Back to Journals » Nature and Science of Sleep » Volume 15
Characteristics of the Attentional Network in Children with Sleep-Disordered Breathing
Authors Wu Y , Wang Y, Wang C, Zhao F, Ma D, Xu Z, Ni X
Received 20 March 2023
Accepted for publication 4 August 2023
Published 20 September 2023 Volume 2023:15 Pages 719—727
DOI https://doi.org/10.2147/NSS.S413330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Yunxiao Wu,1 Yan Wang,2,3 Changming Wang,4 Fujun Zhao,5 Dandi Ma,6 Zhifei Xu,6,7 Xin Ni5,7
1Beijing Key Laboratory of Pediatric Diseases of Otolaryngology, Head and Neck Surgery, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 2Center on Aging Psychology, CAS Key Laboratory of Mental Health, Institute of Psychology, Beijing, People’s Republic of China; 3Department of Psychology, University of Chinese Academy of Sciences, Beijing, People’s Republic of China; 4Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 5Department of Otolaryngology, Head and Neck Surgery, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 6Respiratory Department 1, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 7MOE Key Laboratory of Major Diseases in Children, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China
Correspondence: Zhifei Xu, Respiratory Department 1, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, 56 Nanlishi Road, Xicheng, Beijing, 100045, People’s Republic of China, Tel +8613521660077, Email [email protected] Xin Ni, Department of Otolaryngology, Head and Neck Surgery, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, 56 Nanlishi Road, Xicheng, Beijing, 100045, People’s Republic of China, Tel +8613370115099, Email [email protected]
Purpose: To explore the characteristics of the attentional network and related factors in children with sleep-disordered breathing (SDB).
Patients and Methods: A total 228 children (200 children aged 6– 10 years with snoring or mouth breathing, admitted to our hospital from May 2020 to July 2022, and 28 healthy children recruited from the community as the control group) were enrolled. All participants underwent polysomnography (PSG) and completed the ADHD rating scale and child version of the Attention Network Test. According to their SDB history and obstructive apnea hypopnea index (OAHI), the participants were divided into control (n = 28), primary snoring (PS; n = 67) and obstructive sleep apnea (OSA; n = 133) groups.
Results: The OSA and PS groups were younger than controls (P < 0.05). The proportion of boys was higher in the OSA than control group (P < 0.05). Body mass index was higher in the OSA than control and PS groups (P < 0.01). Attention deficit and hyperactive impulsivity scores were independently associated with the OAHI (P < 0.001). The efficiency of the alerting network was higher in the OSA than in controls (P = 0.020), but was not correlated with OAHI after adjusting for age, sex and SDB history duration (P > 0.05).
Conclusion: Children with OSA have impaired attention, characterized by excessive alerting network activation. However, alerting network efficiency did not change linearly with disease severity. More research is needed to elucidate the neural mechanisms underlying attention deficits in pediatric OSA.
Keywords: child, obstructive sleep apnea, attention, alerting network
Introduction
Sleep disordered breathing (SDB) in children is a syndrome of abnormal upper airway function during sleep that includes primary snoring (PS), upper airway resistance syndrome and obstructive sleep apnea (OSA). Repeated hypoxia and sleep fragmentation in OSA can induce chemical or structural damage to the central nervous system, affect children’s cognition, executive function and behavior, and lead to daytime sleepiness, attention deficits, hyperactivity, impulsivity and other symptoms. More than 30% of children with OSA exhibit attention deficits, which negatively affect learning ability and long-term intellectual development.1
The attention network is divided into three subsystems: alerting, orienting and executive control networks.2 The child version of the Attention Network Test (ANT) can be used for reliable evaluation of the three attentional subnetworks in children.3 The ANT has mainly been used for investigating brain development in normal populations.4 The attentional network develops and matures with age and 6–7 years represents the most important period of development.2 The peak age for OSA occurrence among children is 4–8 years, which coincides with the key period of attentional network development.5 Therefore, it is important to determine whether OSA impairs the development of the attentional network and its subnetworks; this could guide future targeted interventions.
The aim of this study was to explore the characteristics of the attentional network in children with SDB (and related factors), obtain data that could inform the clinical diagnosis and treatment of childhood SDB and associated attention deficits, and shed light on the mechanisms underlying attention deficit.
Materials and Methods
Participants
Children aged 6–10 years with snoring or mouth breathing (breathe with mouth open), who underwent polysomnography (PSG) in the Sleep Center of Beijing Children’s Hospital, Capital Medical University between May 2020 to July 2022, were enrolled in this study. The exclusion criteria were craniofacial deformities, neuromuscular or hereditary diseases, a history of mental disorders (such as autism or epilepsy), systemic chronic diseases that may affect the development of the nervous system, and a history of adenotonsillectomy.
Healthy controls aged 6–10 years were recruited from the community during the same period; OSA was ruled out by PSG in these participants. Other exclusion criteria are same to the SDB group.
Written informed consent and assent were obtained from parents and children (for children >8 years of age).
Study Design
This was a prospective observational study. This study complies with the Declaration of Helsinki. The study was approved by the Ethics Committee of Beijing Children’s Hospital.
Clinical Data
The staff of our sleep center asked the parents how long their children had exhibited snoring or mouth breathing. Height and weight were measured using standard methods (values accurate to within 0.1 kg and 0.1 cm, respectively) before PSG, and body mass index (BMI) was calculated as weight (kg)/height (m2).
Psg
A standard overnight PSG was performed using the E-series system (Compumedics, Melbourne, Australia) and the Alice 5 system (Respironics, Murrysville, PA, USA). The monitoring lasted for more than 7 hours. PSG included electroencephalography (C3/M2, C4/M1, O1/M2, and O2/M1), electrooculography, electromyography, electrocardiography, airflow monitoring (using a nasal pressure cannula and thermistor), and assessment of chest and abdomen respiratory movement, oxygen saturation, snoring, and body position. Sleep stage and respiratory events were scored manually according to the American Academy of Sleep Medicine manual.6 The results were consistent among the various devices used for monitoring. Obstructive sleep apnea-hypopnea index (OAHI) is the number of episodes of obstructive apnea, mixed apnea, and obstructive hypopnea per hour. According to diagnostic and treatment guidelines for pediatric OSA, PS is defined as having snoring symptom, but OAHI ≤1 time /h; OSA is diagnosed if the OAHI is > 1/h, while OAHI values of 5–10/h and > 10/h correspond to moderate and severe OSA, respectively.1
ADHD Rating Scale
Staff at our sleep center interviewed the parents in the face-to-face setting and completed the ADHD rating scale, which comprises 18 items. Nine items are on attention deficit symptoms such as “Inattention to details or carelessness when studying or working”, “It is difficult to arrange daily study and life” and 9 on hyperactivity and impulsivity symptoms such as “Can’t sit still, make small movements or twist in the seat”, “Often interrupts or forces acceptance on others”. One point for a “yes” answer to each item and higher scores indicate more severe symptoms.7
Child Version of the ANT
The international version of the ANT was used in this study.3 It was administered by trained personnel during 2pm-5pm before PSG. E-Prime 2.0 software (Psychology Software Tools, Sharpsburg, PA, USA) was used to program and run the ANT. The task includes four cue types (no cue, central cue, double cue and spatial cue) and three flanker types (neutral, congruent and incongruent). The children were asked to press the left or right mouse buttons when the middle fish faced left and right, respectively. The paradigm consists of 24 practice sessions and 144 formal sessions, and takes about 25 minutes to complete. The experimental materials and flow chart of the task are shown in Figure 1. The staff who administered the ANT test and parent interviews and/or scored the PSG were blind to the other results.
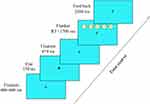 |
Figure 1 Flow chart of the child version of Attention Network Test. Abbreviation: RT, reaction time. Notes: Flanker fishes indicate incongruent type. |
Key Indicators for the ANT
The reaction time (RT) and accuracy rate (ACC) were recorded under different cue type and flanker conditions. The efficiency of the three networks was determined as follows: alerting network = RTno cue - RTdouble cue, where a larger difference indicates better performance of the alerting network; orienting network = RTcentral cue - RTspatial cue, where a larger difference indicates better orientation ability; and executive control network = RTincongruent - RTcongruent, where a larger difference indicates poorer executive control network function.3
Statistical Analysis
SPSS 23.0 software (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. Normally distributed data are expressed as mean ± standard deviation and data with a skewed distribution are expressed as median (P25, P75). To compare the groups, one-way ANOVA with Fisher’s least significant difference post-hoc test was applied to normally distributed data, while the rank-sum test was used for skewed data. For numerical data, the chi-square test was used for group comparisons. Multiple linear regression was used to identify predictors of dependent variables (attention deficit score, hyperactive impulsivity score, efficiency of the alerting network and overall RT). All tests were two-sided and P < 0.05 was considered statistically significant.
Results
In total, 251 children underwent PSG and completed the ANT, whereas 23 were not included in the final analysis because of an ANT ACC < 80%. Except for age (Z = −3.104, P = 0.002) and ACC (Z = −7.760, P < 0.001), there were no differences between children who were and were not included in the final analysis (ie, in sex, BMI, disease severity, SDB history duration, or the attention deficit and hyperactive impulsivity scores; P > 0.05).
The control group included 28 children, the PS group included 67 children, and the OSA group included 133 children. Age and SDB duration were younger and longer, respectively, in the PS and OSA groups compared with the control group (P < 0.05). The proportion of boys in the OSA group was higher than that in the control group (P < 0.05). The BMI in the OSA group was higher than that in the control and PS groups (P < 0.01). The PSG results, ie, the respiratory and oxygen saturation parameters, were as expected (Table 1).
 |
Table 1 Clinical and PSG Data of the Study Groups |
As shown in Table 2, the efficiency of the alerting network was higher in the OSA than control group (P = 0.020) (Figure 2) whereas did not change linearly with disease severity (Figure 3). The efficiency of orienting and executive control network did not differ among the groups (P>0.05). The RT was longer in the PS and OSA groups compared with the control group (P < 0.001 and P = 0.001, respectively). There was no significant difference in overall ACC among the groups.
 |
Table 2 Efficiency of Attentional Networks, Overall Reaction Times and Accuracy Rates of the Study Groups |
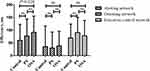 |
Figure 2 Efficiency of the attentional networks of the study groups. Abbreviations: PS, primary snoring; OSA, obstructive sleep apnea; ns, no significance. |
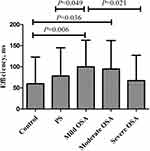 |
Figure 3 Efficiency of the alerting network according to disease severity. Abbreviations: PS, primary snoring; OSA, obstructive sleep apnea. Notes: Control vs PS, P=0.209; Control vs mild OSA, P=0.006; Control vs moderate OSA, P=0.036; Control vs severe OSA, P=0.669; PS vs mild OSA, P=0.049; PS vs moderate OSA, P=0.225; PS vs severe OSA, P=0.439; mild OSA vs moderate OSA, P=0.705; mild OSA vs severe OSA, P=0.021; moderate OSA vs severe OSA, P=0.091. Only the cases with P value < 0.05 are marked in Figure 3. |
Age, sex, SDB duration, BMI and OAHI were included in the multiple regression analysis of attention deficit and hyperactive impulsivity scores, alerting network efficiency and overall RT (Table 3). The attention deficit score was independently associated with BMI (P=0.040) and OAHI (P=0.005) and the hyperactive impulsivity score was independently associated with OAHI (P < 0.001). The overall RT was independently associated with age (P < 0.001). However, the alerting network efficiency was not correlated with any independent variable (P>0.05).
 |
Table 3 Multiple Regression Analysis of Attention Deficit and Hyperactive Impulsivity Scores, Alerting Network Efficiency and Overall Reaction Time |
Discussion
The aim of this study was to investigate characteristics of the attentional network (and related factors) in children with SDB. Children with OSA had high ADHD rating scale attention deficit and hyperactivity impulsivity scores, and the scores were positively correlated with the OAHI. Contrary to our hypothesis, during the ANT, children with OSA exhibited excessive alerting network efficiency whereas that did not change linearly with the severity of the disease.
The attentional network is divided into three sub-networks (alerting, orienting and executive control networks) that differ in function and anatomy. The alerting network underpins the brain’s ability to maintain a state of alertness to receive incoming information; the frontal and parietal regions of the right hemisphere and locus coeruleus are implicated in this network. The orienting network is the process of selecting input sensory information; its substrates are the fronto-parietal lobe, superior colliculus, and temporo-parietal connection. Finally, the executive control network refers to the ability of conflict resolution and coordination control; it is involved in processes including judgments, choices, and attention switching. The substrates of the executive control network include the prefrontal cortex, anterior cingulate gyrus, ventral thalamic nucleus, and basal ganglia.8
The children with OSA in this study showed excessive alerting network activation, which is an unexpected but interesting finding. Why do children with OSA show hyper-alertness? One study on the relation between elevation and attentional network found that, with increased elevation exposure, attention first decreased, then increased, and finally decreased again; the increase was attributed to adaptive compensation.9 Another study observed increased attention-related beta activity in healthy people who migrated from plains to high elevation, reflecting increased cortical excitability. It was speculated that compensatory mechanisms associated with long-term residence at high elevation may have been responsible for this effect, ie, enhanced attention to allow completion of daily activities.10 In a study using event-related potentials (ERPs) to explore the relationship between hypoxia and cognition, the latency of the P1 component in college students in the “plateau” group was significantly shorter than in the “plain” group on a perceptual task, suggesting that long-term hypoxia increased the conduction speed of the nerves responsible for early perception.11 Another study found that, in patients with moderate-to-severe OSA, more neurons were activated in what was described as the “contour global recognition processing stage”. Moreover, there were compensatory effects of the frontal lobe appeared in the visual perception stage.12 Against this background, we speculate that hyper-alertness in children with OSA may also be a compensatory response to long-term chronic intermittent hypoxia, although more research is needed to confirm this. A hyper-alert state is also consistent with the daytime performance of children with OSA; such children tend to be hyperactive and excited during the daytime, unlike adult OSA, which presents with excessive sleepiness. This was confirmed by the ADHD rating scale used in this study, where the hyperactive impulsivity scores of the children with OSA were significantly higher than those of the healthy controls. The neurotransmitter noradrenaline may underlie the hyper-alertness seen in children with OSA, given the regulatory role of noradrenaline in the effects of warning signals on alertness.8 Furthermore, a recent meta-analysis reported that urinary noradrenaline levels were higher in children with SDB, suggesting sympathetic overactivity in such children.13
Further analysis of our data showed that the alerting network efficiency did not change linearly with disease severity; in fact, it was highest in mild OSA cases and was not correlated with the OAHI. This disparity between cognitive function and disease severity was reported in previous study.14 Some studies found that cognitive function was correlated with PSG indicators, while others reported no strong correlations between these variables.15 Therefore, factors other than hypoxia and sleep disruption may also be important with respect to the cognitive function of children with SDB, such as individual susceptibility, brain electrical activity, and brain oxygen saturation regulation. Tamanyan et al16 found that individuals with SDB tended to have higher cerebral oxygenation levels than healthy controls. It was speculated that, although arterial oxygen saturation may decrease during apnea, if cerebral blood flow increases simultaneously, the increased blood flow could compensate for the reduced peripheral oxygenation, thereby maintaining the brain’s oxygen supply (cerebral autoregulation). However, in cases of more severe disease or a longer history thereof, this compensatory mechanism may be impaired, ultimately leading to cognitive deficits. Electrophysiological indicators from resting state and sleep electroencephalograms, as well as ERPs, may also provide new insight into, and biomarkers for, the cognitive impairment seen in children with OSA.17
The executive control network assesses of the ability to resolve conflict and coordinate control when the direction of the target flanker was not congruent with that of the surrounding flankers. Reaction times were longer under incongruent conditions. Hu et al18 found that attention was impaired in OSA patients, which was reflected in a decline in executive control network function. In ERP studies, OSA groups had higher-amplitude and longer-duration P300 components than healthy controls, indicating that more attentional resources are needed by children with OSA to process target stimuli. Moreover, deployment of these resources took longer in the children with OSA.19,20 In our study, there was no difference in executive control network efficiency between the OSA group and control and PS groups, which may be attributable to compensatory effects of hyper-alertness.
In this study, the ADHD rating scale was used to evaluate clinical manifestations of SDB. The attention deficit and hyperactivity impulsivity symptoms of children with OSA were similar to those of the PS group but more obvious than those of the control group. There was no correlation between the efficiency of each attention subnetwork and the attention deficit and hyperactivity impulsivity scores, suggesting that the results of clinical scales and neurobehavioral tests evaluating attention can diverge.
The RT in this study was negatively correlated with age, ie, with an increase in age the RT gradually shortened, consistent with the results of the ANT in healthy children of different ages. This finding suggests that RTs improve in children as the brain develops over time.3
Our study has some limitations. First, the healthy control group had a small sample size. Second, the only performance metrics for the ANT are the RT and ACC. Angelelli et al21 observed OSA patients had greater performance instability than the controls in alertness and fewer valid response in medium/long intervals, particularly 15–20 min in vigilance; therefore, more indexes of attention may be needed. Third, electrical activity and oxygen saturation of the brain were not evaluated, and the mechanisms underlying attention remain unclear.
Conclusion
This study found that children with OSA have an abnormal attentional network, which mainly manifests as excessive activation of the alerting network. Notably, the efficiency of alerting network did not change linearly with disease severity. The mechanism underlying the attention network impairment seen in children with OSA merits further study, and efforts should be made to devise effective treatments and interventions to improve the attention span of such children. Finally, there is a need for greater experience in the application of neurocognitive assessments for the purposes of clinical practice and research.
Acknowledgments
This study was supported by Beijing Natural Science Foundation [7212033], National Natural Science Foundation of China [82070092], the Fundamental Research Funds for the Central Universities [2242022k30036], the Fundamental Research Funds for the central Universities [2242022k30037] and National Clinical Research Center for Respiratory Diseases (HXZX-20210401).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Working Group of Chinese Guideline for the Diagnosis and Treatment of Childhood OSA; Subspecialty Group of Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association; Subspecialty Group of Respiratory Diseases, Society of Pediatrics, Chinese Medical Association. Chinese guideline for the diagnosis and treatment of childhood obstructive sleep apnea (2020). Pediatr Investig. 2021;5(3):167–187. doi:10.1002/ped4.12284
2. Pozuelos JP, Paz-Alonso PM, Castillo A, et al. Development of attention networks and their interactions in childhood. Dev Psychol. 2014;50(10):2405–2415. doi:10.1037/a0037469
3. Rueda MR, Fan J, McCandliss BD, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi:10.1016/j.neuropsychologia.2003.12.012
4. Yang Q, Xie Y. The developmental characteristics of attentional networks in children. China J Health Psychol. 2017;25(5):797–800.
5. Zhao Z, Ye J. Sleep Medicine.
6. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(05):597–619. doi:10.5664/jcsm.2172
7. The Subspecialty Group of Developmental and Behavioral Pediatrics, the Society of Pediatrics, Chinese Medical Association. Consensus on pediatric clinical practice of early identification, standardized diagnosis and treatment of attention deficit hyperactivity disorder. Chin J Pediatr. 2020;58(3):188–193.
8. Davidson MC, Marrocco RT. Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. J Neurophysiol. 2000;83(3):1536–1549. doi:10.1152/jn.2000.83.3.1536
9. An X, Ma H, Han B, et al. Attention network varied along with the time of residence at high altitude. Chin J Clin Psychol. 2017;25(03):502–506.
10. Liu B, Han B, An X, et al. Research progress of impact of acute high altitude hypoxia on the resting-state EEG power. Chin General Pract. 2017;20(29):3683–3688.
11. Guo F, Wang C, Tao G, et al. Effects of long-term exposure to high altitudes on perceptual closure. Chin J Behav Med Brain Sci. 2021;30(5):446–451.
12. Yang C, Wang C, Chen X, et al. Event-related potential assessment of visual perception abnormality in patients with obstructive sleep apnea: a preliminary study. Front Hum Neurosci. 2022;16:895826. doi:10.3389/fnhum.2022.895826
13. Cheng E, Chan R, Chan K, et al. Level of urinary catecholamine in children with sleep disordered breathing: a systematic review and meta-analysis. Sleep Med. 2022;100:565–572. doi:10.1016/j.sleep.2022.10.008
14. Yu PK, Radcliffe J, Gerry TH, et al. Neurobehavioral morbidity of pediatric mild sleep-disordered breathing and obstructive sleep apnea. Sleep. 2022;45(5). doi:10.1093/sleep/zsac035
15. Yu Y, Chen YX, Liu L, et al. Neuropsychological functioning after adenotonsillectomy in children with obstructive sleep apnea: a meta-analysis. J Huazhong Univ Sci Technolog Med Sci. 2017;37(3):453–461. doi:10.1007/s11596-017-1756-2
16. Tamanyan K, Walter LM, Weichard A, et al. Age effects on cerebral oxygenation and behavior in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2018;197(11):1468–1477. doi:10.1164/rccm.201709-1825OC
17. Gorgoni M, D’Atri A, Scarpelli S, et al. Sleep electroencephalography and brain maturation: developmental trajectories and the relation with cognitive functioning. Sleep Med. 2020;66:33–50. doi:10.1016/j.sleep.2019.06.025
18. Hu Y, Pei C, Liu F, et al. A study of attention networks in patients with obstructive sleep apnea-hypopnea syndrome. Zhonghua Jie He He Hu Xi Za Zhi. 2015;38(9):659–663.
19. Gosselin N, Mathieu A, Mazza S, et al. Attentional deficits in patients with obstructive sleep apnea syndrome: an event-related potential study. Clin Neurophysiol. 2006;117(10):2228–2235. doi:10.1016/j.clinph.2006.07.130
20. Kaihua J, Yang Y, Fangqiao Z, et al. Event-related potentials and behavior performance scores in children with sleep-disordered breathing. Brain Dev. 2019;41(8):662–670. doi:10.1016/j.braindev.2019.04.008
21. Angelelli P, Macchitella L, Toraldo DM, et al. The neuropsychological profile of attention deficits of patients with obstructive sleep apnea: an update on the daytime attentional impairment. Brain Sci. 2020;10(6):325. doi:10.3390/brainsci10060325
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
