Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Characteristics of Attention-Deficit/Hyperactivity Disorder Subtypes in Children Classified Using Quantitative Electroencephalography
Authors Ji Y , Choi TY , Lee J , Yoon S, Won GH , Jeong H, Kang SW, Kim JW
Received 18 August 2022
Accepted for publication 11 November 2022
Published 21 November 2022 Volume 2022:18 Pages 2725—2736
DOI https://doi.org/10.2147/NDT.S386774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Taro Kishi
Yoonmi Ji,1 Tae Young Choi,1 Jonghun Lee,1 Seoyoung Yoon,1 Geun Hui Won,1 Hyerin Jeong,2 Seung Wan Kang,2,3 Jun Won Kim1
1Department of Psychiatry, Daegu Catholic University School of Medicine, Daegu, Republic of Korea; 2iMediSync Inc, Seoul, Republic of Korea; 3National Standard Reference Data Center for Korean EEG, Seoul National University College of Nursing, Seoul, Republic of Korea
Correspondence: Jun Won Kim, Department of Psychiatry, Daegu Catholic University School of Medicine, 3056-6 Daemyeong-4 dong, Nam-gu, Daegu, 705-718, Republic of Korea, Tel +82-53-650-4786, Fax +82-53-623-1694, Email [email protected]
Purpose: This study used quantitative electroencephalography (QEEG) to investigate the characteristics of attention-deficit/hyperactivity disorder (ADHD) subtypes in children.
Patients and Methods: There were 69 subjects (42 with ADHD and 27 neurotypical (NT)) in this study. A semi-structured interview was conducted with each participant for psychiatric diagnostic evaluation. We measured the absolute and relative power in 19 channels and analyzed QEEG using the following frequency ranges: delta (1– 4 Hz), theta (4– 8 Hz), alpha 1 (8– 10 Hz), alpha 2 (10– 12 Hz), beta 1 (12– 15 Hz), beta 2 (15– 20 Hz), beta 3 (20– 30 Hz), and gamma (30– 45 Hz). Group analyses and EEG noise preprocessing were conducted using iSyncBrain, a cloud-based, artificial intelligence EEG analysis platform. Analysis of covariance adjusted for IQ, age, and sex was used.
Results: QEEG analysis revealed three ADHD subtypes, characterized by (A) elevated relative fast alpha and beta power, (B) elevated absolute slow frequency (delta and theta power), or (C) elevated absolute and relative beta power. A significant difference was found in the Korean ADHD Rating Scale (K-ARS) among the four groups (df=3, F=8.004, p< 0.001); group C had the highest score (25.31± 11.16), followed by group A (21.67± 13.18). The score of group B (12.64± 7.84) was similar to that of the NT group (11.07± 6.12) and did not reach the cut-off point of the K-ARS. In the Wender–Utah Rating Scale (WURS), group B score (55.82± 23.17) was significantly higher than the NT group score (42.81± 13.26).
Conclusion: These results indicate that children with ADHD do not constitute a neurophysiologically homogenous group. Children with QEEG subtype B (elevated slow frequency) may be difficult to distinguish from normal children using the K-ARS, which is the most common screening tool for ADHD. Moreover, parents of children with this subtype may be less sensitive to observing ADHD symptoms.
Keywords: attention-deficit/hyperactivity disorder, quantitative electroencephalography, subtype, clinical characteristics
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders in children and adolescents and usually occurs in childhood.1 In the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), it is defined as a neurodevelopmental disorder whose core symptoms are inattention and hyperactivity–impulsivity.2 According to a recent systematic review, the global prevalence of ADHD is 2–7%, with an average of about 5%.3 About 60% of patients diagnosed with ADHD in childhood continue to have ADHD symptoms into adulthood.4 In addition to the core symptoms presented in the DSM-5, symptoms such as deficits in executive function and emotional dysregulation negatively affect patients socially, occupationally, in academic performance.4,5 ADHD is so common that 60% of people have one or more comorbidities such as anxiety, depression, conduct disorder (CD), oppositional defiant disorder (ODD), and learning disability.6
Currently, the diagnosis of ADHD is made using interview tools such as the Diagnostic Interview Schedule for Children Version IV7 and the Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime version (K-SADS-PL)8 as well as interviews with clinicians about the symptoms and duration of ADHD based on the DSM-5. In addition, rating scales such as the Child Behavior Checklist (CBCL)9 and ADHD Rating Scale (ARS),10 and neurocognitive tests such as the Continuous Performance Test (CPT),11 are used as an aid in diagnosis. However, there are cases in which an early and accurate diagnosis of ADHD is missed. This is because, without objective biological markers, ADHD must be evaluated through interviews according to the categorical diagnostic criteria of the DSM-5, because ADHD has various other symptoms in addition to the core symptoms, and there are many cases with comorbidities.12,13 Moreover, overdiagnosis also occurs, because the tools for objective diagnosis are not clear.14
Neuroimaging, genetic, and electrophysiological studies have been conducted aiming to discover pathophysiological and biological markers for ADHD.15,16 Quantitative electroencephalography (QEEG) can detect subtle changes in EEG that are difficult to discriminate visually, and it is known to have greater accessibility and safety compared with other examination methods.17,18 Accordingly, there have been attempts to use QEEG to distinguish between normal children and children with ADHD. Moreover, several characteristics of people with ADHD, including increases in delta and theta waves and decreases in alpha and beta waves, are different compared to a control group.19–21 An increase in theta–beta ratio (TBR) was spotlighted as a strong candidate applicable as a biological marker for children with ADHD;22 however, a later study showed that the false-positive rate was high, and increases in the TBR were also found in a control group,23,24 indicating that the TBR has limitations as a diagnostic index.
Specific QEEG patterns have been identified in ADHD children with heterogeneous symptoms,19 and there have been attempts to classify the subtypes of ADHD using QEEG. Clarke et al classified three subtypes of children diagnosed with ADHD. These were the maturational lag type, in which the slow wave increased and the fast wave decreased; the hypoarousal type in which theta wave increased and beta wave decreased (thus, the TBR increased); and the hyperarousal type, in which the beta wave increased.25 A later study added a subtype that had increased alpha waves.26 Loo et al used QEEG to classify ADHD subtypes into delta, theta, alpha, beta, and no spectral elevation groups based on the highest frequency band in each group.27 Although, there was no specific biomarker found only in children with ADHD among the five subtypes, there was a significant difference in behavioral and cognitive functions for each subtype. Accordingly, Loo et al suggested that QEEG measurements could be a useful biomarker for outcomes and post-treatment responses in ADHD, rather than a diagnostic function of ADHD. Recently, Byeon et al analyzed QEEG among children who were either ADHD, ADHD not otherwise specified (NOS), or neurotypical (NT);28 as a result, it is now possible to classify ADHD into subtypes with either high delta power and low theta power, high theta power and low–fast frequency, or elevated alpha waves. The authors suggested that it is necessary to investigate whether the group with the elevated alpha waves are a new subtype of ADHD or whether it reflects the characteristics of childhood depression. The results of the studies analyzing subtypes of ADHD using QEEG suggest that ADHD is a physiologically heterogeneous disease presenting with differences in behavior, emotion, and cognition for each QEEG subtype. However, the number of studies that classify the QEEG subtypes for children with ADHD is small, making it generalization difficult. In addition, there are not much data on the emotional and behavioral characteristics of each QEEG subtype.
In this study, the QEEG of children with ADHD and NT children were recorded, the subtypes of the ADHD group were classified, and the QEEG characteristics for each subtype of ADHD were investigated. Using the clinical assessment scales widely applied in clinical practice, the emotional and behavioral characteristics of the ADHD subtypes were analyzed and compared with those of NT children.
Material and Methods
Participants
Individuals who visited the child and adolescent psychiatric clinic at Daegu Catholic University Hospital from 2018 to 2020 were considered for the study. Participants included in the study were 7–12 years of age and diagnosed with ADHD according to the DSM-5 criteria. The ADHD diagnosis was based on the K-SADS-PL Korean version (K-SADS-PL-K), which is a semi-structured interview tool, and these diagnoses were confirmed by more than one doctor of child and adolescent psychiatry. If a participant did not meet the ADHD diagnostic criteria of the DSM-5 and K-SADS-PL-K, they were assigned to the NT group. Children were excluded from the study if they had a history of brain damage, neurological disorders, genetic disorders, substance dependence, epilepsy, or any other mental disorder. Children were also excluded if they had an IQ of ≤70 on the Korean–Wechsler Intelligence Scale for Children (4th edition) or were receiving drug treatment. Based on these criteria, 69 participants (42 ADHD and 27 NT) were enrolled in this study.
Detailed information regarding the study was provided to the parents and children. Written consent for the medical use of the test results and participation of the children in this study was obtained from each of the participant’s parents. In addition, after receiving a detailed explanation of the study, each of the children participated voluntarily and provided written consent for participation. This study was approved by the Institutional Review Board (IRB) of the Daegu Catholic University Medical Center (DCUMC IRB approval No. MDCR-19-007) and was performed in accordance with the Declaration of Helsinki (World Medical Association: Ethical Principles for Medical Research Involving Human Subjects, 1964).
Measures
K-SADS-PL-K
The K-SADS-PL-K, a semi-structured interview tool, was administered to select participants by a psychiatrist and skilled residents. The K-SADS-PL-K assesses the present and lifetime conditions and disease symptom severity against 32 Axis I diseases, based on the DSM-IV diagnostic criteria developed by Kaufman.8
Korean ADHD Rating Scale (K-ARS)
The ARS is a screening measure for ADHD developed by DuPaul.10 It is designed to help parents and teachers assess attention deficits and hyperactivity in children. In this study, we used the K-ARS, which was translated into Korean and validated for parents and teachers by Kim et al.29 It contains a total of 18 questions scored on a four-point Likert scale from 0 to 3.
Disruptive Behavioral Disorder Scale (DBDS)
The DBDS was developed by Pelham et al to evaluate the symptoms of ADHD, ODD, and CD in children.30 In this study, we used a scale consisting of 8 items related to ODD and 15 items related to CD based on the DSM diagnosis.
Wender–Utah Rating Scale (WURS)
This scale was developed by Ward et al31 and has been widely used in many studies. It is a self-reported evaluation that can be used to measure ADHD-related behaviors retrospectively.
EEG Recordings
All subjects were instructed to relax with their eyes closed and refrain from movement and talking. EEG data were recorded in the resting-state, eyes-closed condition from 19 scalp electrodes positioned over the whole head according to the International 10–20 System (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2), using a 64-channel Comet digital EEG (Grass, Natus Neurology, USA), with a recording frequency of 800 Hz with reference to the ear electrode. A linked-ear reference electrode was noted if present, but not deemed mandatory for the present study, to respect standard internal protocols of the several clinical recording units. Electrode impedance was kept below 10 kΩ. All recorded artifact-free EEG data were re-referenced offline to a common average to normalize the EEG data collected using different reference electrodes.
EEG Preprocessing Pipeline
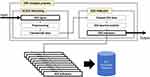
EEG preprocessing was performed to denoise the data and minimize the effects of artifacts. During the first stage of EEG preprocessing, the signals were sampled at 250 Hz and filtered with a bandpass filter (1–45.5 Hz). Figure 1 shows a schematic diagram of the pretreatment. The EEGs were then passed through a notch filter in preparation for downstream processing, including common average referencing, bad epoch rejection using artifact subspace reconstruction, and adaptive mixture independent component analysis (amICA). amICA provides a generic unsupervised approach for identifying and modeling changes in EEG dynamics.32 Finally, artifacts identified via electromyogram, cardiac signal, body movement, or electrooculogram were removed to yield cleaned QEEG normative data. All EEG preprocessing processes, sensor-level data, and extraction were performed using the iSyncBrain cloud-based AI-driven auto-analyzing platform (iMediSync, Inc., https://isyncbrain.com). The iSyncBrain is an AI EEG Analytics Software used in medical institutions such as hospitals/clinics, health check-up centers, and public health centers. It assists in the diagnosis of various neuropsychiatric disorders such as depression and Alzheimer’s disease, through brain mapping. By automating the entire process of EEG analytics, results can be obtained in less than 5 minutes. Recently, several papers using iSyncBrain have been published.33,34
 |
Figure 1 EEG preprocessing flowchart. |
Feature Generation
In each case, relative power was used for features. First, the power spectral density of the EEG rhythms was computed using Fast Fourier Transform (FFT) method with 0.25 Hz of frequency resolution using the iSyncBrain AI-driven auto-analysis system. Then, the signal was decomposed into the following frequency bands: delta (1–4 Hz), theta (4–8 Hz), alpha 1 (8–10 Hz), alpha 2 (10–12 Hz), beta 1 (12–15 Hz), beta 2 (15–20 Hz), beta 3 (20–30 Hz), and gamma (30–45 Hz). For each channel and frequency band, the average power during the recording was calculated and treated as a feature. This yielded 152 total features (19 channels × 8 frequency bands). A standardized index (z-score) for quantitative EEG (QEEG) was used for reference the data to a resting-state, sex- and age-differentiated QEEG normative database (ISB-NormDB). The ISB-NormDB comprises data from 1289 subjects (553 males, 736 females), aged 4.5–81 years, that met strict normative data criteria. A denoising process allowed for stratification between various age ranges of normal healthy men and women based on QEEG variability (Figure 2).34
ADHD Subtype Grouping
ADHD grouping according to QEEG patterns was based on previous studies.28 A determining factor of the ADHD subtype is the topological map pattern of the z-score for each frequency band. According to previous studies,28 four ADHD subtypes can be identified using QEEG feature maps. In order to find out the ADHD phenotypes, we added a step to verify the significance of each group as well as the topological map pattern. In particular, since the group of ADHDs was verified as LDA (Linear Discriminant Analysis) in a previous study, the same method was utilized for the three ADHD phenotypes. We conducted an independent t-test and ANOVA using iSyncBrain for the ADHD subtypes: ADHD (A), ADHD (B), and ADHD (C). The LDA clustering method was applied to the significant features showing differences in the ANOVA post-hoc analysis. In addition, features showing a significant difference were also included in the independent t-test between ADHD (A) and ADHD (C). Thirty-nine sensor-level features were used for LDA clustering analysis. Supplementary Figure 1 shows the results of the clustering analysis. Three ADHD phenotypes are shown in Supplementary Figure 2, including NT, which has a feature map close to a zero z-score. The topomap in Supplementary Figure 2 shows the absolute power z-score for each frequency.
Statistical Analysis
An analysis of covariance (ANCOVA) was performed to test the differences among the three ADHD groups and NT group. Post hoc tests were performed to determine specific group differences. First, the homogeneity of slopes between the groups was assessed using Levene’s test; if slopes were found to be homogeneous, an ANCOVA test was performed to compare the clinical variables among the four groups. When the assumption of sphericity was violated, Greenhouse–Geisser estimates are reported. Statistical significance was set at p<0.05. To control for false positives from multiple comparisons, we used a false discovery rate correction, in which p-values were multiplied by the number of comparisons.35 All data were analyzed using the Statistical Package for the Social Sciences (SPSS) software, version 25.0 (IBM Corp., Armonk, NY, USA). To improve the clarity of the results, topographical plots were created from the results of the statistical comparisons to normative values (z-scores) using iSyncBrain (iMediSync Inc., Republic of Korea; https://isyncbrain.com/), a cloud-based, artificial intelligence EEG analysis platform.
Results
Demographic Characteristics of the Participants
The NT group had 27 participants (18 males, 9 females; mean age: 8.93±1.81 years), and the ADHD group had 42 (38 males, 4 females; mean age: 8.71±1.66 years). There was no statistically significant difference in age between the NT group and the overall ADHD group (p=0.537). The QEEG standardized index (z-score) of the entire ADHD group was calculated based on the resting-state, sex- and age-differentiated QEEG normative database (ISB-NormDB), and three subtypes were classified through this. There were 15 participants in ADHD subgroup A, 11 in subgroup B, and 16 in subgroup C. There were no differences in age (p=0.769), sex (p=0.096), or intelligence (p=0.532) between the NT group and each ADHD subtype (Table 1).
 |
Table 1 Demographic Characteristics of Participants |
QEEG Characteristics by Each ADHD Subtype
Compared with the NT group, the characteristics of the three ADHD subtypes were as follows: ADHD subgroup A had significant differences in the relative power of the alpha 2, beta 2, and beta 3 bands, mainly in the temporal region. In ADHD subgroup B, the absolute power of the delta and theta bands were high in most cerebral regions; however, the relative powers of the beta 2 band in the occipital region and the alpha 2 band in the frontal region were significantly lower than that of the NT group. In ADHD subgroup C, the absolute power of the beta band was significantly higher in most areas of the cerebrum, and the relative power of the beta band was significantly higher in most areas except the occipital region (Figure 3). The results comparing the EEG power values of each ADHD subgroup and the NT group are presented in supplementary Tables 1–3.
Clinical Characteristics of Each ADHD Subtype
To compare the clinical characteristics between the three ADHD subtypes and the NT group, ANCOVA was performed using age, sex, and intelligence as covariates. It was found that there was a significant difference between groups in K-ARS, a measure by which parents evaluated their children’s ADHD symptoms (F=8.004, p<0.001). When Bonferroni post-hoc analysis was performed, the K-ARS scores of ADHD subgroups A (21.67±13.18) and C (25.31±11.16) were significantly higher than that of the NT group (11.07±6.12); the scores of ADHD subgroup B (12.64±7.84) was significantly lower than that of subgroup C (25.31±11.16). There were no significant differences between the scores of ADHD subgroup B and the NT group.
There was a statistically significant difference between the three ADHD subtypes and the NT group for the DBDS, which is used to evaluate children’s behavioral problems (F=3.527, p=0.020). However, there was no significant difference between groups in Bonferroni’s post-hoc test. The DBDS scores of the three ADHD subgroups (A: 8.67±7.01, B: 8.36±6.05, C: 8.50±6.83) were each higher than that of the NT group (3.56±4.85).
There was a significant difference among the four groups in WURS, a measure of parental recall of ADHD-related symptoms when they were young (F=2.872, p=0.043). In Bonferroni’s post hoc test, the WURS score of ADHD subgroup B (55.82±23.17) was significantly higher than that of the NT group (42.81±13.26). There were no significant differences in scores between ADHD subgroup A (45.40±11.68) and the NT group, or subgroup C (44.13±18.37) and the NT group. There were no significant differences between the groups in Children’s Depression Inventory (CDI), Barratt Impulsiveness scale (BIS), Young Internet Addiction Scale (YIAS), Screen for Child Anxiety Related Emotional Disorders, Early Trauma Inventory, Peer-Victimization Scale, and Bullying Behavior Scale, which are the other commonly used children’s clinical assessment scales. In addition, there were no significant differences between groups in the Beck Depression Inventory-II, Beck Anxiety Inventory, BIS, or Korean Adult Attention-Deficit/Hyperactivity Disorder Scale, which are measures that parents used to evaluate themselves (Table 2).
 |
Table 2 Comparison of Clinical Assessment Between the NT and ADHD Subtype Groups |
Discussion
Using the K-SADS-PL-K, QEEG, and clinical assessment scales, three subtypes of ADHD were identified. The clinical characteristics of each subtype are defined through comparison with NT children.
First, there were no differences in age, sex, or intelligence between the NT group and each ADHD subtype. ADHD is more common in males, and depending on the region or sample population; the male to female ratio may range from 2:1 to 16:1.36 In this study, the sex ratio of the ADHD group (38 males and 4 females) was 9.5:1.
Second, using the QEEG, it was possible to classify the study participants into three ADHD subtypes. In group A, significant differences in alpha 2, beta 2, and beta 3 relative powers were found mainly in the temporal region. There are research results in which the alpha wave is increased in the ADHD group compared to a control group,19,26 but there are also studies that found no significant difference;37–39 therefore, the relationship between ADHD and alpha waves is not clearly established. Although local alpha waves were increased significantly in our study, and we recommend that more research results should be accumulated on the relationship between ADHD and alpha waves. In group B, the absolute powers of the delta and theta bands were high in most cerebral regions, and the relative powers of the beta 3 band in the occipital region and alpha 2 bands in the frontal region were significantly lower than those of the NT group. This ADHD subtype was previously named the “maturational lag type”.25 In general, EEGs change with increasing age from a predominantly slow wave to a dominant fast wave,40 but this name was given because the slow wave is dominant compared to normal children of the same age. According to Clarke et al, as the name suggests, this subtype showed a preference for associating with children younger than their age.26 In a study by Loo et al,27 the groups in which delta and theta waves were dominant were actually younger than the groups in which alpha and beta waves were dominant. Additionally, in a study by Markovska-Simoska et al, the absolute power of delta and theta bands increased in children ADHD patients compared to adult ADHD patients.41 Consistent with this study, it was possible to classify the types in which the slow wave increased and the fast wave decreased in children with ADHD; however, there was no age difference between group B and the other groups in our study. In Group C, the absolute and relative powers of the beta band were found to be significantly higher in most areas. In early studies, increases in theta waves and decreases in alpha and beta waves were recognized as typical QEEG findings in ADHD; however, later studies showed excess beta waves in children with ADHD.19,25,27,42 This study accounts for the results of previous studies by classifying subtypes in which excess beta waves are dominant as subgroup C.
Third, for the clinical assessment scale of the QEEG-classified ADHD subtypes, subgroup A did not show any significant difference compared with other ADHD subtypes. Clarke et al found that there was no subtype with increased alpha waves when comorbid psychiatric disorders were excluded25; however, in a subsequent study by the same research team, a subtype that showed an increase in alpha waves in children with ADHD was confirmed when comorbid psychiatric disorders were not excluded.26 These results suggest that the increase in the alpha wave is related to other psychiatric disorders accompanying ADHD; an increase in the alpha wave is also observed in patients with depression.43,44 In a study by Byeon et al, the ADHD group showed the highest rate of being diagnosed with ADHD NOS among the subtypes with an elevated alpha wave.28 Accordingly, the authors suggested that the reduced attention and concentration symptoms of the subtypes were the result of childhood depression, or that ADHD and depression coexisted. In this study, the CDI score, which is a self-reported scale for identifying children’s depressive symptoms, was 11.40±6.33 in group A, which was not different from the other groups. In addition, this score did not reach 15,45 which is the cut-off score for mild depression on the Korean version of the CDI, indicating that there were no clinically meaningful depressive symptoms in group A. However, since the alpha wave in group A increased in a relatively localized brain region, it is difficult to conclude from this result alone that there is no relationship between an elevation of the alpha wave and symptoms of depression.
The value of the K-ARS score of group B was significantly lower than that of groups A and C, but it was similar to the NT group. The K-ARS score of group B (12.64±7.84) was lower than the cut-off score (19) of the K-ARS parent’s version.29 Two factors can be considered for this result. First, it is possible that the parents in group B underestimated their children’s ADHD symptoms. K-ARS has a disadvantage in that scores are not consistent between evaluators even for the same child.29 However, the WURS score of group B (55.82±23.17) was significantly higher than that of the NT group (42.81±13.26) and the WURS cut-off score of 46.31 This suggests that the parents of the group B children experienced more ADHD-related symptoms when they were young than the parents of the NT group did. Parents with ADHD tend to be inattentive and lax in parenting46; accordingly, the parents of group B did not carefully observe or might have neglected their children’s ADHD symptoms, resulting in a K-ARS score that may be underestimated. Second, in the B subtype, the symptoms that can actually be distinguished using K-ARS are not prominent; therefore, it is possible that the score was low. K-ARS is a tool for evaluating inattention and hyperactivity–impulsivity, which are core symptoms of ADHD; it can be easily evaluated in clinical practice, and is the most-often used screening tool for ADHD due to its high sensitivity and predictive accuracy.47 However, since only the narrow symptoms of ADHD defined by the DSM are evaluated, and ADHD is known to have various symptoms other than the core symptoms, it is not easy to evaluate broader symptoms of ADHD using only the K-ARS. Because of these shortcomings, K-ARS in clinical practice is used in conjunction with broadband rating scales such as CBCL or cognitive assessment tools such as CPT.48,49 We found no significant difference between group B and other ADHD subtypes in clinical assessments other than K-ARS and WURS. Therefore, it was not possible to determine whether group B had any symptoms other than those presented in K-ARS. In future studies, it will be necessary to more systematically identify the clinical characteristics of children with the EEG patterns of group B. Considering the clinical characteristics of group B in this study, it is expected that QEEG will be clinically helpful for diagnosing ADHD children of the group B subtype that cannot be detected using K-ARS.
The K-ARS of group C was higher than that of the NT group; there was no significant difference in the post-hoc analysis, but the DBDS score, a measure of behavioral problems, was also higher than that of the NT group. Previously, several studies have suggested a relationship between behavioral problems and an increase in beta activity. Clarke et al found that children of the subtype with increased beta waves showed increased delinquent behavior and a tendency to feel less guilty than other subtypes, suggesting that the increase in the beta wave is related to children’s antisocial behavior.26 Loo et al reported that the diagnosis rate of conduct disorder was highest in the ADHD subtype in which the alpha wave and the beta wave were elevated.27 Another study suggested that an increase in beta activity in adolescent CD patients was associated with psychopathic traits.50 A study on adults found an excessive increase in beta waves in adult males with ADHD with delinquent behavior, as compared to adult males with ADHD without delinquent behavior.51 In general, ADHD is well known as a risk factor for delinquent behavior,52 and the increase in K-ARS and DBDS scores in our study supports this. However, there was no significant difference in the DBDS scores in group C when compared with the other ADHD subtypes. Therefore, although more behavioral problems occur in children with ADHD than in NT children, it seems that it cannot be concluded that this clinical characteristic distinguishes the excessive beta group from other ADHD subtypes; this is inconsistent with previous studies. In addition, a significant increase in group B was not observed in the emotion-related scales of this study; this is a different from Clarke et al42 who found that children of this subtype are moodier and have more temper tantrums than children with other ADHD subtypes. In addition, Park et al53 reported that beta waves were increased in adolescents with ADHD accompanied by internet gaming disorder (IGD); in our study, there was no significant increase in YIAS, a measure related to internet addiction. QEEG studies on ADHD accompanied by IGD are few; therefore, this requires further study.
In addition, in this study, BIS-C, a measure related to children’s impulsivity, and PVS-sum and BBS-sum, which evaluates the behavioral problem between peers, showed no significant difference in any subgroups of NT and ADHD. These results contrast with previous studies that expected high BIS-C scores in ADHD children because impulsivity is a key symptom of ADHD and showed more peer victimization54 and bullying behavior experience55 in ADHD adolescents than in normal controls. The reason for these results is that BIS-C, PVS-sum, and BBS-sum are measures evaluated by the children themselves, so it is possible that the reliability was somewhat low. Therefore, when evaluating these behavioral problems, it is necessary to comprehensively evaluate the information provided by the children and various information providers such as parents, teachers, and peers.
This study has several limitations. First, the number of participants was relatively small, and the number of samples in the NT group and the ADHD group was dissimilar. There were no statistically significant differences, but the age and sex of the NT and ADHD groups did not match exactly. Second, the clinical characteristics of each group were evaluated mainly using a self-reported scale. While this evaluation method has the advantage of requiring less time and simple test execution and scoring, the self-report format may lack objectivity when evaluating symptoms. In addition, since the self-reported scale identifies clinical trends, it is disadvantaged in diagnosing comorbidities of children with ADHD. Third, although the diagnostic interviews were conducted through the K-SADS-PL-K, the NT group comprised children who were contacted through advertisements about ADHD research in the hospital; therefore, they might tend to have lower attention spans than are typical.
This study is meaningful in that it explored the characteristics of QEEG in children with ADHD, and the clinical characteristics of each ADHD subtype was classified based on these characteristics. Unlike previous studies26–28 that identified only the subtypes of children with ADHD or evaluated only the clinical characteristics of children, this study also identified the clinical characteristics of children’s parents for each subtype. If the results on QEEG subtype classification and the relationship between emotion/behavior and a specific EEG band are studied in a sufficient number of children with ADHD, electrophysiological evaluations could improve the current categorical diagnostic criteria for ADHD. Additionally, since EEGs are known to change as children grow,40 it could be misleading to determine the ADHD subtype using an EEG taken at one specific time; the results of QEEG may be different as age increases and the ADHD presentation type may change. Therefore, it is important to identify the QEEG pattern through regular QEEG measurements and determine a therapeutic approach accordingly.
Conclusion
Children with ADHD could be classified into three subtypes based on their QEEG characteristics. Among the three subtypes, the subtype with an increased slow wave and decreased fast wave had a lower K-ARS score and higher parental WURS score compared to other ADHD subtypes. The results of this study suggest that K-ARS has limitations in evaluating the ADHD subtype with an increased slow wave, and that parents of this subtype may have lower sensitivity to ADHD symptoms. Therefore, when using QEEG to evaluate children with ADHD, electrophysiological heterogeneity must be considered, and the use of clinical assessment scales to supplement diagnosis is important.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI19C0844).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387(10024):1240–1250. doi:10.1016/S0140-6736(15)00238-X
2. Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5).
3. Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5(2):175–186. doi:10.1016/S2215-0366(17)30167-0
4. Adler LA, Faraone SV, Spencer TJ, Berglund P, Alperin S, Kessler RC. The structure of adult ADHD. Int J Methods Psychiatr Res. 2017;26(1):e1555. doi:10.1002/mpr.1555
5. Biederman J, Monuteaux MC, Doyle AE, et al. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J Consult Clin Psychol. 2004;72(5):757. doi:10.1037/0022-006X.72.5.757
6. Larson K, Russ SA, Kahn RS, Halfon N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics. 2011;127(3):462–470.
7. Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH diagnostic interview schedule for children version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38.
8. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988.
9. Achenbach TM. Manual for ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth & Families; 2001.
10. DuPaul GJ, Power TJ, Anastopoulos AD, Reid R, McGoey KE, Ikeda MJ. Teacher ratings of attention deficit hyperactivity disorder symptoms: factor structure and normative data. Psychol Assess. 1997;9(4):436. doi:10.1037/1040-3590.9.4.436
11. Corkum PV, Siegel LS. Is the continuous performance task a valuable research tool for use with children with attention‐deficit‐hyperactivity disorder? J Child Psychol Psychiatry. 1993;34(7):1217–1239. doi:10.1111/j.1469-7610.1993.tb01784.x
12. Madsen KB, Ravn MH, Arnfred J, Olsen J, Rask CU, Obel C. Characteristics of undiagnosed children with parent-reported ADHD behaviour. Eur Child Adolesc Psychiatry. 2018;27(2):149–158. doi:10.1007/s00787-017-1029-4
13. Okumura Y, Yamasaki S, Ando S, et al. Psychosocial burden of undiagnosed persistent ADHD symptoms in 12-year-old children: a population-based birth cohort study. J Atten Disord. 2021;25(5):636–645. doi:10.1177/1087054719837746
14. Kazda L, Bell K, Thomas R, McGeechan K, Sims R, Barratt A. Overdiagnosis of attention-deficit/hyperactivity disorder in children and adolescents: a systematic scoping review. JAMA Netw Open. 2021;4(4):. doi:10.1001/jamanetworkopen.2021.5335.
15. Cortese S, Aoki YY, Itahashi T, Castellanos FX, Eickhoff SB. Systematic review and meta-analysis: resting-state functional magnetic resonance imaging studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(1):61–75. doi:10.1016/j.jaac.2020.08.014
16. Balogh L, Pulay AJ, Réthelyi JM. Genetics in the ADHD clinic: how can genetic testing support the current clinical practice? Front Psychol. 2022;13:751041. doi:10.3389/fpsyg.2022.751041
17. Klonowski W, Jernajczyk W, Niedzielska K, Rydz A, Stepien R. Quantitative measure of complexity of EEG signal dynamics. Acta Neurobiol Exp. 1999;59:315–322.
18. van Straaten EC, Stam CJ. Structure out of chaos: functional brain network analysis with EEG, MEG, and functional MRI. Eur Neuropsychopharmacol. 2013;23(1):7–18. doi:10.1016/j.euroneuro.2012.10.010
19. Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biolo Psychiatry. 1996;40(10):951–963. doi:10.1016/0006-3223(95)00576-5
20. Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG analysis in attention-deficit/hyperactivity disorder: a comparative study of two subtypes. Psychiatry Res. 1998;81(1):19–29. doi:10.1016/S0165-1781(98)00072-9
21. Clarke AR, Barry RJ, McCARTHY R, Selikowitz M. Electroencephalogram differences in two subtypes of attention-deficit/hyperactivity disorder. Psychophysiology. 2001;38(2):212–221. doi:10.1111/1469-8986.3820212
22. Snyder SM, Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23(5):441–456. doi:10.1097/01.wnp.0000221363.12503.78
23. Arns M, Conners CK, Kraemer HC. A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord. 2013;17(5):374–383. doi:10.1177/1087054712460087
24. Loo SK, Cho A, Hale TS, McGough J, McCracken J, Smalley SL. Characterization of the theta to beta ratio in ADHD: identifying potential sources of heterogeneity. J Atten Disord. 2013;17(5):384–392. doi:10.1177/1087054712468050
25. Clarke AR, Barry RJ, McCarthy R, Selikowitz M. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clinical Neurophysiol. 2001;112(11):2098–2105. doi:10.1016/S1388-2457(01)00668-X
26. Clarke AR, Barry RJ, Dupuy FE, et al. Behavioural differences between EEG-defined subgroups of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2011;122(7):1333–1341. doi:10.1016/j.clinph.2010.12.038
27. Loo SK, McGough JJ, McCracken JT, Smalley SL. Parsing heterogeneity in attention-deficit hyperactivity disorder using EEG-based subgroups. J Child Psychol Psychiatry. 2018;59(3):223–231. doi:10.1111/jcpp.12814
28. Byeon J, Choi TY, Won GH, Lee J, Kim JW. A novel quantitative electroencephalography subtype with high alpha power in ADHD: ADHD or misdiagnosed ADHD? PLoS One. 2020;15(11):e0242566. doi:10.1371/journal.pone.0242566
29. Kim YS, So YK, Noh JS, Choi NK, Kim SJ, Koh YJ. Normative data on the Korean ADHD Rating Scales (K-ARS) for parents and teacher. J Korean Neuropsychiatr Assoc. 2003;2003:352–359.
30. Pelham WE
31. Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150(6):885–890. doi:10.1176/ajp.150.6.885
32. Hsu SH, Pion-Tonachini L, Palmer J, Miyakoshi M, Makeig S, Jung TP. Modeling brain dynamic state changes with adaptive mixture independent component analysis. Neuroimage. 2018;183:47–61. doi:10.1016/j.neuroimage.2018.08.001
33. Kim NH, Yang DW, Choi SH, Kang SW. Machine learning to predict brain amyloid pathology in pre-dementia Alzheimer’s disease using QEEG features and genetic algorithm heuristic. Front Comput Neurosci. 2021;15:34.
34. Ko J, Park U, Kim D, Kang SW. Quantitative electroencephalogram standardization: a sex- and age-differentiated normative database. Front Neurosci. 2021;15:766781. doi:10.3389/fnins.2021.766781
35. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300.
36. Nøvik TS, Hervas A, Ralston SJ, Dalsgaard S, Rodrigues Pereira R, Lorenzo MJ. Influence of gender on attention-deficit/hyperactivity disorder in Europe--ADORE. Eur Child Adolesc Psychiatry. 2006;15(Suppl 1):I15–24. doi:10.1007/s00787-006-1003-z
37. Bresnahan SM, Anderson JW, Barry RJ. Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biol Psychiatry. 1999;46(12):1690–1697. doi:10.1016/S0006-3223(99)00042-6
38. van Dongen-Boomsma M, Lansbergen MM, Bekker EM, et al. Relation between resting EEG to cognitive performance and clinical symptoms in adults with attention-deficit/hyperactivity disorder. Neurosci Lett. 2010;469(1):102–106. doi:10.1016/j.neulet.2009.11.053
39. Fonseca LC, Tedrus GM, Bianchini MC, Silva TF. Electroencephalographic alpha reactivity on opening the eyes in children with attention-deficit hyperactivity disorder. Clin EEG Neurosci. 2013;44(1):53–57. doi:10.1177/1550059412445659
40. Kaminska A, Eisermann M, Plouin P. Child EEG (and maturation). Handb Clin Neurol. 2019;160:125–142.
41. Markovska-Simoska S, Quantitative P-JN. EEG in children and adults with attention deficit hyperactivity disorder: comparison of absolute and relative power spectra and theta/beta ratio. Clin EEG Neurosci. 2017;48(1):20–32. doi:10.1177/1550059416643824
42. Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Excess beta activity in children with attention-deficit/hyperactivity disorder: an atypical electrophysiological group. Psychiatry Res. 2001;103(2–3):205–218. doi:10.1016/S0165-1781(01)00277-3
43. Dolsen MR, Cheng P, Arnedt JT, et al. Neurophysiological correlates of suicidal ideation in major depressive disorder: hyperarousal during sleep. J Affect Disord. 2017;212:160–166. doi:10.1016/j.jad.2017.01.025
44. Lee PF, Kan DPX, Croarkin P, Phang CK, Doruk D. Neurophysiological correlates of depressive symptoms in young adults: a quantitative EEG study. J Clin Neurosci. 2018;47:315–322. doi:10.1016/j.jocn.2017.09.030
45. Bang YR, Park JH, Kim SH. Cut-off scores of the children’s depression inventory for screening and rating severity in Korean adolescents. Psychiatry Investig. 2015;12(1):23–28. doi:10.4306/pi.2015.12.1.23
46. Park JL, Hudec KL, Johnston C. Parental ADHD symptoms and parenting behaviors: a meta-analytic review. Clin Psychol Rev. 2017;56:25–39. doi:10.1016/j.cpr.2017.05.003
47. Park JI, Shim SH, Lee M, et al. The validities and efficiencies of Korean ADHD rating scale and Korean child behavior checklist for screening children with ADHD in the community. Psychiatry Investig. 2014;11(3):258–265. doi:10.4306/pi.2014.11.3.258
48. Kim JW, Park KH, Cheon KA, Kim BN, Cho SC, Hong KE. The child behavior checklist together with the ADHD rating scale can diagnose ADHD in Korean community-based samples. Can J Psychiatry. 2005;50(12):802–805. doi:10.1177/070674370505001210
49. Won GH, Choi TY, Kim JW. Application of attention-deficit/hyperactivity disorder diagnostic tools: strengths and weaknesses of the Korean ADHD rating scale and continuous performance test. Neuropsychiatr Dis Treat. 2020;16:2397–2406. doi:10.2147/NDT.S275796
50. Calzada-Reyes A, Alvarez-Amador A, Galán-García L, Valdés-Sosa M. QEEG and LORETA in teenagers with conduct disorder and psychopathic traits. Clin EEG Neurosci. 2017;48(3):189–199. doi:10.1177/1550059416645712
51. Meier NM, Perrig W, Koenig T. Is excessive electroencephalography beta activity associated with delinquent behavior in men with attention-deficit hyperactivity disorder symptomatology? Neuropsychobiology. 2014;70(4):210–219. doi:10.1159/000366487
52. Retz W, Ginsberg Y, Turner D, et al. Attention-Deficit/Hyperactivity Disorder (ADHD), antisociality and delinquent behavior over the lifespan. Neurosci Biobehav Rev. 2021;120:236–248. doi:10.1016/j.neubiorev.2020.11.025
53. Park JH, Hong JS, Han DH, et al. Comparison of QEEG findings between Adolescents with Attention Deficit Hyperactivity Disorder (ADHD) without Comorbidity and ADHD Comorbid with Internet Gaming Disorder. J Korean Med Sci. 2017;32(3):514–521. doi:10.3346/jkms.2017.32.3.514
54. Becker SP, Mehari KR, Langberg JM, Evans SW. Rates of peer victimization in young adolescents with ADHD and associations with internalizing symptoms and self-esteem. Eur Child Adolesc Psychiatry. 2017;26(2):201–214.
55. Murray AL, Zych I, Ribeaud D, Eisner M. Developmental relations between ADHD symptoms and bullying perpetration and victimization in adolescence. Aggress Behav. 2021;47(1):58–68.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.