Back to Journals » Infection and Drug Resistance » Volume 16
Characteristics, Outcomes, and Clinical Indicators of Bloodstream Infections in Neutropenic Patients with Hematological Malignancies: A 7-Year Retrospective Study
Authors Wang S , Song Y, Shi N, Yin D, Kang J, Cai W, Duan J
Received 12 April 2023
Accepted for publication 27 June 2023
Published 8 July 2023 Volume 2023:16 Pages 4471—4487
DOI https://doi.org/10.2147/IDR.S413454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Shuyun Wang,1 Yan Song,1 Nan Shi,1,2 Donghong Yin,1 Jianbang Kang,1 Wanni Cai,2 Jinju Duan1
1Department of Pharmacy, Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 2Department of Pharmacy, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China
Correspondence: Jinju Duan, Department of Pharmacy, Second Hospital of Shanxi Medical University, Wuyi Road, Xinghualing District, Taiyuan, Shanxi, People’s Republic of China, Tel +86 13834653172, Email [email protected]
Purpose: The aim of this study was to investigate the current epidemiology, its changes during the study years, and inflammatory biomarkers of bacterial bloodstream infections (BSIs) in neutropenic patients with hematological malignancies. We assessed mortality risk factors and multidrug-resistant (MDR) gram-negative BSI predictors.
Patients and Methods: We conducted a retrospective study from January 2015 to December 2021, which included adult neutropenic oncohematological patients with confirmed BSIs. We used univariable and multivariable analyses to analyze the risk factors. Each index’s reliability for bacterial BSI diagnosis was assessed using the receiver-operating characteristic curve and area under the curve.
Results: A total of 514 isolates were obtained from the 452 patients. The average mortality was 17.71%. Gram-negative organisms were the predominant causes of BSI. Escherichia coli was the most common microorganism (49.90%). The overall variation trend of the isolation rate of MDR and carbapenem-resistant gram-negative bacteria increased. Multivariate analysis indicated that: 1) neutropenia that lasted for more than 7 days, patients ≥ 60 years of age, septic shock, hospitalization for > 20 days, BSI with a carbapenem-resistant strain, and treatment with linezolid or vancomycin in infections lasting less than 30 days were independent mortality risk factors; 2) severe neutropenia exceeding 7 days, unreasonable empirical therapy, and receipt of aminoglycosides or 3rd or 4th generation cephalosporins in infections lasting less than 30 days were independent risk factors of MDR gram-negative bacteria. Procalcitonin, absolute neutrophil count, and white blood cell indicate higher diagnostic accuracy for BSIs. Moreover, bacteria time to detection was better at differentiating Gram-negative and Gram-positive bacterial infections.
Conclusion: We analyzed the risk factors for BSI neutropenic patients with hematological malignancies, the distribution of bacteria, antibiotic resistance, and the changes in clinical parameters. This single-center retrospective study may provide clinicians with novel insights into the diagnosis and treatment of BSI to improve future clinical outcomes.
Keywords: bloodstream infection, neutropenia, hematological malignancy, pathogens distribution, antibiotic susceptibility, risk factors
Introduction
Patients with hematological malignancies (HMs) are exposed to a high risk of infectious complications because they become immunocompromised due to either the underlying disease or the cytotoxic effects of chemotherapy. Bacterial bloodstream infections (BSIs) are one of the most common complications for patients with HMs, which results in high mortality and morbidity.1 This is especially true for patients with chemotherapy-induced neutropenia. BSI, defined as laboratory-confirmed isolation of at least one Gram-negative or Gram-positive bacterial strain or other pathogens from blood samples, is still a common concern for HM patients.2 Bacterial BSIs occur in approximately 20–30% of adult patients with febrile neutropenia and HMs. Patients with neutropenia are especially susceptible to high-risk bacterial infections, which were defined following the Infectious Diseases Society of America (IDSA) guidelines3: prolonged (>7 days duration) and profound neutropenia (absolute neutrophil count <100 cells/μL) and/or significant medical comorbid conditions, including hypotension or hyperlactatemia, ICU requirement, pneumonia or hypoxemia, intravascular catheter infection, and evidence of renal failure (creatinine clearance of ≤30 mL/minute) or hepatic insufficiency (aminotransferase levels >5 times the normal values), which may occur and progress to fulminant progression.3 The literature suggests that the mortality rate associated with BSIs is as high as 34–50%.1,4,5 There are various reasons for increased mortality, including incorrect diagnosis, inadequate and untimely medication, inappropriate empiric therapy, and multidrug-resistant (MDR) bacteria.3,6 Therefore, there is an urgent need to elucidate the relative risks, diagnostic methods, and reasonable use of antibiotics to treat agranulocytosis and reduce disease incidence and mortality. However, there is limited data from Shanxi (China) on neutropenic HM BSI-infected patients and details of the infecting strains and their antibiotic sensitivity.
Blood cultures are regarded as gold criteria of diagnosis of infection diagnosis. It often takes a relatively long time (3–5 days) and has a low positive rate, thus limiting its application in early diagnosis.7 Patients with infection are usually associated with changes in biochemistry, including albumin (ALB), glucose (GLU),8,9 alanine transaminase (ALT), and aspartate transaminase (AST). Moreover, some clinical parameters, such as absolute neutrophil count (ANC), white blood cell (WBC), platelets (PLT), C-reactive protein (CRP), procalcitonin (PCT),10 and d-dimer (D-D),11 are commonly used in the diagnosis of infection. But, they have not been extensively studied in patients with hematological malignancy.
Thus, we sought to systematically and retrospectively determine the current epidemiology, its changes during the study years, and inflammatory biomarkers of bacterial BSIs in neutropenic patients with HMs. Further, we assessed the overall mortality risk factors and MDR Gram-negative BSI predictors.
Research Methods
Setting and Study Design
This study was performed at the second hospital of Shanxi Medical University, an educational 2700-bed inpatient center. This was a single-center, retrospective study of patients (age ≥18 years) with neutropenic HMs from January 2015 to December 2021. In this study, we included patients with positive blood cultures and BSI diagnoses. Only patients with all available clinical and laboratory data were included in this study. The exclusion criteria were as follows: hospitalization for < 24 hours, contaminated blood culture samples, or subjects with insufficient data.
Data Collection
Data were obtained from the Hospital Information System (HIS) and microbiology department records. This study collected the general data of patients with positive blood cultures and information on the strains isolated from the blood culture, including the following variables: ID number, age, gender, underlying disease, neutrophil count, length of neutropenia before BSI, septic shock, hospitalization history 90 days before BSI, history of antibiotic use 30 days before BSI diagnosis, type and antimicrobial drug susceptibility, clinical outcome, length of hospitalization, and peak temperature at the onset of the BSI.
Microbiological Methods
All microbiological-related experimental operations were conducted in standardized microbiology laboratories. Blood culture bottles were incubated in an automated blood culture monitoring BACTEC system (Becton Dickinson Diagnostic Instrument Systems). When a positive alarm occurred in the blood culture instrument, one drop from each bottle was plated on standard bacteriology media. Next, microorganism identification and antimicrobial susceptibility tests were performed using Vitek 2 technology (bioMe’rieux, Marcy l’Etoile, France). Antimicrobial susceptibility data were categorized as “susceptible”, “resistant”, or “intermediate” according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.12
Definitions
A patient is defined as being infected with a recognized bacterial pathogen, which is not included on the commensal list, identified from one or more blood specimens obtained by culture, and with at least one of the following signs or symptoms: fever, chills, or hypotension. Importantly, symptoms must not be related to an infection at another site to be considered a genuine BSI.1 The overall mortality rate was defined as any death recorded during the hospitalization, regardless of cause.
Febrile neutropenia is defined as an oral temperature measurement > 38.5 °C or two consecutive temperatures > 38 °C lasting for 2 h and an ANC< 0.5 × 109 cell/L or expected to fall below < 0.5 × 109 cell/L. Neutropenia was considered to be severe if the ANC was < 100 neutrophils/mcL.13
Time to-Detection (TTD) is defined as the time between the placement of each blood culture bottle in the incubation cabinet and the detection of growth. Methicillin-resistant coagulase-negative staphylococci (MRCoNS) are defined as cefoxitin-resistant or oxacillin-resistant strains. MDR bacteria were determined according to the European Centre for Disease Prevention and Control (ECDC) criteria (https://www.ecdc.europa.eu/en). Pseudomonas aeruginosa and Acinetobacter baumannii isolates (both part of the Enterobacteriaceae family), that are resistant to ceftazidime or cefotaxime are considered extended-spectrum beta-lactamase (ESBL) producers. Carbapenem-resistant (CR) strains were defined as isolates that are intermediate or resistant to one or more carbapenems using the CLSI current breakpoints. However, not all isolates were tested against all carbapenems.1
According to a previous study,6 unreasonable empirical antibiotic therapy was defined as that empirical antibiotic therapy was considered inappropriate when the isolated bacterium was not susceptible to any of the antibiotics used empirically in the first 96 h.
Statistical Analysis
In this study, we used the chi-squared test for a row-by-column contingency table with appropriate degrees of freedom to examine the critical factors that may influence the outcome of patients and the drug-resistant phenotype of strains. We used the survival status to represent patient outcomes. Moreover, the drug-resistant phenotype of blood culture-isolated strains was categorized. The Pearson χ2 test and the Mann–Whitney U-test or the Student’s t-test were used to compare the distribution of categorical and continuous variables, respectively. Variables with a p-value < 0.1 at univariate analysis were entered into the multivariate model and selected according to a stepwise selection. We reported odds ratio (OR) values, as well as the confidence interval (CI) of the odds ratio for each variable. Each index’s reliability for bacterial BSI diagnosis was assessed using the receiver-operating characteristic (ROC) curve and area under the curve (AUC). Diagnostic accuracy, including sensitivity and specificity, was computed using cut-off values. The optimal diagnostic cut-off level was found using Youden’s index. A p value < 0.05 was considered to be statistically significant. All analyses were performed in SPSS version 22 (IBM Corp., Armonk, NY, USA).
Results
Demographics and Epidemiology
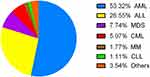
During the study period, 2538 oncohematological patients were diagnosed with a bacterial infection. Further, 731 patients were diagnosed with bacteria BSIs, and 452 patients were found to have bacteria BSIs complicated with neutropenia (Figure 1). A total of 514 bacterial isolates were obtained from the 452 patients enrolled in this study. Of these patients, 250 were male and 202 were female, the mean age was 47.4 years (range: 8–85 years), and the average age of patients has increased in recent years. The distributions of the mean age and sex are shown in Figure 2. Most patients (241; 53.3%) were affected by acute myeloid leukemia (AML) and 120 patients (26.5%) were affected by acute lymphoblastic leukemia (ALL), followed by myelodysplastic syndrome (MDS), and chronic myeloid leukemia (CML). The distribution of underlying diseases is shown in Figure 3. Figure 4 shows the mortality trend. The average mortality rates first decreased and then increased over the years. The lowest death rate occurred in 2017 (11.48%). However, the average mortality was 17.71% (89/514).
 |
Figure 1 Row chart of data collection. |
 |
Figure 2 The distribution of the mean age and male tend. |
 |
Figure 4 The mortality of BSI cases by year. |
Table 1 describes the clinical characteristics of the sample. The majority of patients had no definite focus on infection. The median peak temperature at the onset of the BSIs was 38.9°C ranging from 38.0–41.0°C. Severe neutropenia was observed in 86.7% of cases (446/514). The median days of severe neutropenia before BSI was 7 days, while the median duration of neutropenia was 10 days. Sixty patients experienced septic shock (11.6%). The most reported TTD was less than 12 h (363/514, 70.6%), while 20.2% were detected during 12–24h, 4.1% between 24 h and 36 h, and 4.9% were diagnosed in more than 36 h using the BACTEC system.
 |
Table 1 Clinical Characteristics of 514 Episodes of Bloodstream Infections |
Microbiology
Incidence of Bloodstream Infections in Neutropenic Patients with HMs
During the study period, 394 (76.6%) and 108 (20.1%) Gram-negative and Gram-positive pathogens organisms, respectively, and 12 (2.3%) fungal organisms were isolated from blood cultures. The isolation rate of Gram-negative bacteria was higher than Gram-positive bacteria each year (Figure 5), showing that Gram-negative organisms were the predominant causes of BSIS. Except for 2018, there were no significant differences in the isolation rate of Gram-negative bacteria between any two years in the remaining study period (P>0.05). The overall trend in the annual incidence of BSIs (Gram-negative and Gram-positive) year by year was not significantly different in this study (P>0.05). As shown in Figure 6.
 |
Figure 5 The trend in aetiologies of BSI from 2015 to 2021. Note: *P<0.05, it was considered to be statistically significant. |
 |
Figure 6 The trend in the Gram-negative organism of BSI from 2015 to 2021. Note: *P<0.05, it was considered to be statistically significant. |
Gram-negative infections peaked at 96.4% in 2018 but fell in 2021 to frequencies similar to the start of the study. On the other hand, infections with Gram-positive organisms ranged from 3.5–30.1%.
The Trend in and the Distribution of Etiology of BSI
The Gram-negative organism, Escherichia coli was the most common (203/394, 51.5%), followed by Klebsiella pneumoniae (81/394, 20.5%) and P. aeruginosa (57/394, 14.4%). The overall isolation rates of E. coli and P. aeruginosa were stable and unchanged in the study, but the overall isolation rate of K. pneumoniae was increased (P < 0.05). (Figure 6). Among Gram-positive bacteria, coagulase-negative staphylococci (CoNS) were the most frequently isolated pathogen (51/108, 47.2%), followed by Enterococcus faecium (15/108, 13.8%).
As shown in Figure 7, the frequency of MDR Gram-negative bacteria (MDRGNB) in this study decreased from 2015 to 2017 and then increased from 2017 to 2021. Moreover, it reached its highest level in 2021. Overall, the MDRGNB BSI cases increased between 2015 and 2021 (P < 0.001). The isolation rate of MDRGNB was 48.98% (193/394). Until 2018, carbapenem-resistant Gram-negative bacteria (CRGNB) BSI cases occurred only sporadically but an outbreak occurred after 2018. The isolation rate of CRGNB was 6.09% (24/394) and the overall trend was not statistically significant. Meanwhile, E. coli was the most common MDRGNB and CRGNB (64.30% and 33.33%, respectively), followed by K. pneumoniae (17.50% and 25.00%, respectively). Interestingly, we found that the overall trend in the annual incidence of MDR E. coli, MDR K. pneumoniae, carbapenem resistant (CR) E. coli increased annually (P < 0.05).
Antimicrobial Susceptibilities of Primary BSI Isolates
Antibiotic susceptibility results for the most common Gram-negative bacteria are shown in Table 2. There was only one strain resistant to polymyxin or tigecycline. E. coli showed higher drug resistance rates to cephalosporins of the first and second generations (85.71%), as well as aztreonam (70.44%), and quinolones (67.98%). More than 90% of E. coli isolates were sensitive to carbapenems. In addition, E. coli showed high sensitivity to the other drugs. Still, the resistance rates to 3rd or 4th generation cephalosporins, cephamycin, β-Lactam/β-lactamase inhibitor combinations, Carbapenems, Aztreonam, Aminoglycosides, Quinolones were significant increases in the three time periods (2015–2016, 2017–2019, 2020–2021). Klebsiella pneumoniae had higher resistance rates to cephalosporins, especially cephalosporins of the first and second generations (79.01%). In addition, the resistance rates to 3rd or 4th generation cephalosporins, β-Lactam/β-lactamase inhibitor combinations, Aztreonam, Aminoglycosides, and Quinolones were significant increases in the three time periods (2015–2016, 2017–2019, 2020–2021). The resistance rate of Pseudomonas aeruginosa to β-Lactam/β-lactamase inhibitor and aztreonam was the most higher, at 28.07%. Enterobacter cloacae were highly sensitive to Carbapenems. However, the resistance rate of Enterobacter cloacae to cephalosporins and cephamycin was higher, over 80%. As shown in Figure 8.
 |
Table 2 Antibiotic Resistance Results for the Most Common Isolated Bacteria |
Risk Factors for Mortality and MDR in Patients
Risk Factors for Mortality in Patients
Univariate analyses comparing the clinical characteristics of patients who survived or died are shown in Table 3. Of the study group, there were 426 survivors and 88 deaths, resulting in a death rate of 17.1%. In univariate analysis, we found significant differences between the two groups in the following areas (P<0.05): severe neutropenia for more than 7 days, neutropenia for more than 7 days, hypoproteinemia, a peak temperature at the beginning of BSI ≥39°C, septic shock, hospitalization days >20, receipt of antibiotics 30 days before infection ≥2 different antimicrobial classes, unreasonable empirical treatment. Compared to the survivor group, the proportion of utilization of 3rd or 4th generation cephalosporins, cephamycin, linezolid, vancomycin, and tigecycline before 30 days of BSIs was higher in the non-survivor group (P < 0.05). Moreover, compared with patients in the survivor group, patients in the death group are more likely to have a higher proportion of ESBL-producing strains (44.62% versus 39.51%) and MDR strains (62.5% versus 53.9%). Still, there were no statistically significant differences (P = 0.684 and P = 0.080, relatively). However, CR strains in the survivor group also showed a higher proportion than the group of patients that died (18.18% versus 5.91%) and this was statistically significant (P <0.001).
 |
Table 3 Risk Factors for Mortality |
In this study, variables with P < 0.1 in the univariate logistic regression models and factors considered clinically relevant were selected for the multivariate logistic regression model for mortality. Multivariate analysis indicated that neutropenia lasting more than 7 days (OR, 2.396; 95% Cl, 1.266–4.533; P = 0.007), septic shock (OR, 16.772; 95% Cl, 8.203–34.292; P < 0.001), hospitalization days >20 (OR, 0.282; 95% Cl, 0.131–0.606; P = 0.001), CR strain (OR, 6.506; 95% Cl, 2.360–17.935; P < 0.001), receipt of linezolid within 30 days of infection (OR, 4.614; 95% Cl, 1.826–11.659; P = 0.001), and receipt of vancomycin within 30 days of infection (OR, 2.521; 95% Cl, 1.202–5.285; P = 0.014) were independent mortality risk factors compared to the survivor group (Table 3).
Risk Factors for MDRGNB in Patients
Univariate analyses comparing the clinical characteristics of patients with or without infections caused by MDRGNB are shown in Table 4. The following factors were most frequently detected in patients with MDRGNB, and there were statistically significant differences (P<0.05): more than 7 with severe neutropenia, hospitalization days >15, receipt of antibiotics within 30 days of infection ≥ 2 different antimicrobial classes, unreasonable empirical treatment. Moreover, the proportion of utilization of 1 cephalosporins, aminoglycosides, tigecycline, SMX/TMP, and vancomycin before 30 days of BSIs (P < 0.05).
 |
Table 4 Risk Factors for MDRGNB |
Variables with P < 0.1 in the univariate logistic regression models and factors that were considered clinically relevant were selected for the multivariate logistic regression model for mortality. Multivariate analysis indicated that more than 7 days with severe neutropenia (OR, 2.023; 95% CI, 1.252–3.269; P = 0.004), unreasonable empirical treatment (OR, 0.243; 95% CI, 0.101–0.589; P = 0.002), receipt of aminoglycosides within 30 days of infection (OR, 3.753; 95% CI, 2.123–6.635; P < 0.001) and 3rd or 4th generation cephalosporins (OR, 3.137; 95% CI, 1.674–5.879; P < 0.001) were independent risk factors for MDR infection when compared with the non-MDR group (Table 4).
Diagnostic Accuracy of Indicators for BSI Detection
ROC curves of WBC, PLT, CRP, PCT, D-D, ALB, GLU, ALT, AST, and ANC levels were plotted to diagnose BSI in 514 febrile episodes (Figure 9). Among these indicators, the AUCs of PCT, WBC, and ANC were 0.8056, 0.7515, and 0.7397, respectively, indicating higher diagnostic accuracy. When we analyzed WBC, PLT, CRP, PCT, D-D, ALB, ALT, AST, ANC levels, and bacteria culture time in differentiating G- and G+ bacterial infections, bacteria culture time performed better than PCT with an AUC of 0.8040 (Figure 10). Table 5 shows these indexes’ diagnostic sensitivity and specificity with the best cut-off value.
 |
Table 5 Sensitivity and Specificity of Parameters in Sample |
 |
Figure 9 ROC curves of indicators differentiating blood culture positive and negative groups. |
 |
Figure 10 ROC curves of indicators differentiating Gram- and gram+ groups. |
Discussion
HM patients are more vulnerable to pathogen infection, especially BSIs, which lead to higher mortality. Fever is a typical infectious symptom, although non-infectious febrile episodes are often observed. It is essential to distinguish infectious from non-infectious patients, which can then be used to provide appropriate and immediate antibiotic therapy.
Several studies have been conducted with neutropenic patients, but there is a lack of literature describing distinctions between those with HMs. An additional study included patients with HMs and did not differentiate between neutropenic patients. Few studies have focused on BSI neutropenic patients with HMs.1,2,4,6 Moreover, the epidemiology of microbial pathogens and antimicrobial resistance may differ by geographical region.14–16 Therefore, the novelty of this study is that the sample area and the related diseases in the patients with HMs are not the same as those in other studies. That is, our sample included patients from Shanxi Province in northwest China who were diagnosed with HMs with BSI-associated neutropenia.
In this study, the incidence of bacterial BSI was 28.80% (731/2538), which is higher than other reports from different studies. The reason may be that our subjects were hematological patients who are more predisposed to infection because of their lowered immunity.17–19 The incidence of bacterial BSIs complicated with neutropenia was 17.81% (452/2538), which agreed with other studies.6 We enrolled 452 patients with HM complicated with neutropenia and BSIs, and 514 strains were isolated. The mean age of the patients was 47.48 years, which is similar to that of most other BSI patients with HM.1,2 The proportion of males was higher than that of females, which is consistent with other studies. AML, ALL, and MDS were the most common diseases and are compatible with other studies.13,20–22
In the present study, we observed that the percentage of Gram-negative bacilli (GNB) (76.6%) was higher than that of Gram-positive bacilli (GPB) (20.1%), which is consistent with other reports studied in Iran, Italy, Spain, and Taiwan.1,6,17,23 However, in one Australian study, GPB was more prevalent (50.1%) than GNB (45.6%).24 Reports regarding the etiology of BSIs in cancer patients are rare, and their results are controversial. Some regard GNB as the leading cause of BSIs,25 while others consider it to be GPB.5,24 In an earlier study, patients with BSI and neutropenia who had been treated with prophylactic antibiotics and those with central lines which were more susceptible to Gram-positive infections had been reported.25 In this study, however, most patients had a central line and were treated with prophylactic antibiotics. This difference in primary bacteria may be related to the geographical area. Remarkably, an increase in GNB in recent studies. This change is likely due to improved management, for example, the optimal use of central venous catheters, the reduction in the incidence of severe mucositis, and the discontinuation of quinolone prophylaxis. Furthermore, the chemotherapy regimens may enhance intestinal toxicity and endogenous bacteremia.6,17 In addition, this shift may also be related to the disease prevalence and use of medication.
In this study, E. coli was the most common Gram-negative organism (203/394, 51.5%), followed by K. pneumoniae (81/394, 20.5%) and P. aeruginosa (57/394, 14.4%). Similar findings were seen in earlier studies carried out in Italian, Chinese, and Spanish BSIs with hematological disorders.2,6,17 In comparison, Australia showed that the most frequently isolated pathogen was E. coli, followed by K. pneumoniae.24 This difference in primary bacteria may be related to the geographical area. Regular surveillance of the bacterial epidemiologic status can be used to evaluate antimicrobial strategies and adapt them to mitigate the effects of emerging pathogens.26
Antibiotic susceptibility results in this study showed that the resistance rate of E. coli to cephalosporins, aztreonam, and quinolones were all more than 50%, K. pneumoniae to cephalosporins of the first and second generations nearly up to 80%, which was investigated in our other study. We found that P. aeruginosa showed a higher susceptibility to drugs (all over 70%). This indicates that the abuse of broad-spectrum antibiotics increases drug resistance.2,17,22 Importantly, all Gram-negative bacteria in this study were susceptible to carbapenems, in contrast to other studies.6,27
Neither CoNS, nor antibiotic resistance was higher in penicillin, erythromycin, and clindamycin, which was more than 60%. This was consistent with other studies.2
Differences in susceptibility results may occur even within the same geographical area or within different institutions, regions, and countries, which suggests that antibiotic susceptibility models may not be widely applied on a large scale, thereby highlighting the importance of local surveillance.6,27
Death rates have generally increased throughout the study, with death rates ranging from 11.48% to 26.09%. The highest mortality rate occurred in 2018. Mortality is high among neutropenic cancer patients with BSI caused by Gram-negative organisms.25,28,29 Carbapenems are broad-spectrum antibiotics and are widely used for the treatment of serious infections caused by MDR Enterobacteriaceae. The spread of CR strains is associated with high mortality rates. The China Antimicrobial Surveillance Network (CHINET, www.chinets.com), included the largest tertiary-care teaching hospitals in each province or city and represents 26 provinces or cities, reported that from 2015 to 2021, the resistance rates of Enterobacterales to imipenem or meropenem were increased from 3.8% to 10.0%. Moreover, CR K. pneumoniae was widespread in 2018. In another one of our studies, we showed that during 2017–2019, 90.3% (28/31) of patients infected with CR K. pneumoniae were infected in 2018.30 Furthermore, the first patient in our hospital infected by CR K. pneumoniae was detected in the Hematology ward. Interestingly, in 2018, Gram-negative bacteria, K. pneumoniae, and MDR K. pneumoniae were among the largest number of isolates in our study, which may have contributed to the high death rate in 2018. In August 2018, we established a working group of the Antimicrobial Management Task Force (AMS) to control the infection outbreak, which is the main reason why the mortality rate decreased after 2019 in our study. The AMS workers implemented strict infection control procedures during the transmission time, such as: rapid communication set up to ensure that the results of microbiological testing were shown online in a timely manner; the experts of infection control and clinic pharmacists made timely recommendations on the rational use of drugs, et al.
Days with neutropenia of more than 7 days, age ≥ 60 years, septic shock, hospitalization days>20, and CR strain are independent predictors for mortality, which are in line with other studies.23,31–34 Interestingly, we observed the use of linezolid or vancomycin within 30 days of infection has an association with survival. The possible reason may be that the patient is now suffering from a bloodstream infection, which is mainly due to negative bacteria, while the use of vancomycin or linezolid before indicates the presence of positive bacteria, so the patient has multiple biological infections, which increases the chance of death.
The development of MDRGNB is a global health problem, especially in immunocompromised patients,15,16,35–37 and this poses a significant challenge to the treatment of neutropenic fever.14,38,39 There are few evidence-based data on the prevention and control of infections caused by MDR bacterial strains.35,37 One of the key findings of this study was that the isolation rate for MDRGNB in the GNB group increased from 24% to 70.93%, primarily because of the number of days with severe neutropenia exceeding 7 days, unreasonable empirical therapy, and the use of aminoglycosides or 3rd or 4th generation cephalosporins within 30 days of infection. Therefore, patients who may be at risk of infection by resistant strains must be identified so that the best antibiotic regimen can be selected in patients with febrile neutropenia with HMs.
Investigating the risk factors of death and MDR bacteria may assist clinicians in early intervention to improve patient outcomes, especially for those who are in a critical situation with HMs with neutropenia. The selection of initial empirical antibiotic treatment in patients in this area is often a clinical challenge. Based on our findings, clinicians should take special care of the patient who has independent risk factors for death and MDR bacteria. Several points need to be considered in the treatment of BSI patients. First, carbapenems are the preferred agents for empirical therapy. Despite the presence of carbapenem-resistant bacteria in the region, their detection rate is relatively low. Patients with neutropenia may benefit from empirical antibiotic treatment of carbapenems. Second, to maximize the therapeutic effect of carbapenems, their usage and dosage should be standardized. Adequate concentrations must be achieved at the site of infection to suppress the causative pathogens. Carbapenem exhibits a time-dependent antimicrobial activity, and long-duration infusion (prolonged or continuous infusion) of carbapenem may be able to reach the pharmacodynamic objective more effectively than an intermittent bolus.40 Third, if empirical anti-infective therapy is ineffective after 48–72 h. Clinicians should consider that the pathogens causing BSI may be: 1) CR bacteria and adding tigecycline or polymyxin to the anti-infection therapy; 2) Gram-Positive drug-resistant bacteria. In the current study, the percentage of Gram-positive isolates was 20.1%; among them, 47.2% were coagulase-negative staphylococci, which are more probably contaminated bacteria. If the proportion of MDR Staphylococcus aureus and MRCoNS are over 50% in the Gram-positive bacteria; it is recommended to add vancomycin or teicoplanin to the anti-infection regimen. 3) If both of the aforementioned types of bacteria co-exist, the anti-infectious therapy should be either tigecycline or polymyxin in combination with vancomycin or teicoplanin.
The biomarkers of inflammation are always used as an assistant indicator in diagnosis. In the present study, the level of PCT in the blood culture-positive group was significantly higher than that in the blood culture-negative group, and the results showed that PCT was sensitive and specific for the prediction of bacteremia, in accordance with the previous studies.2,41–45 Engel et al46 measured the peak PCT levels after 32 hours after the onset of fever in over 75% of febrile episodes. In the case of procalcitonin, it was impossible to confirm the antibiotic treatment’s mitigating effect alone.43 Therefore, in all respects, it appears to be important to collect samples within 24 hours of the onset of the fever and following the initiation of treatment with antibiotics.
This study discovered no relevant difference in PCT levels for Gram-positive and Gram-negative infections, which was also observed in Germany. This could be because PCT may be affected by the high level of contamination of gram-positive bacteria and is less sensitive.47
WBC, a standard indicator of inflammation with elevated levels, has been observed to have a lower WBC count in the blood cultures. That is in line with previous studies.2 The probable cause is that the immune function of hematologic patients is decreased because of chemotherapy or bone marrow suppression. Unlike previous studies, WBC was more sensitive and specific, allowing for the diagnosis of infection.
PLT is essential in hemostasis and protection against bacterial infection.48 Platelets can protect against bacteria by recognizing microbial antigens, secreting antimicrobial peptides and kinocidins, enhancing innate immune effectors (including complement and neutrophils), and adaptive coordinate immunity (APS, T cells, and B cells).49 However, in our study, PLT levels decreased in the blood culture-positive group compared with the blood culture-negative group, with an AUC of 0.5411 to differentiate these two groups. The specificity was too low, which was insufficient for diagnosing infection.
TTD significantly rose in the Gram-positive group compared to the Gram-negative group and performed well in differentiating these two groups with AUC above 0.8.
Previous studies have also shown that IL-6 is effective at differentiating between non-infectious infections and in Gram-negative and Gram-positive malignancies with or without neutropenia.2,45 Since this is a retrospective study and given the absence of IL-6 in patients, we did not include this analysis, which is one limitation of our research. This is something we would like to include in future studies.
Our study has some limitations. First, this study are a single-center, retrospective study with a small sample size, Thus, there might be some hidden bias and the results are unlikely to be generalized to other settings. These results were not representative of the situation in Shanxi, China, but only in the survey unit. A larger sample is needed for further research. Secondly, we did not explore the molecular characteristics. This needs to be studied further.
Conclusion
In summary, Gram-negative bacteria are more prevalent in neutropenic patients with HMs and BSIs. Carbapenems are the preferred agents for empirical treatment. In addition, neutropenia lasting more than 7 days, age ≥ 60 years, septic shock, hospitalization days>20, infection with a CR strain, and receipt of linezolid or vancomycin within 30 days of infection were independent mortality risk factors. Further, severe neutropenia exceeding 7 days, unreasonable empirical therapy, and receipt of aminoglycosides or 3rd or 4th-generation cephalosporins within 30 days of infection were independent of MDR Gram-negative bacteria risk factors. PCT, ANC, and WBC indicate higher diagnostic accuracy of BSI. Bacteria culture time performed better in differentiating Gram-negative and Gram-positive bacterial infections.
Ethics Statement
Our study complied with the Declaration of Helsinki. This study was approved by the Ethics Committee of the second hospital of Shanxi Medical University (Code 2021 YX-183). The data of patients’ clinical variables were collected from their medical records and did not contain names, addresses, or other personal information. The patient’s written informed consent was exempt. Informed consent is waived in the study because this research uses previous medical records, and meets all the following conditions: (1) the purpose of the study is important; (2) the risk of the study to the subjects was not greater than the minimal risk; (3) the exemption of informed consent will not adversely affect the rights and health of the subjects; (4) the privacy and personal identity information of the subjects were protected; (5) the study could not be carried out if informed consent was required.
Acknowledgments
We thank Second Hospital of Shanxi Medical University, for supporting this research.
Funding
This study was supported by the Shanxi Province Natural Science Foundation (Grant number 201901D111390). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Amanati A, Sajedianfard S, Khajeh S, et al. Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance. BMC Infect Dis. 2021;21(1):636. doi:10.1186/s12879-021-06243-z
2. Ma Y, Wang S, Yang M, Bao J, Wang C. Analysis of risk factors and clinical indicators in bloodstream infections among patients with hematological malignancy. Cancer Manag Res. 2020;12:13579–13588. doi:10.2147/CMAR.S289291
3. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42(8):1749–1755. doi:10.1097/CCM.0000000000000330
4. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266. doi:10.1002/cncr.21847
5. De Socio GV, Garcia-Vidal C, Cardozo-Espinola C, et al. Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PLoS One. 2018;13(6):e0199531.
6. Mattei D, Baretta V, Mazzariol A, et al. Characteristics and outcomes of bloodstream infections in a tertiary-care pediatric hematology-oncology unit: a 10-year study. J Clin Med. 2022;11(3):880. doi:10.3390/jcm11030880
7. Ma Y, Wen X, Kong Y, et al. Identification of new peptide biomarkers for bacterial bloodstream infection. Proteom Clin Appl. 2020;14(2):e1900075. doi:10.1002/prca.201900075
8. Shao I, Elkind M, Boehme A. Risk factors for stroke in patients with sepsis and bloodstream infections. Stroke. 2019;50(5):1046–1051. doi:10.1161/STROKEAHA.118.023443
9. El Haddad H, Chaftari A, Hachem R, Chaftari P, Raad I. Biomarkers of sepsis and bloodstream infections: the role of procalcitonin and proadrenomedullin with emphasis in patients with cancer. Clin Infect Dis. 2018;67(6):971–977. doi:10.1093/cid/ciy331
10. Mercier J, Ouldali N, Melki I, et al. Severe acute respiratory syndrome coronavirus 2-related multisystem inflammatory syndrome in children mimicking Kawasaki disease. Archiv Cardiovasc Dis. 2021;114(5):426–433. doi:10.1016/j.acvd.2021.04.005
11. Schwameis M, Steiner M, Schoergenhofer C, et al. D-dimer and histamine in early stage bacteremia: a prospective controlled cohort study. Eur J Intern Med. 2015;26(10):782–786. doi:10.1016/j.ejim.2015.10.024
12. Lameire N, Van Biesen W, Vanholder R. Electrolyte disturbances and acute kidney injury in patients with cancer. Semin Nephrol. 2010;30(6):534–547. doi:10.1016/j.semnephrol.2010.09.002
13. Di Domenico E, Marchesi F, Cavallo I, et al. The impact of bacterial biofilms on end-organ disease and mortality in patients with hematologic malignancies developing a bloodstream infection. Microbiol Spectr. 2021;9(1):e0055021. doi:10.1128/Spectrum.00550-21
14. Ramphal R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis. 2004;39(s1):S25–S31. doi:10.1086/383048
15. Chen C, Tang J, Hsueh P, et al. Trends and antimicrobial resistance of pathogens causing bloodstream infections among febrile neutropenic adults with hematological malignancy. J Form Med Assoc. 2004;103(7):526–532.
16. Rolston K. Challenges in the treatment of infections caused by gram-positive and gram-negative bacteria in patients with cancer and neutropenia. Clin Infect Dis. 2005;40(Supplement_4):S246–S252. doi:10.1086/427331
17. Marin M, Gudiol C, Ardanuy C, et al. Bloodstream infections in neutropenic patients with cancer: differences between patients with haematological malignancies and solid tumours. J Infect. 2014;69(5):417–423. doi:10.1016/j.jinf.2014.05.018
18. Shao S, Cong H, Wang M, Liu P. The diagnostic roles of neutrophil in bloodstream infections. Immunobiology. 2020;225(1):151858. doi:10.1016/j.imbio.2019.10.007
19. Zhu S, Kang Y, Wang W, Cai L, Sun X, Zong Z. The clinical impacts and risk factors for non-central line-associated bloodstream infection in 5046 intensive care unit patients: an observational study based on electronic medical records. Crit Care. 2019;23(1):52. doi:10.1186/s13054-019-2353-5
20. Zhang Y, Guo L, Song W, Wang Y, Dong F, GJBid L. Risk factors for carbapenem-resistant K. pneumoniae bloodstream infection and predictors of mortality in Chinese paediatric patients. BMC Infect Dis. 2018;18(1):248.
21. Lien M, Chou C, Lin C, et al. Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: a retrospective cohort study. PLoS One. 2018;13(6):e0197851. doi:10.1371/journal.pone.0197851
22. Yao J, Li N, Jiang J. Clinical characteristics of bloodstream infections in pediatric acute leukemia: a single-center experience with 231 patients. Chin Med J. 2017;130(17):2076–2081. doi:10.4103/0366-6999.213411
23. Chen CY, Tien FM, Sheng WH, et al. Clinical and microbiological characteristics of bloodstream infections among patients with haematological malignancies with and without neutropenia at a medical centre in northern Taiwan, 2008–2013. Int J Antimicrob Agents. 2017;49(3):272–281. doi:10.1016/j.ijantimicag.2016.11.009
24. Carvalho AS, Lagana D, Catford J, Shaw D, Bak N. Bloodstream infections in neutropenic patients with haematological malignancies. Infect Dis Health. 2020;25(1):22–29. doi:10.1016/j.idh.2019.08.006
25. R F. Bloodstream infections in cancer patients with febrile neutropenia. Int J Antimicrob Agents. 2008;32:S30–S33. doi:10.1016/j.ijantimicag.2008.06.017
26. Mutnick A, Kirby J, Jones R. CANCER resistance surveillance program: initial results from hematology-oncology centers in North America. Chemotherapy alliance for neutropenics and the control of emerging resistance. Ann Pharmacother. 2003;37(1):47–56. doi:10.1345/aph.1C292
27. Knight T, Glaser D, Ching N, Melish M. Antibiotic susceptibility of bloodstream isolates in a pediatric oncology population: the case for ongoing unit-specific surveillance. J Pediatr Hematol Oncol. 2019;41(5):e271–e276. doi:10.1097/MPH.0000000000001498
28. Gustinetti G, Mikulska M. Bloodstream infections in neutropenic cancer patients: a practical update. Virulence. 2016;7(3):280–297. doi:10.1080/21505594.2016.1156821
29. Nesher L, Rolston K. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection. 2014;42(1):5–13. doi:10.1007/s15010-013-0525-9
30. Zhu J, Li Q, Li X, et al. Successful control of the first carbapenem-resistant Klebsiella pneumoniae outbreak in a Chinese hospital 2017–2019. Antimicrob Resist Infect Cont. 2020;9(1):91. doi:10.1186/s13756-020-00757-y
31. Righi E, Peri A, Harris P, et al. Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72(3):668–677. doi:10.1093/jac/dkw459
32. Andria N, Henig O, Kotler O, et al. Mortality burden related to infection with carbapenem-resistant Gram-negative bacteria among haematological cancer patients: a retrospective cohort study. J Antimicrob Chemother. 2015;70(11):3146–3153. doi:10.1093/jac/dkv218
33. Moghnieh R, Estaitieh N, Mugharbil A, et al. Third generation cephalosporin resistant Enterobacteriaceae and multidrug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis. Front Cell Infect Microbiol. 2015;5:11. doi:10.3389/fcimb.2015.00011
34. Tofas P, Skiada A, Angelopoulou M, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: analysis of 50 cases. Int J Antimicrob Agents. 2016;47(4):335–339. doi:10.1016/j.ijantimicag.2016.01.011
35. Blennow O, Ljungman P. The challenge of antibiotic resistance in haematology patients. Br J Haematol. 2016;172(4):497–511. doi:10.1111/bjh.13816
36. Trecarichi E, Tumbarello M. Antimicrobial-resistant Gram-negative bacteria in febrile neutropenic patients with cancer: current epidemiology and clinical impact. Curr Opin Infect Dis. 2014;27(2):200–210. doi:10.1097/QCO.0000000000000038
37. Perez F, Adachi J, Bonomo R. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin Infect Dis. 2014;59(suppl_5):S335–339. doi:10.1093/cid/ciu612
38. Trecarichi E, Pagano L, Candoni A, et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect. 2015;21(4):337–343. doi:10.1016/j.cmi.2014.11.022
39. Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, et al. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin Infect Dis. 2020;70(6):1068–1074. doi:10.1093/cid/ciz319
40. Yu Z, Pang X, Wu X, Shan C, S J, Miyamoto A. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: a meta-analysis. PLoS One. 2018;13(7):e0201667. doi:10.1371/journal.pone.0201667
41. Yang M, Choi S, Lee J, et al. Serum procalcitonin as an independent diagnostic markers of bacteremia in febrile patients with hematologic malignancies. PLoS One. 2019;14(12):e0225765. doi:10.1371/journal.pone.0225765
42. Svaldi M, Hirber J, Lanthaler A, et al. Procalcitonin-reduced sensitivity and specificity in heavily leucopenic and immunosuppressed patients. Br J Haematol. 2001;115(1):53–57. doi:10.1046/j.1365-2141.2001.03083.x
43. Holzheimer R. Oral antibiotic prophylaxis can influence the inflammatory response in aortic aneurysm repair: results of a randomized clinical study. J Chemother. 2003;15(2):157–164. doi:10.1179/joc.2003.15.2.157
44. de Bont E, Vellenga E, Swaanenburg J, Kamps W. Procalcitonin: a diagnostic marker of bacterial infection in neutropenic cancer patients with fever? Infection. 2000;28(6):398–400. doi:10.1007/s150100070014
45. von Lilienfeld-Toal M, Dietrich MP, Glasmacher A, et al. Markers of bacteremia in febrile neutropenic patients with hematological malignancies: procalcitonin and IL-6 are more reliable than C-reactive protein. Eur J Clin Microbiol Infect Dis. 2004;23(7):539–544. doi:10.1007/s10096-004-1156-y
46. Engel A, Steinbach G, Kern P, Kern W. Diagnostic value of procalcitonin serum levels in neutropenic patients with fever: comparison with interleukin-8. Scand J Infect Dis. 1999;31(2):185–189. doi:10.1080/003655499750006254
47. Ruokonen E, Nousiainen T, Pulkki K, Takala J. Procalcitonin concentrations in patients with neutropenic fever. Eur J Clin Microbiol Infect Dis. 1999;18(4):283–285. doi:10.1007/s100960050277
48. Sartori M, Zurlo C, Bon M, et al. Platelet-derived microparticles bearing PF4 and anti-GAGS immunoglobulins in patients with sepsis. Diagnostics. 2020;10(9):627.
49. Hannachi N, Lepidi H, Fontanini A, et al. A novel approach for detecting unique variations among infectious bacterial species in endocarditic cardiac valve vegetation. Cells. 2020;9(8). doi:10.3390/cells9081899
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.