Back to Journals » Drug Design, Development and Therapy » Volume 14
Challenges for Mesenchymal Stem Cell-Based Therapy for COVID-19
Received 25 June 2020
Accepted for publication 6 September 2020
Published 29 September 2020 Volume 2020:14 Pages 3995—4001
DOI https://doi.org/10.2147/DDDT.S269407
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Chenghai Li,1,2,* Hua Zhao,3,4,* Bin Wang5
1Stem Cell Program of Clinical Research Center, Henan Provincial People’s Hospital and People’s Hospital of Zhengzhou University, Zhengzhou 450003, People’s Republic of China; 2Henan Key Laboratory of Stem Cell Differentiation and Modification, Zhengzhou 450003, People’s Republic of China; 3Reproductive Medicine Institute, Henan Provincial People’s Hospital and People’s Hospital of Zhengzhou University, Zhengzhou 450003, People’s Republic of China; 4People’s Hospital of Henan University, Zhengzhou 450003, People’s Republic of China; 5Department of Neurosurgery, Henan Provincial People’s Hospital and People’s Hospital of Zhengzhou University, Zhengzhou 450003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Wang; Chenghai Li Email [email protected]; [email protected]
Abstract: The coronavirus disease 2019 (COVID-19) global pandemic continues and antiviral agents and vaccines are currently under investigation. Mesenchymal stem cell (MSC)-based therapy can be a suitable option for management of patients with COVID-19 at the urgent time of virus outbreak. Currently, MSCs are being explored against the novel infectious disease due to their therapeutic properties of anti-inflammation, immunomodulation and tissue repair and regeneration, albeit the precise mechanisms of MSC action toward COVID-19 remain unclear. To date, rigorous results from clinical trials using MSCs in human have been weakly positive. The pervasive uncertainty of using MSC therapeutic products as an effective combatant against COVID-19 requires rigorous resolution on several fronts, including MSC fate after infusion, safety issue, homing capability, and MSC resistance to the disease microenvironment. Focusing on these facets, a few important ones will be critically analyzed and addressed in this article for the development of safe and effective MSC-based therapies for COVID-19.
Keywords: COVID-19, mesenchymal stem cell, immunomodulation, tissue regeneration
Background
The explosive spread of novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is of great global concerns and the origin of the virus remains actively controversial. Patients with COVID-19 will experience mild to moderate respiratory illness and about 26% hospitalized patients with severe COVID-19 require to be treated in the intensive care unit because of complications, including acute respiratory distress syndrome (ARDS), sepsis and septic shock.1,2 ARDS, a major cause of acute respiratory failure, is one of major complications in these patients during hospitalization and mortality remains morbidly high due to no effective pharmacotherapy.1,2 Sepsis and septic shock, an urgent indication of multi-organ dysfunction, are the most frequently observed complications of the severe COVID-19.2 To address the urgency of combating the COVID-19 pandemic, antiviral agents and vaccines are currently under study but the challenges are numerous. The urgency should also be met with a concurrent exploration of other effective biological treatments against COVID-19. Accounting for the immunomodulatory and tissue regenerative properties of mesenchymal stem cells (MSCs),3–5 they become a new hope for the treatment and management of COVID-19 pandemic.
MSCs are multipotent stem cells and can be isolated and expanded from multiple tissue sources, including bone marrow, adipose tissue, peripheral blood and various neonatal birth-associated tissues.3 MSCs can be induced in vitro to differentiate to osteoblasts, adipocytes, chondrocytes, and other cell types. Importantly, MSCs possess their “immune-privileged” property due to low expression of major histocompatibility antigens. Human MSCs have drawn increasing attention for stem cell-based therapy in translational medicine. While many pre-clinical studies propose that MSCs have immunomodulatory properties, rigorous results from clinical trials have been less positive.6 So far, there are limited clinical data available, but an increasing number of clinical trials are still underway using MSCs for the treatment of COVID-19.
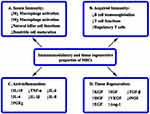
The underlying mechanisms by which MSCs exert a therapeutic influence include anti-inflammation, immunomodulation and tissue remodeling (Figure 1). MSCs have been shown to exhibit these potential capabilities through the release of soluble paracrine factors. MSCs play a major role in the preservation of both epithelial and endothelial barrier function in ARDS and sepsis.7 They can enhance alveolar fluid clearance and regulate epithelial and endothelial permeability through secretion of angiopoietin-1 and keratinocyte growth factor.8,9 A preclinical mouse model suggests that MSCs seem to attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase the level of interleukin (IL)-10.10 Importantly, MSCs are known to exert profound immunomodulatory effects on individual immune cell subset via soluble paracrine factors and direct cell-cell contact to modulate innate and acquired immune responses.
Clinical Investigations of MSC Administration in COVID-19
MSC products are being explored as clinical therapeutic agents, representing a new biological approach for the treatment of COVID-19. At the time of writing of this manuscript, there are 17 clinical trials using MSCs to treat COVID-19 ongoing in China (http://apps.who.int/trialsearch/Default.aspx). Additionally, there are 42 other MSC-based clinical investigations worldwide registered on the NIH clinicaltrials.gov. Among these studies, two (ChiCTR2000030261 and NCT04276987) take the route of therapeutic agent by aerosol inhalation of the MSC-derived exosomes.
Two recent clinical studies have reported the safety and effectiveness of MSC-based therapy for COVID-19 pneumonia.11,12 The first study conducted by Leng et al is a pilot clinical trial in China (ChiCTR2000029990) to assess whether MSC transplantation can improve the outcome of patients with COVID-19 pneumonia.11 In this trial, 7 confirmed COVID-19 patients, including 1 critically severe, 4 severe and 2 non-severe, received a single intravenous infusion of MSCs at a dose of 1×106 cells/kg body weight, respectively, and 3 other severe types were followed for placebo control. These MSCs were negative for angiotensin-converting enzyme 2 (ACE2), indicating that they cannot be infected by the virus. Compared with the control cohort, patients treated with MSCs showed significant improvement of clinical outcomes and there were no reports of MSC infusion-associated adverse events. However, this pilot study is limited by the small sample size. The second clinical study by Guo et al reported that human umbilical cord-derived MSC (UC-MSC) therapy improved the clinical outcomes in 31 patients with severe COVID-19 pneumonia.12 Clinical data and laboratory parameters showed that the oxygenation improved and the cytokine storm syndrome, a hyper-inflammatory state, was attenuated in these patients treated with UC-MSC intravenous infusion at a dose of 1×106 cells/kg. Interestingly, there is a nonrandomized open-label cohort study using exosomes secreted from bone marrow-derived MSCs (BM-MSCs) as treatment for severe COVID-19.13 Sengupta et al conducted this study that presented the clinical and laboratory data following a single intravenous dose of exosomes derived from BM-MSCs to address a promising therapeutic candidate for severe COVID-19.13 Aforementioned, these pilot clinical studies indicate beneficial effects of MSCs and MSC-derived exosomes in COVID-19, but the safety and consistent efficacy need validation from large studies.
Considering the limited data available from current clinical studies for COVID-19, we can extrapolate our discussion on the potential therapeutic benefit of MSCs in ARDS and sepsis in the present manuscript. Two Phase I and one phase IIa clinical trials have revealed that MSC therapy could be used as an effective option for ARDS.14–16 One randomized, placebo-controlled phase I clinical trial was to assess the safety and effects of allogeneic adipose-derived MSCs (AD-MSCs) in a small size of cohort of six patients with moderate to severe ARDS.14 This study demonstrated that AD-MSCs appeared to be safe and feasible in the patients who received one intravenous dose of 1×106 cells/kg, respectively.14 However, there was no significant difference in oxygenation index between MSCs and placebo groups at any time point, albeit significant improvement in oxygenation index from baseline was observed in the MSCs group but not in the placebo group. Meanwhile, ARDS biomarkers in serum, such as IL-6, IL-8, and surfactant protein D (SP-D), showed no significant changes between the two groups at day 5 after infusion in this trial. Another phase I clinical trial registered on ClinicalTrials.gov (NCT01775774) was to test the safety of the dose-escalation allogeneic BM-MSCs in 9 patients with moderate to severe ARDS, using 1, 5, and 10×106 cells/kg, respectively.15 There were no MSC-related severe adverse events after infusions at any dosage. Based on this phase I experience, the same team conducted a subsequent double-blind, multicentre and randomized phase IIa clinical study to further assess treatment with one intravenous dose of BM-MSCs at the high dose (10×106 cells/kg) in 40 patients with moderate to severe ARDS.16 This phase IIa clinical trial showed that no MSC infusion-related haemodynamic or respiratory adverse events were identified, but the MSCs group had higher disease severity scores than the placebo group at baseline. However, the both trials conducted by this team administered freshly thawed frozen BM-MSC products for treatment and, therefore, it remains a matter of debate whether using freshly cultured MSCs will alleviate the disease severity induced by freshly thawed MSCs.15,16
In addition, there are two phase I clinical trials of MSCs in patients with severe sepsis and septic shock.17,18 He et al conducted a phase I clinical trial using UC-MSCs for treatment of severe sepsis.17 A single intravenous infusion of UC-MSCs of low (1×106 cells/kg), intermediate (2×106 cells/kg), and high (3×106 cells/kg) dosage cohorts were confirmed safe and well tolerated in 15 participants with severe sepsis, respectively.17 Another similar phase I dose-escalation trial of BM-MSCs in septic shock (NCT02421484) conducted by Mclntyre et al showed no adverse safety signals in the three BM-MSC dose cohorts of three participants each receiving a single dose of 0.3, 1.0 and 3.0×106 cells/kg, respectively.18 The same team proceeded to a subsequent phase I clinical study in septic shock patients receiving the same dose-escalation BM-MSC infusion to investigate the safety and biological effects of BM-MSC treatment.19 No significant increase in pro-inflammatory cytokines was detected in plasma samples from septic shock patients within the 72-hour time course after BM-MSC infusion, suggesting that MSC treatment may alter underlying sepsis biology. As mentioned above, these clinical safety data are conducive to and may provide useful insight on the progressive clinical trials of MSCs in the COVID-19 clinical setting.
To help accelerate the development of MSC products as therapeutic options, a few stem cell biotechs are joining forces, such as Mesoblast, Athersys, Hope Biosciences and Aspire Health Science. Mesoblast has made decision to evaluate the therapeutic effects of an allogeneic MSC product candidate (Remestemcel-L) in COVID-19 patients with ARDS (http://www.mesoblast.com/). Athersys, another stem cell biotechnology company in Cleveland, Ohio, has been developing a possible therapy for ARDS caused by COVID-19 (https://www.athersys.com/news-and-media/default.aspx). Indeed, Athersys developed MSC product MultiStem and began testing it on ARDS patients in 2015. The FDA has already cleared an investigational new drug application for Mesoblast to treat ARDS patients caused by COVID-19 with intravenous infusion of Remestemcel-L.
Potential Challenges for MSC-Based Approaches for COVID-19
MSC-based therapy against COVID-19 may be a very effective option at the time of virus outbreak. However, several immediate and urgent challenges have to be addressed for the development of the more safe and effective MSC-based approaches for COVID-19.
First, a key issue is the fate of MSCs after intravenous injection. The long-term fate of MSCs after infusion is not fully understood. It is important to note that the majority of therapeutic cells are entrapped in the microvasculature of the lung after systemic MSC administration and they have a short life span. Only a small number of the infused MSCs after administration are distributed to other tissues. When MSCs are systemically infused, they are rapidly removed from the circulation in the lung, where they may also easily cause emboli. MSCs may elicit an innate immune attack, termed instant blood-mediated inflammatory reaction (IBMIR).20 Triggering of IBMIR is potentially augmented after embolization in the microvasculature. Higher cell doses and higher passage numbers of MSCs promote their prothrombotic profile. This may cause MSC infusion-associated adverse events in COVID-19 patients who suffer from a hypercoagulopathy. Therefore, to avoid thrombotic risk, a lower dose of MSCs harvested in low passage may prove to be a more suitable candidate as a treatment option.
Second, whether the circulating MSCs will be cleared from the body after systemic infusion by the humoral components (eg, complements) and immune cell subsets (eg, macrophages) of the immune system should be carefully considered. It should be appreciated that the lung microenvironment can profoundly influence the properties of MSCs and their beneficial effects on ARDS upon initiating MSC administration.21 Previous study by Islam et al showed that stimulation of human MSCs with plasma of ARDS patients resulted in the decrease of CD105 and CD90 for 5 days, suggesting a phenotype shift.21 This observation also suggests the need to consider whether ACE2 on MSC surface may be altered by the inflammatory environment.
Third, emerging evidence indicates that MSCs have a crucial role in drug resistance. MSC-mediated compound/drug resistance frequently occurs in MSC-based anti-cancer therapy,22,23 resulting from tumor microenvironment protecting cancer cells against treatment. Likewise, disease microenvironment can modulate MSC function.24 One previous pre-clinical research indicated that BM-MSCs from osteoporotic donors of ovariectomized (OVX) mice failed to prevent bone loss due to resistance of the disease microenvironment, while AD-MSCs showed protective effects on bone.25 This pre-clinical study highlights the influence of the disease microenvironments on the clinical response of MSCs, which can manifest low therapeutic efficiency.
Fourth, little is currently known about MSC homing to the sites of inflammation or injury in vivo. Research on homing capability of MSCs in vitro is inconclusive, because in vitro assays do not mimic the cells’ journey in vivo. Theoretically, the small blood vessels may be the site of adhesion and migration of MSCs into the tissues, but exogenous MSC engraftment seems to be rare due to the physical properties of MSCs. Thus, this suggests that the limited number of homing MSCs in vivo in turn hampers their potential therapeutic efficacy. There are several factors that affect MSC homing to the target tissues. It is well known that homing-related molecules (eg, chemokines, chemokine receptors and adhesion molecules) and matrix metalloproteinases (MMPs) regulate MSC homing to specific sites. Of special note, aging MSCs downregulate chemokine receptors, such as CCR7, CXC3CR1 and CXCR5, and MMP9,26 which may influence MSC homing capability. High culture confluence has been shown to inhibit transendothelial migration of expanded MSCs by an upregulation of MMP-inhibitor TIMP3.27 MSCs might lose certain surface receptors during serial MSC passaging, which may also affect MSC homing ability. For example, CXCR4, a chemotactic receptor for stromal cell-derived factor-1, is usually lost on the surface of culture-expanded MSCs.28 Interestingly, hypoxic culture condition can promote MSC migration by upregulating CXCR4 and MMPs.28 As mentioned already, it is necessary to address such variability of MSC properties in order to improve MSC homing. Extracellular vesicles (EVs), carrying a variety of substance including bioactive molecules and enzymes, are being actively investigated as a potential clinical candidate.29 MSCs are known to secrete soluble paracrine factors, some of which are contained in EVs (eg, exosome). MSC-derived exosomes can significantly increase the migration of MSCs in a time- and dose-dependent manner.30 Importantly, the release of exosomes from MSCs is increased through a hypoxia-induced paracrine mechanism.30 One recent clinical study suggests that MSC-exosome administration is a therapeutic candidate for severe COVID-19.13 However, MSC-exosome stability, storage, motility, distribution and response in vivo need further optimization.
Finally, whilst a variety of strategies have emerged to optimize MSC-base therapies, genetically modified MSCs for ARDS are very attractive in translational medicine.31 This may provide additional benefits of MSCs in the treatment and management of COVID-19. For example, CXCR4 transgene expression in genetically modified MSCs enhances MSC homing toward the infarct region of the myocardium and improves cardiac performance in a rat myocardial infarction model.32 One pre-clinical study has reported the modified MSCs to overcome the detrimental lung microenvironment in acute lung injury.21 In that study, treatment with modified MSCs carrying either human hepatocyte growth factor or IL-10 resulted in impressive reductions in lung injury state, fibrosis and inflammation. Although genetic engineering strategies can improve therapeutic potential of MSCs, safety issues remain under debate, such as MSC transformation, trans-differentiation and confliction of MSC innate properties.
Expert Opinions on Optimizing MSC Therapeutic Regimens
Optimizing MSC therapeutic regimens is necessary to derive beneficiary clinical outcomes with minimal jeopardy to the patient. The timing of MSC administration is critical, as life-threatening injury (eg, cytokine storm syndrome) to organs often develops quickly. The current clinical study suggests that the window period for cell transplantation is the time when symptoms/signs are progressively getting worse.11 The optimized dose of MSCs, however, has not been formally established. The intravenous dose of 1×106 cells per kilogram of body weight suggested in the previous clinical studies may be considered as a starting guide.11,14,15 The proposed dosing strategy is using up to 4 separate MSC infusions with 3 days apart each time, but optimized dosage regimens are currently undetermined. The population under study will include patients with moderate to severe COVID-19 to administer MSCs intravenously. The route of administration is intravenous infusion of MSCs. Aerosol inhalation of MSC-derived exosomes (ChiCTR2000030261 and NCT04276987) may be an apt route of administration to avoid the impact of virus on MSCs. However, criteria for optimization of exosome products have not been established so far, such as exosome isolation, purification, characterization and storage. With the primary goal of safety and possible efficacy needing to be met, optimizing MSCs must consider other inherent MSC properties, including cell source, donor age, donor disease history, preparation, passage, and cryopreservation and thawing of MSC products.
Conclusions
In conclusion, MSCs are currently being explored as a new treatment option for COVID-19. Given the urgency of COVID-19 pandemic, it is of great importance to appreciate learning more about MSCs and COVID-19 to guide more safe and effective MSC-based therapies. Several immediate and urgent challenges have to be addressed for the development of the MSC-based approaches for COVID-19. While the precise mechanisms of MSC action towards COVID-19 are not fully understood, the therapeutic benefits of MSCs for the treatment and management of COVID-19 are expected to be better achieved as quickly as possible.
Acknowledgments
The authors are thankful to Dr. A.H. Rezwanuddin Ahmed (City College of City University of New York, USA) for his assistance in drafting, discussing and editing the manuscript. This work was supported by Henan Key Laboratory of Stem Cell Differentiation and Modification.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests for this work.
References
1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
3. Molina ER, Smith BT, Shah SR, Shin H, Mikos AG. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J Control Release. 2015;219:107–118. doi:10.1016/j.jconrel.2015.08.038
4. Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020. doi:10.1183/13993003.00858-2020
5. Liu S, Peng D, Qiu H, Yang K, Fu Z, Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020;11(1):169. doi:10.1186/s13287-020-01678-8
6. Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi:10.1016/j.stem.2018.05.004
7. Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2(12):1016–1026. doi:10.1016/S2213-2600(14)70217-6
8. Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913–919.
9. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125.
10. Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49.
11. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2− mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. doi:10.14336/AD.2020.0228
12. Guo Z, Chen Y, Luo X, He X, Zhang Y, Wang J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit Care. 2020;24(1):420. doi:10.1186/s13054-020-03142-8
13. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi:10.1089/scd.2020.0080
14. Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15(1):39. doi:10.1186/1465-9921-15-39
15. Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X. Mesenchymal stem (stromal) cells for treatment of ARDS: a Phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32. doi:10.1016/S2213-2600(14)70291-7
16. Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–162. doi:10.1016/S2213-2600(18)30418-1
17. He X, Ai S, Guo W, et al. Umbilical cord-derived mesenchymal stem (stromal) cells for treatment of severe sepsis: a phase 1 clinical trial. Transl Res. 2018;199:52–61.
18. McIntyre LA, Stewart DJ, Mei SHJ, et al. Cellular immunotherapy for septic shock (CISS): a phase I clinical trial. Am J Respir Crit Care Med. 2018;197(3):337–347.
19. Schlosser K, Wang JP, Dos Santos C, et al. Effects of mesenchymal stem cell treatment on systemic cytokine levels in a phase 1 dose escalation safety trial of septic shock patients. Crit Care Med. 2019;47(7):918–925.
20. Moll D, Rasmusson-Duprez I, von Bahr L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30:1565–1574. doi:10.1002/stem.1111
21. Islam D, Huang Y, Fenelli V, et al. Identification and modulation of microenvironment is crucial for effective MSC therapy in acute lung injury. Am J Respir Crit Care Med. 2019;199:1214–1224. doi:10.1164/rccm.201802-0356OC
22. Plava J, Cihova M, Burikova M, Matuskova M, Kucerova L, Miklikova S. Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol Cancer. 2019;18(1):67. doi:10.1186/s12943-019-0960-z
23. Daverey A, Drain AP, Kidambi S. Physical intimacy of breast cancer cells with mesenchymal stem cells elicits Trastuzumab resistance through Src activation. Sci Rep. 2015;5:13744.
24. Sui BD, Hu CH, Liu AQ, Zheng CX, Xuan K, Jin Y. Stem cell-based bone regeneration in diseased microenvironments: challenges and solutions. Biomaterials. 2019;196:18–30. doi:10.1016/j.biomaterials.2017.10.046
25. Zheng CX, Sui BD, Liu N, et al. Adipose mesenchymal stem cells from osteoporotic donors preserve functionality and modulate systemic inflammatory microenvironment in osteoporotic cytotherapy. Sci Rep. 2018;8(1):5251.
26. Bustos ML, Huleihel L, Kapetanaki MG, et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189(7):787–798. doi:10.1164/rccm.201306-1043OC
27. De Becker A, Van Hummelen P, Bakkus M, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92(4):440–449. doi:10.3324/haematol.10475
28. Karp JM, Teo GSL. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi:10.1016/j.stem.2009.02.001
29. Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9:28–38.
30. Salomon C, Ryan J, Sobrevia L, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8(7):e68451. doi:10.1371/journal.pone.0068451
31. Han J, Liu Y, Liu H, Li Y. Genetically modified mesenchymal stem cell therapy for acute respiratory distress syndrome. Stem Cell Res Ther. 2019;10:386.
32. Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16(3):571–579. doi:10.1038/sj.mt.6300374
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.