Back to Journals » Infection and Drug Resistance » Volume 16
Characteristics of a Carbapenem-Resistant Acinetobacter baumannii Strain Causing Community-Acquired Pneumonia in a Young Healthy Women
Received 2 October 2023
Accepted for publication 1 December 2023
Published 23 December 2023 Volume 2023:16 Pages 7819—7826
DOI https://doi.org/10.2147/IDR.S439614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yan Chen,1 Liqun Xu,2 Jianfeng Wang3,4
1Department of General Practice, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Emergency Department, Affiliated Hospital of Hangzhou Normal University, Hangzhou, Zhejiang Province, People’s Republic of China; 3Department of Respiratory Diseases, The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, Zhejiang Province, People’s Republic of China; 4Institute of Respiratory Diseases of Traditional Chinese Medicine, Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, People’s Republic of China
Correspondence: Jianfeng Wang, Department of Respiratory Diseases, The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang Province, 310018, People’s Republic of China, Tel +86 571-87060305, Email [email protected]
Background: Multidrug-resistant Acinetobacter baumannii rarely causes community-acquired pneumonia. Here, we report the clinical and genomic characteristics of a multidrug-resistant A. baumannii strain responsible for community-acquired pneumonia in a 31-year-old healthy young women.
Methods: A. baumannii strain W2LL was recovered from the alveolar lavage fluid sample of a hospitalized patient with pulmonary infection. Growth rate studies were conducted under various conditions, and virulence assessments were performed using Galleria Mellonella larvae. Whole Genome Sequencing (WGS) was carried out using Oxford Nanopore MinIon and Illumina HiSeq. In silico multilocus sequence typing (MLST), plasmid replicons, antimicrobial resistance genes, and virulence genes were determined using the BacWGSTdb webserver. Phylogenetic analysis between strain W2LL and other closely related A. baumannii genomes retrieved from NCBI database was performed.
Results: WGS identified strain W2LL as a rare sporadic lineage sequence type (ST) 1431. In addition to the detection of the β-lactamase gene (blaOXA-98) on the chromosome, blaOXA-58 was found on a 92,034 bp plasmid. Antimicrobial susceptibility testing revealed this strain was resistant to cephalosporins and carbapenems, with initial treatment using cefoxitin proving ineffective. Subsequent treatment with piperacillin-sulbactam combined with levofloxacin led to gradual improvement. Compared to A. baumannii ATCC 17978, W2LL exhibited similar growth rates at 37°C and 42°C, as well as in the presence of zinc. However, strain W2LL exhibited higher virulence phenotype compared to ATCC 17978 in G. mellonella model. The closest relative of A. baumannii W2LL was CAM180_1, another isolate recovered from Cambodia, which differed by 191 SNPs.
Conclusion: W2LL is a rare ST1431 carbapenem-resistant A. baumannii strain recovered from a patient with no prior hospitalization or typical risk factors. This underscores the growing menace posed by carbapenem-resistant A. baumannii, no longer limited to hospitalized patients, potentially impacting the broader, younger population.
Keywords: A. baumannii, carbapenem resistant, community-acquired pneumonia, whole-genome sequencing
Introduction
Acinetobacter baumannii, a widely distributed Gram-negative coccus, has become a major nosocomial pathogen, contributing to 20% of global ICU infections.1 Its global spread, coupled with multidrug resistance and virulence factors, poses a significant threat to public health.2–4 In the Antimicrobial Testing Leadership and Surveillance (ATLAS) program for 2020, results indicate a persistently high overall resistance rate of A. baumannii in South Korea, India, and China. This includes resistance to penicillin, cephalosporins, carbapenems, quinolones, and aminoglycosides, with rates exceeding 84.0%, 96.0%, 98.0%, 88.0%, and 87.0%, respectively.5 Notably, China exhibits the lowest susceptibility to tigecycline among A. baumannii isolates when compared to other countries. Infections caused by A. baumannii are associated with a notably high mortality rate, with an overall in-hospital mortality rate reaching 56%.6
A key factor to its success is the adaptable genome, rapidly mutating in response to stress, enabling its persistence in challenging environments, especially within healthcare settings.7,8 The mechanisms of antimicrobial resistance primarily involve the regulation of antibiotic transport through bacterial membranes, alterations in antibiotic target sites, and enzyme modifications that result in the neutralization of antibiotics.9 Molecular traits promoting environmental persistence include resistance to desiccation, biofilm formation, and motility. While A. baumannii is implicated in nosocomial and community-acquired infections, its primary natural host remains poorly understood.10
Historically, A. baumannii has been considered an opportunistic pathogen primarily associated with hospital-acquired infections.11 However, recent studies have documented cases of community-acquired pneumonia caused by A. baumannii, particularly in tropical and subtropical regions. Most of these cases involved patients with underlying health conditions, making the infection of healthy young individuals a rare occurrence. Community-acquired pneumonia caused by A. baumannii is characterized by its severity, high mortality, and fulminant nature.12,13
In this study, we delineate the clinical attributes of an A. baumannii strain responsible for community-acquired pneumonia in a healthy young woman. We conducted extensive investigations into its growth patterns, antimicrobial resistance, and genomic characteristics.
Materials and Methods
Patient Clinical Data
A 31-year-old female patient was admitted to a tertiary teaching hospital in Hangzhou on July 12, 2018, with a complaint of “cough and fever for 5 days”. Chest CT examination on admission showed pneumonia in the left lung (Figure 1). Following a comprehensive examination, the patient was diagnosed with community-acquired pneumonia. Notably, the patient had no prior history of underlying medical conditions. A. baumannii W2LL was isolated from the patient’s alveolar lavage fluid sample shortly after admission.
 |
Figure 1 The patient’s lung CT scan displayed a significant area of pneumonia predominantly in the left lung. |
Upon admission, laboratory testing revealed the following results: blood routine and high-sensitivity C-reactive protein: White blood cell count 6.12×10^9/L, neutrophil percentage 72.1%, high-sensitivity C-reactive protein level 54.10 mg/L, and erythrocyte sedimentation rate (microtube method) of 50 mm/h. Biochemical assessments, indicative of abnormal liver function, included alanine aminotransferase at 125 U/L, aspartate aminotransferase at 63 U/L, alkaline phosphatase at 99 U/L, and γ-glutamyltransferase at 116 U/L. Additionally, the C-reactive protein level was measured at 43.8 mg/L. Quantitative evaluation of procalcitonin, blood coagulation function, and IgM detection of respiratory pathogens yielded no significant abnormalities. However, it is important to note that treatment with cefoxitin, initiated after admission, proved to be ineffective in improving the patient’s condition.
Bacterial Isolates and Antimicrobial Susceptibility Testing
A. baumannii W2LL was isolated from the alveolar lavage fluid sample on July 16, 2018. W2LL was identified as A. baumannii using Vitek GNI card. Bacterial cultures were maintained in Luria-Bertani (LB) medium at 37°C. Antimicrobial susceptibility was determined using Etest strips and interpreted according to CLSI breakpoints (Clinical and Laboratory Standards Institute, 28th edition).14
Bacterial Growth Curve Test
Both strain W2LL and ATCC 17978 were cultured overnight in MH broth, diluted 1:1000 into fresh MH broth, and aliquoted into flat-bottomed 100-well plates in six replicates. Using Bioscreen A CMBR (Growth Curves AB Ltd, Oy, Finland) we measured the OD600 of each culture every 5 min for 16 h. Growth rates were estimated by an R script.15 Using GraphPad Prim 9 performed statistical analysis. Differences between means were assessed using a t-test. P values <0.05 were considered statistically significant.
Galleria mellonella Infection Test
A. baumannii strains were cultured overnight in an orbital shaker at 37°C and 200 rpm. Subsequently, the overnight culture was diluted 100-fold into fresh medium and incubated for 4 hours. Following incubation, cells were harvested via centrifugation (5 minutes at 5000 × g), washed once with phosphate-buffered saline (PBS), and then resuspended in PBS to achieve a final OD600 of 1.0. Further dilutions were prepared in PBS. The quantification of bacterial cells in the injected samples was carried out by plating 10-fold serial dilutions on MH agar plates, and the colony-forming units (CFU) were enumerated after an overnight incubation.
For the infection assay, G. mellonella larvae weighing between 200 and 300 mg were employed.16 Briefly, a bacterial sample of 10 μL with a concentration of 105 CFU/mL was injected into the hind left leg of each larva using a 10 μL glass syringe fitted with a 30G needle. Control groups consisted of larvae injected with 10 μL of sterile PBS. The injected larvae were then placed in an incubator at 37°C and monitored for mortality every 2 hours during the initial 24 hours, and subsequently every 8 hours for an additional 48 hours, resulting in a total observation period of 72 hours. Larvae were considered deceased if they failed to respond to physical stimulation. Each experiment included three replicates for the survival groups, with 10 larvae in each group, and the presented curves represent the most representative outcomes. Statistical analysis of survival differences was conducted using the logrank test in GraphPad Prism 9, with a significance threshold set at P < 0.05.
Whole Genome Sequencing and Analysis
Bacteria from a single colony were cultivated overnight at 37°C in MH broth. Genomic DNA was extracted using the QIAamp DNA minikit. The quality and quantity of the extracted genomic DNA were assessed through agarose gel electrophoresis and a NanoDrop spectrophotometer. Subsequently, DNA libraries were prepared employing the Nextera XT kit, and sequencing was carried out using a 2×150 bp paired-end sequencing protocol. Moreover, WGS was also performed on the Oxford Nanopore MinION platform following the manufacturer’s instructions.
The complete genome of W2LL was assembled by combining the sequences obtained from HiSeq and MinION platforms using a hybrid assembly approach facilitated by Unicycler. To assist in the analysis, a comprehensive one-stop platform BacWGSTdb was employed for rapid bacterial whole genome sequence typing, bacterial source tracing, identification of plasmid replicons, identification of antimicrobial resistance genes, and recognition of bacterial virulence factors.17–19 The globally distributed strains most closely related to A. baumannii were chosen for phylogenetic analysis. Phylogenetic trees were constructed using Snippy, relying on recombination-free core genome SNPs (https://github.com/tseemann/snippy). To visualize and interpret the phylogenetic tree and illustrate the presence/absence of various categories of antimicrobial resistance genes, we utilized the Interactive Tree of Life (iTOL) V5 web server 19.
Results
Antimicrobial Susceptibility Testing
W2LL displayed resistance to cephalosporins and carbapenems but was still susceptible to levofloxacin (Table 1). Clinical improvement was observed after switching to piperacillin-sulbactam combined with levofloxacin.
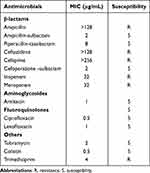 |
Table 1 Antimicrobial Susceptibility Profile of A. baumannii Strain W2LL |
Growth Rate and Virulence of W2LL
There was no significant difference (P > 0.05) in the growth rate of W2LL strain and ATCC 17978 at 37°C, 42°C and when Zn2+ was added (Figure 2). The W2LL strain was more virulent in vitro than ATCC 17978 (P < 0.05), as it killed 40% of G. mellonella larvae within 72 hours, while the ATCC 17978 strain killed 10% within 72 hours larvae (Figure 3).
Genome Characteristics and Phylogenetic Analysis
Whole genome sequencing (WGS) unveiled W2LL as an uncommon ST1431 lineage, bearing two plasmids. One plasmid, measuring 92034bp, exhibits a 99.9% similarity to pCAM180A, previously identified in A. baumannii CAM180-1 from Battambang, Cambodia, dated January 13, 2016. The second plasmid, sized at 76008bp, mirrors the sequence of pC54_002, detected in A. pittii C54 originated from Sydney, Australia, on July 14, 2014.
Notably, the WGS data corroborated the results of the antimicrobial susceptibility testing. Additionally, the β-lactamase gene (blaOXA-98) was detected on the chromosome, while the β-lactamase gene (blaOXA-58) was identified on the plasmid. Furthermore, aminoglycoside resistance genes aph(3’)-I, aac(3)-II, and the tetracycline resistance gene tet(39) were also detected. The presence of 35 virulence genes revealed by WGS, including Type IV pili, Hsp60, SodB, AdeFGH efflux pump, CsrA, Phospholipase C, LPS, OmpA.
Utilizing the full-length 16S ribosomal RNA gene sequence extracted from the WGS data, NCBI BLAST confirmed the species identification, with a match exceeding 99.8% for A. baumannii and less than 99.0% for other Acinetobacter species. The closest related strains to A. baumannii were CAM180-1 (CP044356.1), LRT (CP121375.1), LRB (CP121370.1), AB405E4 (ANND01000001.1), TG31306 (RFEE01000001.1), XH1056 (CP045645.1), ZWS1219 (AMGS01000001.1), 36–1512 (CP059386.1), and FDAARGOS_540 (CP033754.1). The standard bacterial strain ATCC 19606 (CP045110.1) and the prevalent nosocomial strain MDR-ZJ06 (CP001937.2) in China were also included for comparison. Among these strains, CAM180-1 and TG31306 were found to be the most closely related to A. baumannii (Figure 4). However, the genetic dissimilarities between W2LL and A. baumannii CAM180_1, marked by 191 distinct SNPs, underscore substantial genetic disparities between these two strains.
 |
Figure 4 Phylogenetic analysis of A. baumannii W2LL and other publicly available strains based on cgSNP strategy. |
Discussion
In the past two decades, there has been a growing studies detailing severe community-acquired pneumonia linked to A. baumannii (CAP-AB) in individuals without medical exposure or common risk factors for this pathogen. The majority of these cases have been documented in northern Australia and Asia, specifically in regions like Thailand, India, and South Korea.20–22 To date, 19 cases of CAP-AB have been reported in North America. Among these cases, 11 necessitated mechanical ventilation, resulting in a mortality rate as high as 42%.23
With the exception of a limited number of case reports from South Korea and Iran, A. baumannii strains isolated from natural environments typically show susceptibility to a variety of antibiotics.24 Whole-genome phylogenetic analysis revealed no discernible phylogenetic distinctions between community-onset and nosocomial strains. Nevertheless, the bacterial characteristics of community-acquired A. baumannii remain inadequately understood when compared to nosocomial strains.25 In contrast to the usual cases of community-acquired A. baumannii infection associated with underlying conditions such as diabetes or prolonged alcohol consumption, this report highlights a rare occurrence. It involves a carbapenem-resistant strain of A. baumannii causing community-acquired pneumonia in a young healthy woman. Considering the bacterium’s impressive genetic adaptability and the intricate treatment challenges it presents, this incident underscores the need for increased vigilance.
With the rising popularity of whole-genome sequencing (WGS), it has been employed to trace phylogenetic relationships and investigate outbreaks.26 While W2LL shows the closest relationship to CAM180_1 from Cambodia, there still exist 191 SNPs differences between the two isolates. Core genome multilocus sequence typing (cgMLST), a widely used genome-level typing method based on WGS data, comprises hundreds to thousands of core genes, significantly surpassing the loci used in traditional MLST (seven gene loci). The implementation of cgMLST proved highly effective in molecularly typing the strains within this study, significantly streamlining comparisons with similar strains globally. It stands as an exceptional molecular traceability tool, greatly facilitating comprehensive analyses and comparisons with strains worldwide.
A. baumannii carbapenem resistance is often linked to the horizontal transfer of the blaOXA-23 gene, frequently accompanied by the presence of the ISAba1 insertion sequence upstream.27–29 Notably, it carries the β-lactamase gene blaOXA-98 on its chromosome and blaOXA-58 on a plasmid. This signifies that carbapenem-resistant A. baumannii, harboring the blaOXA-58 resistance gene on a plasmid, suggesting caution regarding the potential emergence of highly virulent strains of carbapenem-resistant A. baumannii within the community.
The growth curve analysis of this strain and the G. mellonella experiment demonstrated that its growth rate is comparable to that of A. baumannii ATCC 17978. In fact, the G. mellonella experiment revealed that its lethality was more than that of the wild-type strain. Previous research has indicated that the pathogenicity of A. baumannii is linked to factors such as membrane porins, capsular polysaccharides, protein secretion systems, biofilm formation, metal acquisition systems, and potentially changes in its affinity for lung epithelial cells.30–32 The experimental results presented in this report suggest that the in vitro pathogenicity of this strain is stronger than the standard strain of A. baumannii. Further genome analysis has not identified any distinctive known virulence factors. It is possible that this strain possesses a strong ability to invade lung epithelial cells, but additional experiments are required to elucidate the associated virulence mechanisms.
In conclusion, while A. baumannii is typically regarded as an opportunistic pathogen, our study underscores its capacity to induce severe pneumonia in otherwise healthy individuals, particularly those with multidrug resistance. Considering the genetic adaptability of these species, the substantial risk of broad transmission, particularly from community to hospital environments, warrants serious attention. Further research is essential to understand its pathogenic and transmission mechanisms for the establishment of effective prevention and control measures.
Ethical Approval
Alveolar lavage fluid samples and clinical isolates of A. baumannii W2LL were procured during routine procedures in the hospital laboratory. The study strictly adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of Zhejiang Provincial Hospital of Chinese Medicine in China (ref#KY-ZC-2020-64). The patient provided written informed consent, encompassing permission for the publication of case details.
Funding
This research received support from the Zhejiang Provincial Medical and Health Science and Technology Plan (2020KY670, 2021KY827, 2023KY860), the General Scientific Research Project of Zhejiang Provincial Department of Education (Y202045219), and the Zhejiang Provincial Traditional Chinese Medicine Science and Technology Plan (Project Number: 2024ZL381).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Squire MS, Townsend HA, Actis LA. The influence of blue light and the BlsA photoreceptor on the oxidative stress resistance mechanisms of Acinetobacter baumannii. Front Cell Infect Microbiol. 2022;12:856953. doi:10.3389/fcimb.2022.856953
2. Mea HJ, Yong PVC, Wong EH. An overview of Acinetobacter baumannii pathogenesis: motility, adherence and biofilm formation. Microbiol Res. 2021;247:126722. doi:10.1016/j.micres.2021.126722
3. Nguyen M, Joshi SG. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: a scientific review. J Appl Microbiol. 2021;131:2715–2738. doi:10.1111/jam.15130
4. Garnacho-Montero J, Timsit JF. Managing Acinetobacter baumannii infections. Curr Opin Infect Dis. 2019;32(1):69–76. doi:10.1097/QCO.0000000000000518
5. Chen CH, Wu PH, Lu MC, Ho MW, Hsueh PR. Geographic patterns of carbapenem-resistant, multi-drug-resistant and difficult-to-treat Acinetobacter baumannii in the Asia-Pacific region: results from the Antimicrobial Testing Leadership and Surveillance (ATLAS) program, 2020. Int J Antimicrob Agents. 2023;61(2):106707. doi:10.1016/j.ijantimicag.2022.106707
6. Diep DTH, Tuan HM, Ngoc KM, et al. The clinical features and genomic epidemiology of carbapenem-resistant Acinetobacter baumannii infections at a tertiary hospital in Vietnam. J Glob Antimicrob Resist. 2023;33:267–275. doi:10.1016/j.jgar.2023.04.007
7. Eze EC, Falgenhauer L, El Zowalaty ME. Draft genome sequences of extensively drug resistant and pandrug resistant Acinetobacter baumannii strains isolated from hospital wastewater in South Africa. J Glob Antimicrob Resist. 2022;31:286–291. doi:10.1016/j.jgar.2022.08.024
8. Stracquadanio S, Bonomo C, Marino A, et al. Acinetobacter baumannii and Cefiderocol, between Cidality and Adaptability. Microbiol Spectr. 2022;10(5):e0234722. doi:10.1128/spectrum.02347-22
9. Kyriakidis I, Vasileiou E, Pana ZD, Tragiannidis A. Acinetobacter baumannii antibiotic resistance mechanisms. Pathogens. 2021;11(1):10. doi:10.3390/pathogens11010010
10. Kasperski T, Romaniszyn D, Jachowicz-Matczak E, Pomorska-Wesołowska M, Wójkowska-Mach J, Chmielarczyk A. Extensive drug resistance of strong biofilm-producing Acinetobacter baumannii strains isolated from infections and colonization hospitalized patients in Southern Poland. Pathogens. 2023;12(8):975. doi:10.3390/pathogens12080975
11. Shadan A, Pathak A, Ma Y, Pathania R, Singh RP. Deciphering the virulence factors, regulation, and immune response to Acinetobacter baumannii infection. Front Cell Infect Microbiol. 2023;13:1053968. doi:10.3389/fcimb.2023.1053968
12. Denissen J, Reyneke B, Waso-Reyneke M, et al. Prevalence of ESKAPE pathogens in the environment: antibiotic resistance status, community-acquired infection and risk to human health. Int J Hyg Environ Health. 2022;244:114006. doi:10.1016/j.ijheh.2022.114006
13. Riddles T, Judge D. Community-acquired, bacteraemic Acinetobacter baumannii pneumonia: a retrospective review of cases in Tropical Queensland, Australia. Trop Med Infect Dis. 2023;8(8):419. doi:10.3390/tropicalmed8080419
14. Liu C, Chen K, Wu Y, et al. Epidemiological and genetic characteristics of clinical carbapenem-resistant Acinetobacter baumannii strains collected countrywide from hospital intensive care units (ICUs) in China. Emerg Microbes Infect. 2022;11(1):1730–1741. doi:10.1080/22221751.2022.2093134
15. Mu X, Wang N, Li X, et al. The effect of colistin resistance-associated mutations on the fitness of Acinetobacter baumannii. Front Microbiol. 2016;7:1715. doi:10.3389/fmicb.2016.01715
16. Ten KE, Muzahid NH, Rahman S, Tan HS, Leung SY. Use of the waxworm Galleria mellonella larvae as an infection model to study Acinetobacter baumannii. PLoS One. 2023;18(4):e0283960. doi:10.1371/journal.pone.0283960
17. Wu Y, Jiang T, Bao D, et al. Global population structure and genomic surveillance framework of carbapenem-resistant Salmonella enterica. Drug Resist Updat. 2023;68:100953. doi:10.1016/j.drup.2023.100953
18. Ruan Z, Feng Y. BacWGSTdb, a database for genotyping and source tracking bacterial pathogens. Nucleic Acids Res. 2016;44(D1):D682–D687. doi:10.1093/nar/gkv1004
19. Feng Y, Zou S, Chen H, Yu Y, Ruan Z. BacWGSTdb 2.0: a one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021;49(D1):D644–D560. doi:10.1093/nar/gkaa821
20. Eugenin EA. Community-acquired pneumonia infections by Acinetobacter baumannii: how does alcohol impact the antimicrobial functions of macrophages?. Virulence. 2013;4(6):435–436. doi:10.4161/viru.25747
21. Iwasawa Y, Hosokawa N, Harada M, et al. Severe community-acquired pneumonia caused by Acinetobacter baumannii successfully treated with the initial administration of meropenem based on the sputum gram staining findings. Intern Med. 2019;58(2):301–305. doi:10.2169/internalmedicine.0787-18
22. Xu A, Zhu H, Gao B, et al. Diagnosis of severe community-acquired pneumonia caused by Acinetobacter baumannii through next-generation sequencing: a case report. BMC Infect Dis. 2020;20(1):45. doi:10.1186/s12879-019-4733-5
23. Serota DP, Sexton ME, Kraft CS, Palacio F. Severe community-acquired pneumonia due to Acinetobacter baumannii in North America: case report and review of the literature. Open Forum Infect Dis. 2018;5(3):ofy044. doi:10.1093/ofid/ofy044
24. Ababneh Q, Abu Laila S, Jaradat Z. Prevalence, genetic diversity, antibiotic resistance and biofilm formation of Acinetobacter baumannii isolated from urban environments. J Appl Microbiol. 2022;133(6):3617–3633. doi:10.1111/jam.15795
25. Meumann EM, Anstey NM, Currie BJ, et al. Genomic epidemiology of severe community-onset Acinetobacter baumannii infection. Microb Genom. 2019;5(3):e000258. doi:10.1099/mgen.0.000258
26. Li T, Yang Y, Yan R, et al. Comparing core-genome MLST with PFGE and MLST for cluster analysis of carbapenem-resistant Acinetobacter baumannii. J Glob Antimicrob Resist. 2022;30:148–151. doi:10.1016/j.jgar.2022.06.014
27. Mabrouk A, Chebbi Y, Raddaoui A, et al. Clonal spread of PER-1 and OXA-23 producing extensively drug resistant Acinetobacter baumannii during an outbreak in a burn intensive care unit in Tunisia. Acta Microbiol Immunol Hung. 2020;67(4):222–227. doi:10.1556/030.2020.01208
28. Sawant AR, Pagal S, Amar AK, et al. Coexistence of blaNDM-1, blaOXA-51, blaOXA-23, and armA in conjunction with novel mutations detected in RND efflux pump regulators in tigecycline resistant clinical isolates of Acinetobacter baumannii. Pathog Dis. 2022;80(1):ftac020. doi:10.1093/femspd/ftac020
29. Zhang Y, Ding F, Luo Y, et al. Distribution pattern of carbapenemases and solitary contribution to resistance in clinical strains of Acinetobacter baumannii. Ann Palliat Med. 2021;10(8):9184–9191. doi:10.21037/apm-21-1805
30. Geisinger E, Huo W, Hernandez-Bird J, Isberg RR. Acinetobacter baumannii: envelope determinants that control drug resistance, virulence, and surface variability. Annu Rev Microbiol. 2019;73(1):481–506. doi:10.1146/annurev-micro-020518-115714
31. Asrat H, Samaroo-Campbell J, Ata S, Quale J. Contribution of iron-transport systems and β-lactamases to cefiderocol resistance in clinical isolates of Acinetobacter baumannii Endemic to New York City. Antimicrob Agents Chemother. 2023;67(6):e0023423. doi:10.1128/aac.00234-23
32. Cook-Libin S, Sykes EME, Kornelsen V, Kumar A, Richardson AR. Iron acquisition mechanisms and their role in the virulence of Acinetobacter baumannii. Infect Immun. 2022;90(10):e0022322. doi:10.1128/iai.00223-22
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


