. Plasma membrane expression of GnRH receptors: regulation by antagonists in breast, prostate, and gonadotrope cell lines. J Endocrinol. 2008;196(2):353–367. | ||
Kakar SS, Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995;98(1):57–62. | ||
Jacobson JD, Crofford LJ, Sun L, Wilder RL. Cyclical expression of GnRH and GnRH receptor mRNA in lymphoid organs. Neuroendocrinology. 1998;67(2):117–125. | ||
Tiong JDR, Pakiam JG, Wray S. Gonadotropin releasing hormone-1 expression in incisors of mice. Endocrinology. 2004;145(8):3608–3612. | ||
Angi M, Versluis M, Kalirai H. Culturing uveal melanoma cells. Ocul Oncol Pathol. 2015;1(3):126–132. | ||
White JS, Becker RL, McLean IW, Director-Myska AE, Nath J. Molecular cytogenetic evaluation of 10 uveal melanoma cell lines. Cancer Genet Cytogenet. 2006;168(1):11–21. | ||
Seitz S, Buchholz S, Schally AV, et al. Triple negative breast cancers express receptors for LHRH and are potential therapeutic targets for cytotoxic LHRH-analogs, AEZS 108 and AEZS 125. BMC Cancer. 2014;14(1):847. | ||
Keller G, Schally A, Gaiser T. Human malignant melanomas express receptors for luteinizing hormone releasing hormone allowing targeted therapy with cytotoxic luteinizing hormone releasing hormone analogue. Cancer Res. 2005;65(13):5857–5863. | ||
Engel J, Emons G, Pinski J, Schally AV. AEZS-108: a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs. 2012;21(6):891–899. | ||
Engel JB, Schally AV. Drug insight: clinical use of agonists and antagonists of luteinizing-hormone-releasing hormone. Nat Clin Pract Endocrinol Metab. 2007;3(2):157–167. | ||
Engel JB, Schally AV, Dietl J, Rieger L, Höni A. Targeted therapy of breast and gynecological cancers with cytotoxic analogues of peptide hormones. Mol Pharm. 2007;4(5):652–658. | ||
Kovacs M, Schally AV, Csernus B, Busto R, Rekasi Z, Nagy A. Targeted cytotoxic analogue of luteinizing hormone-releasing hormone (LH-RH) only transiently decreases the gene expression of pituitary receptors for LH-RH. J Neuroendocrinol. 2002;14(10):5–13. | ||
Liu SV, Tsao-Wei DD, Xiong S, et al. Phase I, dose-escalation study of the targeted cytotoxic LHRH analog AEZS-108 in patients with castration- and taxane-resistant prostate cancer. Clin Cancer Res. 2014;20(24):6277–6283. | ||
Emons G, Kaufmann M, Gorchev G, et al. Dose escalation and pharmacokinetic study of AEZS-108 (AN-152), an LHRH agonist linked to doxorubicin, in women with LHRH receptor-positive tumors. Gynecol Oncol. 2010;119(3):457–461. | ||
Emons G, Weiß S, Ortmann O, Gründker C, Schulz KD. LHRH might act as a negative autocrine regulator of proliferation of human ovarian cancer. Eur J Endocrinol. 2000;142(6):665–670. | ||
Aguilar-Rojas A, Huerta-Reyes M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells (review). Oncol Rep. 2009;22(5):981–990. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.