Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Characteristics of Patients with Chronic Obstructive Pulmonary Disease Treated with Long-Acting Bronchodilators in a Real-World Setting in Singapore: A Single-Center Observational Study
Authors Sim M , Yii A , Xu X , Bahety P , Loh CH , Navarro Rojas AA, Milea D, Tee A
Received 10 January 2022
Accepted for publication 19 May 2022
Published 9 June 2022 Volume 2022:17 Pages 1349—1363
DOI https://doi.org/10.2147/COPD.S357820
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Marcus Sim,1 Anthony Yii,1 Xiaomeng Xu,2 Priti Bahety,3 Chee Hong Loh,1 Aldo Amador Navarro Rojas,4 Dominique Milea,2 Augustine Tee1
1Department of Respiratory and Critical Care Medicine, Changi General Hospital, Singapore, Singapore; 2Value Evidence & Outcomes, Greater China and Intercontinental, GSK, Singapore, Singapore; 3Medical Affairs, GSK, Singapore, Singapore; 4Respiratory & Specialty Medical Lead, Greater China and Intercontinental, GSK, Singapore, Singapore
Correspondence: Marcus Sim, Department of Respiratory and Critical Care Medicine, Changi General Hospital, 2 Simei Street 3, Singapore, 529889, Singapore, Tel +65 6788 8833, Email [email protected]
Introduction: There is limited real-world evidence regarding clinical practice for chronic obstructive pulmonary disease (COPD) in Singapore. We compared baseline clinical characteristics and evaluated outcomes in patients with COPD who initiated treatment with either a long-acting muscarinic antagonist (LAMA) or a LAMA and a long-acting β2-agonist (LAMA+LABA).
Methods: This was a single-center observational study at Changi General Hospital, Singapore. Routine clinical data (hospital visits, case management, lung function, laboratory/imaging results, medication orders) were collected and compiled into a data warehouse. Eligible patients with COPD were ≥ 40 years old and newly prescribed LAMA or LAMA+LABA during the enrollment period. Patient characteristics in the baseline period (6 months) were compared between treatments. Clinical worsening was measured as a composite endpoint, defined as the first of a change in maintenance treatment class or a moderate-to-severe exacerbation during follow-up (12 months).
Results: In total, 261 patients were included in the baseline period (LAMA: 73; LAMA+LABA: 188). In the baseline period, patients receiving LAMA+LABA versus LAMA had significantly lower body mass index, higher COPD Assessment Test score and worse lung function, and numerically higher exacerbation history. Prevalence of comorbidities was similar between treatment groups. In follow-up, high rates of clinical worsening were observed regardless of treatment regimen (LAMA: 38/73 [52%]; LAMA+LABA: 86/188 [46%]). Median time-to-clinical worsening was 340 days for the LAMA cohort and the raw median 154 days (interquartile range: 44– 225) for the LAMA+LABA cohort. Median medication dispensation rate (0.86; interquartile range: 0.56– 1.00) was similar between treatments.
Conclusion: Patients initiating treatment with LAMA+LABA had more severe COPD than patients prescribed LAMA. The proportion of patients experiencing clinical worsening was similarly high in both cohorts, suggesting that early identification and treatment optimization are necessary.
Keywords: COPD treatment, database analysis, dual bronchodilator, LABA, LAMA, inhaler prescription
Plain Language Summary
Why was the study done?
Guidelines for the treatment of patients with chronic obstructive pulmonary disease (COPD) are largely based on the results of clinical trials. However, clinical practice can be influenced by factors that are not taken into consideration in clinical trials. This leads to a gap between clinical practice and treatment guidelines. Some guidelines recommend starting treatment with a long-acting muscarinic antagonist (LAMA) medication only, while others recommend starting treatment with a combination of a LAMA and a long-acting β2-agonist medication (LAMA+LABA). More studies with real-world data are needed to address the gap between clinical practice and treatment guidelines.
What did the researchers do and find?
We collected clinical data, including inpatient and emergency department visits, from patients with COPD in a hospital in Singapore. Data were collected for 1 year after patients started treatment with either LAMA or LAMA+LABA. We generated a data warehouse and used it to compare the clinical characteristics of patients with COPD who had started treatment with LAMA or LAMA+LABA. Before they started treatment, patients who were prescribed LAMA+LABA had worse lung function, symptoms and health status than those who were prescribed LAMA. Approximately half of patients experienced health decline, regardless of their treatment regimen.
What do these results mean?
The results of this study indicate that patients starting treatment with LAMA+LABA have more severe COPD than those starting LAMA alone. However, many patients in both treatment groups experienced health decline, highlighting the need for early identification to ensure patients receive optimal treatment.
Introduction
Chronic obstructive pulmonary disease (COPD) represents a substantial health burden and is currently the third leading cause of death worldwide.1 In Singapore, COPD was the tenth leading cause of death in 2019,2 and in 2017 it was the ninth leading cause of years of life lost.3 In 2010, among chronic respiratory diseases, COPD was the largest contributor to health burden and disability in Singapore.4
Long-acting muscarinic antagonists (LAMA) and long-acting β2-agonists (LABA) play an important role in the pharmacotherapy of COPD. LAMAs and LABAs bind to muscarinic and beta-2 adrenergic receptors, respectively, inducing smooth muscle relaxation and bronchodilation.5 It has been shown that maintenance therapy with long-acting bronchodilators improves lung function, reduces dyspnea, improves quality of life, and increases exercise tolerance in patients with COPD.6–9 Combined use of LAMA+LABA is thought to produce a synergistic effect, providing greater efficacy than the individual monocomponents.6,10
There is uncertainty and variability in real-world clinical practice concerning initiation of treatment with LAMA+LABA combination or monotherapy with either LAMA or LABA. This is reflected in the conflicting recommendations provided by different COPD treatment guidelines. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2021 strategy report and the 2018 Singapore Appropriate Care Guide for COPD recommend initiation with mono-bronchodilator therapy, followed by a stepwise escalation to dual therapy for patients who have persistent dyspnea or experience exacerbations.5,11 In contrast, the American Thoracic Society (ATS) 2020 clinical practice guidelines recommend that patients who complain of dyspnea or exercise intolerance should initiate maintenance treatment with LAMA+LABA dual therapy.12
Furthermore, guideline recommendations on COPD pharmacotherapy are not consistently implemented by healthcare professionals. In the UK, a retrospective study of 24,957 patients with COPD showed that treatments were often not prescribed according to the most recent recommendations and guidelines; for example, 17% of patients were not receiving any treatment despite experiencing symptoms or exacerbations.13 Similarly, a US study found that only approximately 36% of 4234 patients with COPD were prescribed GOLD-adherent pharmacotherapy.14 An observational study across South East Asia and Australasia, which included three sites in Singapore, found that only 80% of emergency department (ED)-diagnosed patients with COPD were treated with bronchodilators.15 This suggests that compliance with local treatment guidelines is suboptimal.
Treatment guidelines are predominantly based on evidence from randomized clinical trials, which represent the gold standard methodology for establishing efficacy and safety of treatments. However, randomized clinical trials typically include narrowly defined populations which do not always represent the whole spectrum of patients with COPD encountered in clinical practice. Diverse characteristics of patients in clinical practice and other real-world factors can influence treatment selection and outcomes, leading to a gap between guideline recommendations and clinical practice. Local systemic factors may influence real-world prescribing patterns, such as medication costs and availability of different formulations; for example, in Singapore, LAMA+LABA combinations in a single inhaler are currently priced to patients similarly or slightly cheaper than their monocomponent inhalers, and government subsidies for certain LAMA+LABA formulations may have increased access to these medications.16 Real-world studies utilizing data generated in routine clinical practice can help address this gap by providing insight on local prescribing patterns.
An innovative data warehouse, linking information collected during the routine management of patients with COPD at Changi General Hospital (CGH) in Singapore, has been developed to help monitor clinical practice with regard to management and treatments. The main aim of this study was to describe the population of patients initiating treatment with LAMA versus those initiating with LAMA+LABA and compare the baseline clinical characteristics associated with each treatment regimen using this data source. The secondary objectives of this study were to describe clinical outcomes in this population (measured using a composite endpoint) and the dispensation rate of each treatment.
Materials and Methods
Study Design
This single-center, observational study analyzed hospital data collected prospectively during routine medical practice. Data were collected from CGH, a 1000-bed tertiary hospital with an established Respiratory Medicine department serving eastern Singapore. Hospitalizations for COPD were managed predominantly by respiratory specialists or other physicians, such as geriatricians or internists, while outpatient follow-ups were managed almost exclusively by respiratory specialists.
Patients were identified as starting treatment with either LAMA or LAMA+LABA during the enrollment period; the index date was defined as the date of the first prescription during the enrollment period. The baseline period prior to the index date was used to assess characteristics of patients and the follow-up period after the index date was used to assess treatment outcomes. To ensure that a sufficient number of exacerbations were observed for analysis, the follow-up period was 12 months. Due to potentially low patient numbers and limited data availability, two analyses were conducted based on different baseline period durations. For the main analysis, to ensure data availability for a higher number of patients, baseline data were extracted in the 6 months (1 October 2017 to 31 March 2018) before the start of the enrollment period (1 April 2018 to 30 June 2019). To further explore whether the findings of the main analysis with regard to baseline characteristics were robust, a sensitivity analysis using a longer baseline period was carried out. For the sensitivity analysis, the baseline data collection period was extended to 12 months (from 1 October 2017 to 30 September 2018) and the enrollment period spanned 1 October 2018 to 30 June 2019 (Figure 1).
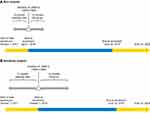 |
Figure 1 Study design for (A) the main analysis and (B) the sensitivity analysis. Abbreviations: LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist. |
The data warehouse was compiled using existing patient information and electronic record systems at CGH, in collaboration with GSK. To be included in the data warehouse, patients had to carry a primary or secondary diagnostic code of COPD according to the International Classification of Diseases (10th revision, Australian modification) (Supplementary Table S1) in either inpatient or ED visits from CGH. The data warehouse included data on hospital visits, case management, lung function, laboratory/imaging results and medication orders related to these patients; these data were updated prospectively on a rolling basis (from October 2017 to October 2020).
Study Population
Eligible patients were aged 40 years or older with a primary or secondary diagnostic code of COPD and a new inpatient or outpatient prescription for LAMA (tiotropium, umeclidinium or glycopyrronium) or LAMA+LABA (tiotropium+olodaterol, umeclidinium+vilanterol or glycopyrronium+indacaterol) within the enrollment period.
Patients were excluded from the analysis if they had a prior inpatient or outpatient prescription of the same class as the index medication, were not attending CGH regularly (<1 encounter, including any ED visit, hospitalization, or outpatient visit) or died during the 12-month follow-up period.
Baseline Characteristics
Baseline characteristics were assessed in the baseline period before the index date. Smoking history, body mass index (BMI) and lung function (pre-bronchodilator forced expiratory volume in 1 second [FEV1] and pre-bronchodilator FEV1% predicted) were taken from the most recent values before the index date. Variables recorded at baseline included asthma diagnosis, COPD Assessment Test (CAT) score, Elixhauser comorbidities, absolute blood eosinophil count, dispensation rate for COPD-related medications, and COPD-related exacerbations.
Moderate-to-severe COPD-related exacerbations were defined as an inpatient hospitalization or ED visit with a COPD exacerbation diagnosis code in the primary diagnosis.
Clinical Worsening
Patient outcomes were assessed using a composite endpoint developed to reflect clinical worsening in routine clinical practice. Clinical worsening was defined as either any change in class of prescribed maintenance treatment or a moderate-to-severe exacerbation requiring ED visit or hospitalization, whichever occurred first (ie, patients were not counted twice). The proportions of patients who experienced each individual component of the composite endpoint (treatment change and moderate-to-severe exacerbation) were also reported.
For the analysis of time-to-clinical worsening, patients were censored if they did not have an event during the follow-up period. Patients who died during the follow-up period were not initially included in the analysis as the cause of death is unknown and could introduce bias. However, analyses including patients who died are presented in Supplementary Tables S3 & S4.
Dispensation Rate
The dispensation rate for COPD-related medications was defined as the ratio of number of inhalers dispensed to the number of inhalers prescribed in the follow-up period. Based on this, the percentage of patients who collected their prescribed medication was estimated as a proxy measure of inhaler adherence.
Sample Size Calculation and Data Analysis
Power analysis was carried out to determine the number of patients needed to detect a statistically significant difference between treatment groups for baseline characteristics. Based on the minimal important differences for baseline variables, the required sample sizes to detect a statistically significant effect with at least 0.8 power was determined for each characteristic (Supplementary Table S2).
Basic descriptive analyses were carried out for all variables. Categorical variables were described using the number of observations and percentage in each category, parametric numeric variables were described using the mean and standard deviation (SD), and nonparametric variables were described using median and interquartile range (IQR; 25th and 75th percentiles).
Baseline characteristics were compared between patients initiated with LAMA versus those initiated with LAMA+LABA using Student’s t-test, the Chi-squared test or Mann–Whitney U-test for parametric, categorical and nonparametric tests, respectively.
The median time-to-clinical worsening was calculated using Kaplan–Meier survival analysis. If the cumulative probability of clinical worsening did not reach 0.5, the censored cases were excluded and the raw median time-to-clinical worsening was calculated instead. Hazard ratios were calculated using Cox proportional hazards regression.
Results
Study Population
In the main analysis, there were 457 patients who had a prescription of LAMA or LAMA+LABA therapy during the enrollment period (Figure 2). A total of 261 patients were eligible for the main analysis, with 71 patients excluded due to being prescribed the same class in the 6 months prior, 86 patients died during the 12-month follow-up period (of whom 26 [26.3%] had a LAMA prescription and 60 [24.2%] had a LAMA+LABA prescription) and 39 patients did not attend CGH regularly (Figure 2). Of those who were eligible, 73 (28%) patients had a LAMA prescription and 188 (72%) patients had a LAMA+LABA prescription (Figure 2), of which, 18/188 (10%) were previously on LAMA monotherapy as maintenance treatment.
In the sensitivity analysis, there were 350 patients who had a prescription of LAMA or LAMA+LABA therapy during the enrollment period (Figure 2). Of these, 159 patients were eligible, of which 40 (25%) had a LAMA prescription and 119 (75%) had a LAMA+LABA prescription (Figure 2). A total of 9/119 (5%) patients who had a LAMA+LABA prescription were previously on LAMA monotherapy as maintenance treatment. Of those patients who were excluded, 117 had been prescribed the same class in the 12 months prior, 49 died during the 12-month follow-up period (of whom 6 [13.0%] had a LAMA prescription and 43 [26.5%] had a LAMA+LABA prescription) and 25 did not attend CGH regularly (Figure 2).
Baseline Characteristics
Overall, in the main analysis, although 51% patients did not have CAT scores and 42% of patients did not have lung function data, the available data suggest that patients in the main analysis were moderately symptomatic (median CAT score: 10) and had mild/moderate lung function impairment (26% GOLD grade 1, 49% GOLD grade 2; Table 1). In the main analysis, patients treated with LAMA+LABA had a significantly lower BMI, significantly higher CAT score, and significantly worse lung function than those treated with LAMA (Table 1). Similar significant differences between treatment groups were observed in the sensitivity analysis; in addition, significantly more patients who initiated treatment with LAMA+LABA had a history of ≥1 moderate-to-severe exacerbation compared with those receiving LAMA (Table 1). However, in the sensitivity analysis, lung function and GOLD grade were not significantly different between treatments (Table 1).
 |
Table 1 Baseline Characteristics |
There were no significant differences in the prevalence of comorbidities or the overall comorbidity burden, measured by the Elixhauser index (Table 2). In the main analysis, the comorbidity with the highest prevalence was uncomplicated hypertension (42.1%), followed by ischemic heart disease (36.4%) (Table 2). The prevalence of heart failure was 18.0% and that of asthma was 5.7% (Table 2). The prevalence of these comorbidities was similar in the sensitivity analysis (Table 2).
 |
Table 2 Prevalence of Comorbiditiesa in the Study Population |
When patients who died were included in the main and sensitivity analyses, similar trends in baseline characteristics were observed, except for the proportion of former smokers, that was greater in the group of patients including those who died (Supplementary Table S3). The mean number of exacerbations were also greater in the group of patients including those who died; however, the number of patients with ≥1 exacerbations were similar.
Clinical Worsening and Dispensation Rates
Overall, in the main analysis, 48% (124/261) of patients experienced clinical worsening during the 12-month follow-up period; this included 52% (38/73) of those treated with LAMA and 46% (86/188) of those treated with LAMA+LABA (Table 3). A greater proportion of patients changed treatment in the LAMA cohort (26% [19/73]) than in the LAMA+LABA cohort (6% [12/188]) (Table 3); and moderate-to-severe exacerbations were reported for 23% (17/73) of patients in the LAMA cohort versus 38% (72/188) of patients in the LAMA+LABA cohort (Table 3). Notably, 3% (2/73) of patients in the LAMA cohort and 1% (2/188) of patients in the LAMA+LABA cohort had a moderate-to-severe exacerbation and changed treatment on the same day. The median time-to-clinical worsening for the LAMA cohort was 340 days (Figure 3). The median time-to-clinical worsening for LAMA+LABA cohort was not determined as the cumulative probability of worsening did not reach 0.5. The raw median (IQR) time-to-clinical worsening was 93 days (40–234) for the LAMA cohort and 154 days (44–225) for the LAMA+LABA cohort (Figure 3). The hazard ratio (95% CI) for time-to-clinical worsening with LAMA+LABA versus LAMA was 0.82 (0.56, 1.20); the study was not powered for this post hoc analysis.
 |
Table 3 Proportion of Patients with Clinical Worsening, a Moderate-to-Severe Exacerbation or a Change in Treatment by Index Maintenance Therapy |
The median medication dispensation rate (IQR) was 0.86 (0.56–1.00); this was similar between treatments (LAMA: 0.85 [0.52–1.00] vs LAMA+LABA: 0.83 [0.55–1.00]; p=0.74).
Overall, in the sensitivity analysis, 48% (77/159) of patients experienced clinical worsening during the 12-month follow-up period. This included 45% (18/40) of those receiving LAMA and 50% (59/119) of those receiving LAMA+LABA (Table 3). Moderate-to-severe exacerbations were reported for 20% (8/40) versus 41% (49/119) of patients receiving LAMA and LAMA+LABA, respectively. As seen in the main analysis, there was a greater proportion of patients in the LAMA cohort requiring a change in treatment regimen than in the LAMA+LABA cohort (23% [9/40] vs 8% [9/119]) (Table 3). In total, 2% (1/40) patients in the LAMA cohort and 1% (1/119) of patients in the LAMA+LABA cohort had a moderate-to-severe exacerbation and changed treatment on the same day. The median time-to-clinical worsening was not reached as the cumulative probability of worsening did not reach 0.5 for either cohort. The raw median (IQR) time-to-clinical worsening was 86 days (25–181) for the LAMA cohort and 125 days (40–209) for the LAMA+LABA cohort (Figure 3). In an unpowered post hoc analysis, the hazard ratio (95% CI) for time-to-clinical worsening with LAMA+LABA versus LAMA was 1.11 (0.65, 1.87).
The median medication dispensation rate (IQR) was 0.88 (0.61–1.00), and as for the main analysis this was similar between treatments (LAMA: 0.88 [0.60–1.00] vs LAMA+LABA: 0.85 [0.62–1.00]; p=0.76).
When deaths were included in the composite endpoint (post hoc analysis), trends in clinical worsening were similar to when deaths were excluded (Supplementary Table S4). In the main analysis, the median time-to-death or clinical worsening was 211 days for the LAMA cohort and 257 days for the LAMA+LABA cohort. The hazard ratio (95% CI) for time-to-death or clinical worsening with LAMA+LABA versus LAMA was 0.87 (0.65, 1.17); p=0.35.
In the sensitivity analysis, the median time-to-death or clinical worsening for the LAMA cohort was not reached as the cumulative probability of worsening did not reach 0.5; the raw median (IQR) time-to-clinical worsening was 71 days (19–157). The median time-to-death or clinical worsening was 207 days for the LAMA+LABA cohort. The hazard ratio (95% CI) for time-to-death or clinical worsening with LAMA+LABA versus LAMA was 1.32 (0.84, 2.00); p=0.23.
Discussion
In this study, real-world data from an innovative data warehouse were used to gain insight on a population of patients with COPD initiating treatment with either LAMA or LAMA+LABA in current clinical practice in a hospital setting. At baseline, patients prescribed LAMA+LABA had on average more severe disease than those prescribed LAMA, as demonstrated by lower BMI, higher CAT score, and worse lung function in the main analysis, whether patients who died during the study period were included or excluded. Of those patients who initiated treatment with LAMA+LABA, only 5–10% of patients were stepped up from LAMA monotherapy, suggesting that dual therapy is being prescribed as initial maintenance treatment in more severe patients. The GOLD 2017 and 2018 strategy reports17,18 and the most recent local guidelines available at the time of the study19 recommended a stepwise escalation from LAMA or LABA monotherapy to dual therapy for patients with persistent breathlessness or exacerbations while on monotherapy. LAMA+LABA was recommended as initial maintenance therapy for Group D patients, defined as those with CAT score ≥10 and elevated risk of exacerbations (history of ≥2 exacerbations or ≥1 leading to hospital admission). In this study, LAMA+LABA initiators had more severe disease than LAMA initiators, but on average did not meet the criteria for Group D patients, in whom initial LAMA+LABA therapy would be indicated according to the applicable guidelines at the time of the study. For example, in the sensitivity analysis, the average rate of exacerbations requiring ED visit or hospitalization among LAMA+LABA initiators was 0.76 per year, and 50% had a median CAT score ≤10. While there was a discordance between prescribing practices observed in the present study and applicable guidelines in 2017 and 2018, the prescribing patterns described here are aligned with the ATS guidelines issued in 2020, which recommend first-line treatment with LAMA+LABA dual therapy for all patients who present with dyspnea or exercise intolerance.12 At baseline, patients were on average approximately 70 years of age. The age of the patients was likely related to the inclusion criteria, as patients had to have an ED visit to or a hospitalization in CGH to be included in the study. The representativeness of the patient population is supported by a previously published outpatient cohort including patients from the same center, which also had an average age of 72 years.20 Taking into account that older patients with COPD tend to have a greater disease burden compared with younger patients,21 this may indicate that there was a delay in prescription of maintenance treatment for COPD symptoms in this patient population. Evidence from a real-world observational study in the US suggested that delay in initiating LAMA+LABA treatment could lead to higher treatment costs and greater risk of a first severe exacerbation.22 However, it should be noted that age is not an indication for starting bronchodilators. It is also notable that approximately half of the patient population were current smokers when first prescribed a LAMA or a LAMA+LABA; smoking cessation is an essential component of COPD management.
The sensitivity analysis used a longer baseline period than the main analysis (1 year vs 6 months), providing further insight regarding the baseline characteristics of the population and better precision with regards to the evaluation of characteristics that vary over time, such as exacerbations. The findings were broadly in agreement with the main analysis; at baseline, patients in the LAMA+LABA cohort had lower BMI and higher CAT score than those in the LAMA cohort, although both cohorts had similar lung function. A greater proportion of patients who initiated treatment with LAMA+LABA experienced one or more moderate-to-severe exacerbations than in the LAMA cohort, although it should be noted that this study was not powered to detect treatment differences on exacerbations, and the treatment cohorts had different baseline exacerbation rates. Taken together, these findings suggest that dual therapy was preferred for these patients and that the choice between dual and monotherapy as initial maintenance treatment is influenced by disease severity. As expected, due to the longer baseline period, the number of moderate-to-severe exacerbations reported in the sensitivity analysis was higher than in the main analysis. However, this did not appear to affect prescribing patterns in this patient population, supporting the findings of the main analysis and further suggesting that assessment of disease severity and thus prescription of LAMA+LABA is the result of multimodal clinical assessment. Furthermore, adherence as assessed using the medication dispensation rate was high across both groups, suggesting that differences in severity were not related to differing rates of adherence. It has been shown that LAMA+LABA dual therapy provides greater improvements in lung function, symptoms and quality of life than LAMA monotherapy,6 further supporting the consideration of LAMA+LABA dual therapy as initial maintenance treatment for patients with COPD. Relative pricing may also be an influencing factor when choosing between dual and monotherapy; for example, ATS guidelines highlight potential health-equity challenges due to the higher cost of LAMA+LABA compared with monotherapy.12 However, in Singapore LAMA+LABA and LAMA bronchodilators are priced similarly, so early LAMA+LABA dual therapy can represent better value for payers than LAMA monotherapy.11 This suggests that disease severity is likely to be a prominent factor when choosing between therapies.
In both the main and sensitivity analyses, similarly high rates of clinical worsening were observed in the LAMA and LAMA+LABA treatment groups. Exacerbations were more common in the LAMA+LABA cohort, likely because these patients had more exacerbations at baseline, which are a predictor of future exacerbations.23 However, patients who initiated treatment with LAMA+LABA were less likely to change treatment compared with those who initiated treatment with LAMA, despite the higher average severity of the LAMA+LABA cohort at baseline. At baseline, approximately 5% of patients suffered from comorbid asthma and may have been eligible for additional treatment with an inhaled corticosteroid (ICS) component and specialist referral, as outlined in the most recent local guidelines available at the time of the study.19 In the local context, there are several considerations for the escalation of treatment to include an ICS component. Physicians may be reluctant to escalate from dual therapy and add a standalone ICS inhaler to LAMA+LABA due to uncertainty around ICS prescribing and dose selection, increased medication costs,16 lack of regular blood eosinophil count monitoring as recommended to support treatment escalation,5,24 or the challenges involved in identifying specific patients who would benefit from the addition of ICS to their treatment.25 Moreover, addition of a standalone ICS component may require use of multiple inhalers with different inhalation techniques, and as such may require patient education on correct use of the new device for successful treatment.26
A further insight provided by this study is that CAT score and lung function assessments are underutilized in clinics in Singapore, since almost half of the patients included in the study did not have recorded assessments for these measures.27–31 Spirometry data are required to establish a diagnosis of COPD, while CAT is recommended as a tool to assess symptoms, and these assessments play a key role in the monitoring of patients with COPD.5 However, in clinical practice, CAT score and spirometry measures may be difficult to assess in patients who are noncommunicative, hearing impaired, cognitively impaired, critically or terminally ill, who refuse assessment, or who are unable to perform spirometry tests. Spirometry assessments have also been previously reported as lacking or inconsistently used in COPD diagnosis in other regions.27–31 In the absence of CAT or lung function information, clinicians may be relying on presenting symptoms, radiological findings, or clinical intuition to make prescribing decisions. However, it should be noted that clinicians may have used other measures to assess symptoms or health state, such as the Medical Research Council (MRC) dyspnea scale which were not investigated in this study.
A strength of this study is the additional sensitivity analysis, which provided a longer baseline, and as such extended the period of patient characterization. This approach increased the likelihood that patients were true treatment initiators. However, the reduced sample size due to the need for patients to have a full year of available baseline data may make some of the results less robust than those of the main analysis, particularly for the composite endpoint of clinical worsening. This study also reflects the gap between guidelines and real-world clinical practice. Guidelines are largely formulated based on results from randomized controlled trials, which are not always representative of real-world populations or clinical settings. For example, in LAMA efficacy clinical trials eligible patients typically had a CAT score ≥10;32,33 however, in this study the median CAT score was 10, suggesting that the average symptom severity was lower than that observed in clinical trials and demonstrating the scarcity of clinical data for a typical population of patients receiving LAMA monotherapy. This highlights the need for observational studies of real-world data to close the gap between guideline recommendations and clinical practice.
This study is not without its limitations. Firstly, the data warehouse was based on observations at a single center, and so the findings may not be generalizable to other hospitals or healthcare systems. Visits to other healthcare facilities were not captured; however, due to the geographical position of CGH and the availability of linked electronic records, it is highly likely that the majority of healthcare visits, ED visits and hospitalizations were included in the data warehouse. The data warehouse used in the study was compiled with data from patients who had been diagnosed COPD and had either an inpatient stay or an ED visit to CGH. As such, this patient population is specifically representative of the patient population with COPD in Singapore who have attended ED or been hospitalized for exacerbations, rather than the wider patient population, as younger and less severe patients were likely to be managed as outpatients in the local setting. Secondly, only moderate-to-severe exacerbations were evaluated during the follow-up period, as mild exacerbations not resulting in ED visits or hospitalization were not captured in the data warehouse. Thirdly, the sample size was relatively small, as expected for a single-center study, in part due to patients who died in the follow-up period (approximately 25%). These patients were excluded from this analysis, as their reason for death was unknown and therefore cannot be considered as COPD-related, which may have introduced bias into the analysis. Furthermore, the findings for symptoms and lung function are based on a subset of patients, since almost half of the study population had missing CAT score and lung function data. Baseline characteristics comparisons between treatments were nonrandomized and there may also have been other confounding factors not included. Additionally, the composite endpoint used in this study has not been validated as an outcome of clinical worsening. Finally, the follow-up period extended into the beginning of the COVID-19 pandemic, which may have influenced hospitalization exacerbation rates; for example, patients may have avoided hospital and ED visits, and more frequent use of face-coverings may have reduced virus-triggered exacerbations.
Conclusion
The results from this real-world study of patients in Singapore suggest that patients prescribed LAMA+LABA had more severe COPD compared with patients prescribed LAMA monotherapy. Symptom severity, lung function impairment, BMI and number of exacerbations were factors that were likely to be associated with starting treatment with LAMA+LABA. The proportion of patients with clinical worsening was high in both treatment groups and the proportion of patients who had a change of therapy was high in the LAMA monotherapy group, suggesting that early identification and treatment optimization are necessary to improve outcomes for patients with COPD. The data warehouse compiled for this study represents a valuable source of real-world data in patients with COPD in Singapore subject to further validation.
Abbreviations
ATS, American Thoracic Society; BMI, body mass index; CAT, COPD Assessment Test; CGH, Changi General Hospital; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; IQR, interquartile range; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; SD, standard deviation.
Data Sharing Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com. Availability of data owned by CGH may be subject to restrictions.
Ethics Approval and Informed Consent
This study complied with all applicable laws regarding personal data protection. No direct patient contact or primary collection of individual human patient data occurred. Study results were captured in a tabular form and aggregate analyses omitted patient identification. Informed consent, ethics committee or Institutional Review Board approval was not required as deidentified data were used. This study was granted exemption by the Singhealth Centralised Institutional Review Board (2018/2698). In accordance with Singhealth personal data protection policies, the data were submitted to a trusted third party for deidentification and/or anonymization prior to analysis.
Consent for Publication
Patient consent is not required as all data are anonymized.
Acknowledgments
Editorial support (in the form of writing assistance during development of the initial draft, assembling tables and figures, collating authors comments, grammatical editing, and referencing) was provided by Maria Guillermina Casabona, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
Author Contributions
All authors took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The data warehouse used in this study was funded by GlaxoSmithKline (GSK study 215242). GSK employees were involved in the study design, the analysis of the aggregated data, and the writing of the manuscript. GSK was not involved in data collection or patient management and did not have access to any identifiable patient data.
Disclosure
MS reports non-financial support from GSK for manuscript writing support, during the conduct of the study. AY reports grants, non-financial support from GSK, during the conduct of the study. AT reports grants from GSK for supporting the systems integration of the COPD database, during the conduct of the study. XX and PB are employees of GSK. AANR and DM are employees of GSK and hold stock and shares at GSK. The authors report no other conflicts of interest in this work.
References
1. World Health Organisation (WHO). The top 10 causes of death; 2021. Available from: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. Ministry of Health Singapore. Principal causes of death; 2020. Available from: https://www.moh.gov.sg/resources-statistics/singapore-health-facts/principal-causes-of-death.
3. Epidemiology & Disease Control Division, Ministry of Health Singapore, Institute for Health Metrics and Evaluation. The Burden of Disease in Singapore, 1990–2017: An Overview of the Global Burden of Disease Study 2017 Results. Seattle, WA: IHME; 2019. Available from: https://www.healthdata.org/sites/default/files/files/policy_report/2019/GBD_2017_Singapore_Report.pdf.
4. Epidemiology & Disease Control Division, Ministry of Health Singapore. Singapore Burden of Disease Study 2010. Singapore: MoHS; 2014. Available from https://www.moh.gov.sg/docs/librariesprovider5/resources-statistics/reports/singapore-burden-of-disease-study-2010-report_v3.pdf.
5. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. GOLD; 2021. Available from https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
6. Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71(1):15–25. doi:10.1136/thoraxjnl-2014-206732
7. O’Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi:10.1183/09031936.04.00116004
8. Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62. 5/25mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi:10.1016/j.rmed.2013.06.001
9. Maltais F, de la Hoz A, Casaburi R, O’Donnell D. Effects of Tiotropium/Olodaterol on Activity-Related Breathlessness, Exercise Endurance and Physical Activity in Patients with COPD: narrative Review with Meta-/Pooled Analyses. Adv Ther. 2021;38(2):835–853. doi:10.1007/s12325-020-01557-x
10. Calzetta L, Rogliani P, Matera MG, Cazzola M. A Systematic Review. with Meta-Analysis of Dual Bronchodilation With LAMA/LABA for the Treatment of Stable COPD. Chest. 2016;149(5):1181–1196. doi:10.1016/j.chest.2016.02.646
11. Agency for Care Effectiveness (ACE), Ministry of Health Singapore. Managing Stable Chronic Obstructive Pulmonary Disease. Singapore: MoHS; 2018. Available from: https://www.ace-hta.gov.sg/docs/default-source/acgs/managing-stable-copd—focusing-on-inhalers-(sep-2018).pdf.
12. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic Management of Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST
13. Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi:10.2147/COPD.S62750
14. Mannino DM, Yu TC, Zhou H, Higuchi K. Effects of GOLD-Adherent Prescribing on COPD Symptom Burden, Exacerbations, and Health Care Utilization in a Real-World Setting. Chronic Obstr Pulm Dis. 2015;2(3):223–235. doi:10.15326/jcopdf.2.3.2014.0151
15. Kelly AM, Van Meer O, Keijzers G, et al. Get with the guidelines: management of chronic obstructive pulmonary disease in emergency departments in Europe and Australasia is sub-optimal. Intern Med J. 2020;50(2):200–208. doi:10.1111/imj.14323
16. Agency for Care Effectiveness (ACE), Ministry of Health Singapore. Long-Acting Muscarinic Antagonist (LAMA) Monotherapy and Combination Therapy with Long-Acting beta2 Agonists (LAMA/LABA) for the Treatment of Chronic Obstructive Pulmonary Disease. Singapore: ACE; 2018. Available from: https://www.ace-hta.gov.sg/docs/default-source/drug-guidances/lama-lama-laba-for-copd-(2-jul-2018).pdf.
17. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. GOLD; 2017. Available from: https://goldcopd.org/wp-content/uploads/2017/02/wms-GOLD-2017-FINAL.pdf.
18. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. GOLD; 2018. Available from: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf.
19. Ministry of Health Singapore. Chronic Obstructive Pulmonary Disease Clinical Practice Guideline; 2017. Available from: https://www.moh.gov.sg/docs/librariesprovider4/guidelines/copd.pdf.
20. Yii ACA, Loh CH, Tiew PY, et al. A clinical prediction model for hospitalized COPD exacerbations based on “treatable traits”. Int J Chron Obstruct Pulmon Dis. 2019;14:719–728. doi:10.2147/COPD.S194922
21. Kobayashi S, Yanai M, Hanagama M, Yamanda S. Burden of chronic obstructive pulmonary disease in the elderly population. Respir Investig. 2014;52(5):296–301. doi:10.1016/j.resinv.2014.04.005
22. Buikema AR, Brekke L, Anderson A, et al. The effect of delaying initiation with umeclidinium/vilanterol in patients with COPD: an observational administrative claims database analysis using marginal structural models. Multidiscip Respir Med. 2018;13:38. doi:10.1186/s40248-018-0151-6
23. Agusti A, Edwards LD, Celli B, et al. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J. 2013;42(3):636–646. doi:10.1183/09031936.00195212
24. Suissa S, Dell’Aniello S, Ernst P. Comparative Effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: cohort Study in Real-World Clinical Practice. Chest. 2020;157(4):846–855. doi:10.1016/j.chest.2019.11.007
25. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52(6):1801219. doi:10.1183/13993003.01219-2018
26. Dudvarski Ilic A, Zugic V, Zvezdin B, et al. Influence of inhaler technique on asthma and COPD control: a multicenter experience. Int J Chron Obstruct Pulmon Dis. 2016;11:2509–2517. doi:10.2147/COPD.S114576
27. Yu WC, Fu SN, Tai EL, et al. Spirometry is underused in the diagnosis and monitoring of patients with chronic obstructive pulmonary disease (COPD). Int J Chron Obstruct Pulmon Dis. 2013;8:389–395. doi:10.2147/COPD.S48659
28. Arne M, Lisspers K, Stallberg B, et al. How often is diagnosis of COPD confirmed with spirometry? Respir Med. 2010;104(4):550–556. doi:10.1016/j.rmed.2009.10.023
29. Perez X, Wisnivesky JP, Lurslurchachai L, Kleinman LC, Kronish IM. Barriers to adherence to COPD guidelines among primary care providers. Respir Med. 2012;106(3):374–381. doi:10.1016/j.rmed.2011.09.010
30. Ragaisiene G, Kibarskyte R, Gauronskaite R, et al. Diagnosing COPD in primary care: what has real life practice got to do with guidelines? Multidiscip Respir Med. 2019;14(1):28. doi:10.1186/s40248-019-0191-6
31. Vanjare N, Chhowala S, Madas S, Kodgule R, Gogtay J, Salvi S. Use of spirometry among chest physicians and primary care physicians in India. NPJ Prim Care Respir Med. 2016;26:16036. doi:10.1038/npjpcrm.2016.36
32. Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20(1):238. doi:10.1186/s12931-019-1193-9
33. Buhl R, de la Hoz A, Xue W, Singh D, Ferguson GT. Efficacy of Tiotropium/Olodaterol Compared with Tiotropium as a First-Line Maintenance Treatment in Patients with COPD Who Are Naive to LAMA, LABA and ICS: pooled Analysis of Four Clinical Trials. Adv Ther. 2020;37(10):4175–4189. doi:10.1007/s12325-020-01411-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


