Back to Journals » Journal of Multidisciplinary Healthcare » Volume 12
Challenges And Factors Associated With Poor Glycemic Control Among Type 2 Diabetes Mellitus Patients At Nekemte Referral Hospital, Western Ethiopia
Authors Fekadu G , Bula K, Bayisa G, Turi E , Tolossa T , Kasaye HK
Received 27 September 2019
Accepted for publication 24 October 2019
Published 22 November 2019 Volume 2019:12 Pages 963—974
DOI https://doi.org/10.2147/JMDH.S232691
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ginenus Fekadu,1 Kejela Bula,2 Getu Bayisa,1 Ebisa Turi,3 Tadesse Tolossa,3 Habtamu Kebebe Kasaye4
1Department of Pharmacy, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia; 2School of Medicine, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia; 3Department of Public Health, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia; 4Department of Midwifery, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia
Correspondence: Ginenus Fekadu
Clinical Pharmacy Unit, Department Of Pharmacy, Institute Of Health Sciences, Wollega University, Nekemte, Oromia, Ethiopia
Tel +251917137145
Fax +251576617980
Email [email protected]
Background: Diabetes is increasing at an alarming rate throughout the world, and ∼80% of diabetics live in developing countries. Similar to the rest of sub-Saharan African countries, Ethiopia is experiencing a significant burden of diabetes, with increased prevalence, complications, and mortality, as well as life threatening disabilities. Reasons for poor glycemic control among type 2 diabetes patients are complex and multivariable. Hence, this study aimed to identify challenges and factors associated with poor glycemic control among type 2 diabetes patients.
Method: A hospital-based cross-sectional study was conducted among type 2 diabetic patients attending the diabetic clinic of Nekemte Referral Hospital (NRH) from February 1 to April 30, 2018. Fasting blood glucose levels of the last three clinic visits were obtained and the mean fasting blood glucose measurement was used to determine the level of glycemic control. Analysis included both descriptive and inferential statistics with SPSS version 20.0. Predictor variable P<0.05 was considered statistically significant.
Results: Out of the total 228 included type 2 diabetes mellitus (DM) patients, 51.8% were males. The mean age of patients was 43±12.4 years and 154 (67.5%) were found to not be following their general dietary program correctly. Nearly one third, 73 (32%), of patients never attended diabetic education and 52 (22.8%) of the patients had greater than 10 years’ duration on treatment. The majority, 148 (64.9%), of patients had poor blood glucose control. Age 40–60 years (AOR=2.01, 95% CI=0.04–0.06, P=0.044), being illiterate (AOR=3.12, 95% CI=1.52–8.50, P=0.001), having informal education only (AOR=2.28, 95% CI=2.14–32.60, P=0.024), longer duration of diabetes treatment (>10 years) (AOR=3.94, 95% CI=1.51–27.83, P=0.012), inadequate physical exercise (AOR=3.19, 95% CI=1.05–19.84, P=0.019), and smoking (AOR=4.51, 95% CI=0.00–0.50, P=0.022) were independent predictors of poor glycemic control on multivariable logistic regression analysis.
Conclusion: Nearly two-thirds of patients had poorly controlled diabetes. Age, exercise, level of education, duration of the treatment, and smoking were significantly associated with poor glycemic control. Health facilities should provide continuous education, and barriers of glycemic control should be explored with further research.
Keywords: diabetes mellitus, factors, challenges, glycemic control, type 2 diabetes mellitus, Ethiopia
Background
Diabetes mellitus (DM) is one of the top metabolic disorders characterized by chronic hyperglycemia caused by multiple etiologies including defects in insulin secretion, action, or both.1–6 Globally it is one of the commonest non-communicable chronic-degenerative diseases1,7,8 and it is estimated that between 5–10% of the population suffer from this disease.5 Type 2 diabetes is the predominant diabetes, which accounts for 85−95% of all diabetes.2–4,9
All forms of diabetes have very serious effect on health, with increased risk of disabling and life threatening problems.1–3,5,10 With this, diabetes is a major cause of morbidity and mortality through both direct and indirect clinical effects.5,10–12 Additionally, a persistent higher blood glucose level can result in serious health problems to the heart, blood vessels, kidneys, nerves, and other organs.1–4,13 Currently, type 2 DM has become a public health problem globally. Reasons for poor glycemic control in type 2 diabetes are complex and multivariable.14 This inadequate glycemic control contributes to increased rates of both macrovascular and microvascular diabetic complications that are risk to the health of the public.9,15 Controlling the glycemic level is considered the main therapeutic intervention to prevent diabetes complications and further organ damage.16
In 2013, globally there were ~382 million people living with diabetes, with a global prevalence of 8.3%.1 According to the international diabetes federation (IDF), in 2015 ~415 million people were affected and, by 2040, this number could reach 642 million.17,18 Diabetes has historically had a higher burden in high-income countries, but the disease is growing rapidly in developing countries, accounting for ~80% of all global diabetic cases.1,19 In Africa the IDF estimated that ~19.8 million adults were estimated to have diabetes and in 2018 there were more than 500 million prevalent cases of type 2 diabetes worldwide.1 The prevalence of diabetes will increase in future in all countries, mostly to developing countries.20 This is due to modernization, economic well-being, and a westernized lifestyle; the burden of diabetes and its complications increases significantly in Africa.21 As new lifestyles, imported dietary practices, and globalization take roots in the developing world, diabetes and its complications are considered an epidemic in Africa.22 Diabetes in Africa presents a rising public health challenge, and many cases are probably undetected.23
Healthcare systems in sub-Saharan Africa (SSA) also vary widely.21 There is poor health seeking behaviors in low resource countries because of inaccessible quality healthcare that increases the risk of DM complications.24 The management of DM is complex, and good glycemic control significantly reduces the risk of complications.25 This management of DM is not readily available in low resource settings.26,27 With limited resources and health budgets, along with a sharp rise in the prevalence of type 2 diabetes, staffing levels are inadequate to handle and care for the patients appropriately.28 Lack of awareness and facilities for monitoring leads to a high prevalence of diabetic complications.29 On the other hand, a lack of national guidelines, poverty, and ignorance result in complications.22 Therefore, there is a serious threat to the health of individuals and the health systems capacity as a whole.30
Similar to the rest of SSA countries, Ethiopia is experiencing a significant burden of diabetes with increased prevalence, complications, and mortality, as well as life threatening disabilities.31,32 The World Health Organization (WHO) estimated the number of cases of diabetics in Ethiopia to be 800,000 in 2000 and projected that it would increase to 1.8 million by the year 2030.8,32 Previous findings in Ethiopia also reported that the rate of poor glycemic control was high,17,33,34 most importantly due to non-compliance to existing medications.32 Despite the prevalence of type 2 DM is increasing rapidly in Ethiopia, data regarding glycemic control is scarce, and little is known about the factors contributing to poor glycemic control.35 Identification of the challenges and factors associated with poor glycemic control is important in order to institute appropriate interventions to improve glycemic control, and prevent target organ damage and other chronic complications arising from diabetes.16,36 This study was aimed to determine the status of glycemic control and identify factors associated with poor glycemic control among diabetic patients at Nekemte referral hospital (NRH).
Methods And Participants
Study Setting And Design
The study was conducted at Nekemte Referral Hospital (NRH), located in Nekemte town, 331 km away from Addis Ababa to the west. NRH provides curative and preventive services for ~80,000–120,000 individuals per year. This institution-based cross-sectional study was conducted from February 1 to April 30, 2018.
Eligibility Criteria
Patients who were diagnosed to have type 2 DM, had at least a 6 months follow-up, with at least three consecutive blood glucose measurements, and who were willing to participate were included in the study. Newly-diagnosed, with psychiatric disorders, pregnant women, those hospitalized and/or with critical illness, and those patients unable to sign the informed consent form were excluded from the study.
Sample Size And Sampling Technique
The estimated sample size was determined by using the single proportion formula, where n=the desirable sample size; Z (α/2)=the confidence interval (95%) level of significance (1.96); p=proportion of patients with poor glycemic control, and d=precision of measurement (acceptable marginal error). The values were p=0.5 and d=0.05.
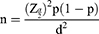
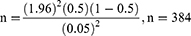
NF=n/(1+n/N) If n<10,000
Where N is the total number of type 2 DM patients=507
Using reduction formula, nf=384/(1+384/507)=218.
By taking contingency=5%
218*5%=218*0.05=10; with this the final sample size was 228 patients.
A systematic random sampling technique was employed to select the participants every two patients (k=2) during their attendance by taking the even number randomly using lottery method.
Study Variables
Dependent Variable
- Glycemic control
Independent Variables
- Sociodemographic variables such as: age, sex, marital status, religion, educational status, and income
- Medical and clinical characteristics
- Adherence to diabetic self-management
- Knowledge of blood glucose target
- Diabetes education attended
- Medications used
- Behavioral conditions of the patients like alcohol consumption and smoking.
Data Collection Instrument And Technique
Data was collected from the medical cards to know their blood glucose level and patients were interviewed by using a semi-structured questionnaire developed by reviewing different literatures. The data collection format contained information on the socio-demographic characteristics, clinical characteristics of patients such as diagnosis, duration of illness, dosage regimen of medications, comorbidities, diabetes complications, and blood glucose measurements. Weight, height, and Fasting blood glucose were measured at the time of the clinical examination performed and recorded by using a structured format. Body mass index (BMI) was calculated as a patient’s weight in kilograms, divided by height in meters squared (kg/m2). BMI was categorized as underweight if BMI was <18.5 kg/m2, normal if BMI was 18.5–24.9 kg/m2, overweight if BMI was 25–29.9 kg/m2, and obese if BMI was ≥30 kg/m2. Fasting blood glucose was measured using a glucose meter (one touch basic monitor) after 8 hours of fasting. Glycemic control was based on American Diabetic Association (ADA) recommendation into two groups as good glycemic control with fasting blood glucose of 70–130 mg/dL and poor glycemic control with fasting blood glucose of <70 mg/dL and >130 mg/dL.37 Fasting blood sugar records of the last three clinic visits (3 months) were obtained from patients’ medical cards and the mean was used to determine the level of glycemic control.
Data Processing And Analysis
The collected data was analyzed using SPSS version 20. Descriptive statistics were calculated to describe the independent variables. Variables with P<0.25 on a bivariate logistic regression analysis were entered into a multivariate logistic regression analysis model to identify the independent predictors of poor glycemic control. The data was summarized using odds ratio (OR) and 95% confidence interval. Predictor variable with P<0.05 was considered statistically significant.
Results
Socio-Demographic Characteristics
Of 228 type 2 DM patients included in the study, 51.8% were males. The mean age of patients was 43±12.4 years (ranging from 18–86 years). One hundred and ninety-two (84.2%) were Oromo in ethnicity, and more than two thirds (69.7%) of the patients were married. Less than one third (27.6%) of the patients were illiterate and 28.9% were unemployed (Table 1).
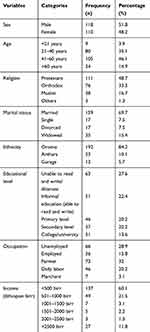 |
Table 1 Socio-Demographic Characteristics Of Type 2 Diabetes Patients At NRH, 2018 |
Self-Care Behaviors Of The Patients
Of the study participants, 154 (67.5%) were found to not be following their general dietary program correctly. One hundred and ninety three (84.6%) were not having adequate physical exercise and 74 (32.5%) patients were not testing their blood glucose level adequately within the last 3 months of the study period. Fifteen (6.6%) were smokers and 30 (13.2%) had a history of alcohol drinking (Table 2).
 |
Table 2 Self-Care Behaviors Of The Type 2 DM Patients At NRH, 2018 |
Clinical And Medication Characteristics
The mean BMI of the patients was 22.24±5.12 kg/m2. Of the total patients, 2.3% of them were underweight, 58.2% of them had normal line BMI, 33.2% of them were overweight, and 6.3% of the patients were obese. Medications were prescribed to manage diabetes. Seventy-four (32.5%) of the patients were on anti-diabetic medication for less than 5 years, 102 (44.7%) were on the treatment for 5–10 years, and the remaining 52 (22.8%) patients had greater than 10 years’ duration on treatment. About 24 (10.5%) had less than six, 17 (11.8) had six-to-ten, and 177 (77.6) had 11–12 follow-ups last year. One hundred and seventy-nine (78.5%) of the study patients did not know their target blood glucose level for diabetes management. Nearly one third, 73 (32%), of respondents never attended diabetic education. The majority, 190 (83.3), of the patients had less than two diabetic education sessions last year and 53 (22.4%) of them had less than ten follow -ups to the clinic per year. Seventy-five (33%) patients had at least one type of comorbidity. Hypertension was the major type of comorbidity, detected in 60 (26.3%) patients. The most common anti-diabetic drug was oral anti-diabetic agents, 136 (59.6%), followed by the combination treatment of oral hypoglycemic agents and insulin, 53 (23.2%), and insulin only, 39 (17.1%). Seventy-seven (33.8%) of the patients were taking the combination of metformin and glibenclamide. More than half, 133 (58.3%) of the patients were on combination therapy (two drug treatments), and the remaining patients were on monotherapy. Seventy-five (32.9%) patients had concomitant medication for the treatment of comorbidities. Enalapril was the most common prescribed concomitant medication.
Glycemic Control And Factors Affecting Glycemic Control
Fasting blood sugar records of the last three clinic visits were obtained from patients’ medical cards and the mean was used to determine the level of glycemic control. With this, the majority, 148 (64.9%), of patients had poor blood glucose control. Out of uncontrolled glycemic control, 22 (9.60%) and 126 (55.3%) had blood glucose level <70 mg/dL and >130 mg/dL, respectively. Age, educational level, duration of diabetes treatments, physical exercise, and smoking had a significant association with glycemic control up on a multivariable logistic regression analysis.
The relative odds of poor glycemic control was 2-times (AOR=2.01, 95% CI=0.04–0.06, P=0.044) higher among patients in the age range of 40–60 years compared to the ages of greater than 60 years. The association of poor glycemic control was about 3- (AOR=3.12, 95% CI=1.52–8.50, P=0.001) and 2-times (AOR=2.28, 95% CI=2.14–32.60, P=0.024) higher among patients who were illiterate and with informal education, respectively, than those with college/higher educational levels. Compared to those who had shorter duration of diabetes treatment (<5 years), patients who had a longer duration of diabetes treatment (>10 years) were ~ 4-times (AOR=3.94, 95% CI=1.51–27.83, P=0.012) more likely to have poor glycemic control. Compared to patients who had adequate physical exercise, respondents who had inadequate physical exercise were ~ 3-times (AOR=3.19, 95% CI=1.05–19.84, P=0.019) more likely to have poor glycemic control. On the other hand, the odds of poor glycemic control was 4.5-times higher among smokers (AOR=4.51, 95% CI=0.00–0.50, P=0.022) compared to non-smokers (Table 3).
 |
Table 3 Multivariable Logistic Regression Analysis Of Factors Associated With Poor Glycemic Control Among Type 2 DM Patients At NRH, 2018 |
Discussion
The study found that overall glycemic control among the study subjects was far below the internationally recommended standards and guidelines. Only fasting blood sugar was used to monitor glycemic control in this hospital similar to a previous study done in Addis Ababa, Ethiopia.38 This was due to the unavailability of the service and high cost of the glycated hemoglobin (HbA1c) determination in the governmental hospitals of Ethiopia. In developed countries glycemic management is primarily assessed with the A1C test that reflects average glycemia over ~3 months.39
Glycemic control knowledge is very crucial to control blood sugar level. The majority of the patients did not have sufficient knowledge of target blood glucose levels for diabetes management. This indicates that patients depend on their healthcare provider’s support to control and treat their diabetes. It is great problem for patients to take appropriate interventions without knowing the target level of diabetes management. Unless patients understand the chronic progressive nature of the disease and are actively involved in their management process, it would be difficult to achieve adequate glycemic level.
About two-thirds (64.9%) of patients had poor blood glucose control. The proportion of poor glycemic control was comparably similar with the studies conducted in Amman Jordan,11 Shanan Gibe hospital,33 Dessie referral hospital,34 Jimma university teaching hospital,35 and university of Gondar referral hospital,40 where the rates of poor glycemic control were 65.1% 59.2%, 70.8%, 70.9%, and 64.7%, respectively. But the level of poor blood glucose control in our finding was lower when compared to previous studies conducted at Jimma university specialized hospital41 and in MGM medical college, Navi Mumbai,36 where 81.9% and 91.8% of patients had not achieved an adequate level of glycemic control, respectively. The study also revealed that poor glycemic control was higher when compared with studies conducted in Najran armed force hospital,42 Ambo hospital,43 and Ayder referral hospital,9 where 22%, 50%, and 48.7% of the patients had poor glycemic control, respectively. Studies from western and Asian countries have also shown similar findings with respect to the quality of diabetes care and the glycemic outcome among the diabetic population of different countries.44–46 This highlights the progressive difficulty of maintaining optimal glycemic control among type 2 diabetes patients, as only a few patients achieve the desired glycemic goals. The possible reason for this difference could be due to a difference in knowledge of glycemic control, the available health service, income, behavioral and clinical characteristics of the patients, as well as the lack of uniform guidelines. These finding highlights the need to work more on appropriate management of diabetes, as maintaining optimum glycemic control is the main therapeutic goal for all patients.
The mean age of the patients was 43±12.4 years, with the majority of them in the age group of 41–60 years. Patients in the age range of 41–60 years constitute a higher proportion of patients with poor glycemic control when compared with those in the age group of <40 years and >60 years, similar to a previous study conducted in Dar es Salaam.16 The presence of an association between age and poor glycemic control in our study was consistent with previous study findings9,11,16 that reported younger age was associated with poor glycemic control. However, a study done in MGM medical college, India, revealed that age was not statistically significantly associated with glycemic control.36 The observed variation of association between age and poor glycemic control could be explained by the differences in population pyramids and distribution of age in different studies. Younger individuals are more likely to have more barriers to self-management behaviors such as healthy low-fat diet, glucose testing, and compliance with their diet and medications.
The young age of patients in this study was striking. Previously, type 2 DM was predominantly a disease of middle-aged and older people. However, recent reports indicated that type 2 DM is becoming an increasingly prevalent disorder among young in all ethnicities driven by lifestyle factors.47–49 This is linked to the global economic growth and changes in lifestyle as well as dietary habits. This rising is in parallel with the incidence of overweight and obesity, suggesting a possible causal relationship, particularly when the obesity is central and in relation to decreased physical activity.47–50 Genetic and familial factors, low birth weight, fetal environmental factors, particularly maternal gestational diabetes and intrauterine growth retardation and lack of physical activity during childhood and adolescence were the other contributing factors. All of these are associated with insulin resistance, although decreased insulin secretion is also required.47,48,51 Despite the young age of onset and shorter duration of diabetes, this group tends to develop diabetes-related complications such as nephropathy and cardiovascular disorders early in the disease process.52 Type 2 DM in the young can be controlled to a large extent through lifestyle modification measures. It is important to screen this disease condition, and identify the at-risk cases.50 Patient and family education for a young person with type 2 DM is very important and will focus on behavioral changes (diet and activity).51
There were almost equal proportions of patients among the two sexes (51.8% vs 48.2%). Compared to some previous studies, the proportion of women was low in our study findings. The steep rise and associated complications of type 2 DM go along with mounting evidence of clinically important sex and gender differences. Large sex-ratio differences across countries are observed. Diversities in biology, culture, lifestyle, environment, and socioeconomic status impact differences between males and females in predisposition, development, and clinical presentation. Genetic effects and epigenetic mechanisms, nutritional factors, and sedentary lifestyle affect risk and complications differently in both sexes.53 In the first half of the last century the prevalence of type 2 DM was higher among women than among men, but this trend has shifted, so more men than women are now diagnosed with type 2 diabetes. This change in the gender distribution of type 2 diabetes is mainly caused by a more sedentary lifestyle, particularly among men, resulting in increased obesity.54 Men are more insulin resistant than women, which can be explained by their higher proportion of visceral and hepatic fat compartments.54,55 Even one meta-analysis demonstrated that, compared with the corresponding women, the men in eastern, middle, and southern Africa had a significantly higher prevalence of impaired fasting glycemia.56 In our setup economic issue is a challenge for both sexes to follow the medical care. Thus, the prevalence of diabetes mellitus was found to be lower or higher in women than in men when analyzed by African sub-regions. Sex-based differences in the relationship between individual socioeconomic status and diabetes mellitus still need to be investigated in developing countries.
Being illiterate and having lower education was independently associated with poor glycemic control, which complies with previous studies.19,34,35,41 This was unlike the study in Dar es Salaam where education of patients was not associated with glycemic control.16 Low education level is associated with poor health, low glycemic diabetes knowledge, low self-management behaviors, lower self-efficacy, and lower continuity of care. Additionally, a shortage of availabile health services may also negatively affect glucose control. Moreover, a higher education level is correlated with better knowledge of diabetes complications and greater adherence to diet and medications.
The duration of the first diagnosis of >10 years was significantly associated with poor glycemic control which was consistent with previous studies that reported the length of duration of diabetes was associated with poor glycemic control.11,36,44,57,58 Patients with the shortest duration of disease may be relatively adherent to medication and recommended diets. From the pathophysiology of the disease, longer duration of diabetes is associated with progressive impairment of insulin secretion, increased insulin resistance, and eventually a decrease in insulin secretion. In earlier disease stages, the task of reaching glycemic goal is aided by residual ß-cell function, whereas in advanced stages there is progressively less endogenous insulin secretion.11,14,16 Therefore, as the disease progresses most patients require an increase in their medications to maintain glycemic control. However, as in a study done at Shanan Gibe Hospital Southwest Ethiopia, diabetes treatment for 5–10 years was one independent predictor of glycemic control among type 2 diabetes patients33 due to poor medication adherence, poor lifestyle conditions, and failure to adhere to regular follow-up at the diabetes clinic.
The storage and quality of the drugs are a critical issue in Africa, including our set-up. Due to the pocket expense, there were situations where some patients could not afford the cost and failed to purchase the drugs, which leads to non-compliance. For example, most patients were not able to store insulin below 8°C due to the lack of refrigerators and other storage facilities. Even the storage of oral medications did not comply with the recommended standard guidelines. While some of the participants’ anti-diabetic medications-related perceptions appeared to be similar to those expressed by western patients, there were perceptions that were different, including the exaggerated concerns towards the medications that could potentially lead to intentional non-adherence and affect health outcomes. Consensus was that some patients do not want to take medication long-term. Back home, medication was used on a short-term basis to “cure” something and then it was stopped. Taking medication over a long period of time as a means to prevent damage from chronic disease may be unfamiliar and difficult to understand. There are several recurring reasons people did not want to take medication, including a generalized fear of side-effects. Patients often stopped taking medications without informing their healthcare provider.
Regarding the feeding habits, patients in our country do not follow the dietary recommendation physicians and nutritionists ordered because of the background economy to afford it. The typical diet the patients followed were mostly “injera” prepared from locally produced teff, sorghum, and maize. There is no quality of water as most of rural patients were using from groundwater/well water, but urban patients were using tap water. The majority of the patients did not have devices (glucometer) to monitor glucose in the blood. Some were illiterate, some patients failed to afford, and others were inaccessible to the devices. This is the current challenge in the majority of societies in our country.
Although physical activity was shown to be protective among patients with type 2 DM,19 only a small proportion of patients participated in regular planned physical activity. There were statistically significant differences between patients who did not perform regular physical activity in terms of glycemic control and those who were participating in regular physical exercise. A study by Alramadan et al59 in Saudi Arabia reported that a low level of physical activity was one independent risk factor for inadequate glycemic control, and a study in Ayder referral hospital, Mekelle town, Ethiopia showed that patients participating in regular exercise were less likely to be poorly controlled.9 However, a lack of relationship between exercise behavior/physical activity and poor glycemic control was observed in other studies.14,16,19 This difference possibly is explained by the difference in study population, culture, economy, environment, and sample size. Most rural patients walk the majority of their daily life for occupation-related reasons, but this was not associated with aerobic/planned physical activity. Some patients were not willing to do planned aerobic physical activity as physicians ordered (at least 150 minutes per week). This was due to most patients not having the time, having no experience of what to do, failing to understand the required procedures, and a lack of a field area to practice it.
On the other hand, the odds of poor glycemic control were 4-times higher among smokers compared to non-smokers. This complies with a previous study that reported current smokers had an increased risk of poor glycemic control.60 Also, a study by Willi et al61 reported that the risk of diabetes is shown to be 45% higher in smokers than among non-smokers. Additionally, a study by Ohkuma et al62 reported that HbA1c levels increased progressively with increases in both the number of cigarettes per day and pack-years of cigarette smoking compared with never smokers. Smoking and its cessation showed dose- and time-dependent relationships with glycemic control and insulin resistance in patients with type 2 diabetes mellitus. Smoking increases the risk of central obesity and insulin resistance, as well as nicotine exposure having several other deleterious effects.63
There are different challenges in glycemic control in Africa, including our country Ethiopia. Very few countries in sub-Saharan Africa can afford to screen and treat the complications of diabetes.64 These resource-limited countries are unable to provide even minimum care in some instances.21 Poorly skilled healthcare staff, a delay in seeking medical attention, and a lack of access to affordable drugs contribute to the high rate of diabetes-related mortality.29,65 Even the newer classes of drugs are unaffordable for the majority of the population. One of the major challenges facing insulin-treated patients in sub-Saharan Africa is the lack of a constant supply of insulin at an affordable cost.66 The supply of insulin is erratic, even at large hospitals, and the prospects for people with diabetes are poor.64,67
Achieving glycemic control in patients with diabetes is of paramount importance to their overall health and survival.47 Poor glycemic control is a risk factor for both micro and macro vascular complications of diabetes and a major factor in the burden of the disease.23 Self-management is a key element for the proper management, but strategies are currently lacking in the developing countries context.22,30 Self-monitoring of blood glucose was rarely used, mainly because of the cost of testing supplies in 90% and the unavailability of testing supplies in 70% of the countries in Africa.21 Patients with diabetes often struggle to achieve glycemic control targets, as self-monitoring of blood glucose, physical activity and risk reduction behavior are insufficient.30,47 Even if treatment guidelines are available, they are hardly used and are not up to date.21 Even in many European countries patients may find this degree of disease management difficulty, with a corresponding negative impact on adherence and glycemic control.44
In general there are parcels of problems encountered in the management of diabetes in sub-Saharan Africa. This includes problems related to diagnosis, medical care, drug supply, monitoring, diabetes education, cost of medication, dietary advice, and management infections associated with diabetes. Additionally, poor patient attendance, short consultation time, inadequate infrastructure, poor evaluation of complications of diabetes, poor record keeping, disproportionate distribution of healthcare facilities, and lack of adequately trained healthcare professionals to care for and treat diabetic patients are top challenges observed in African countries including our country. In our set-up patients are not supplied with medications, rather they purchase from the hospital at their own expense. If drugs are not available or in case of stock out from the governmental hospital pharmacy, patients purchase drugs from the private and community pharmacies. Only the few patients that have a written certificate stating their inability to purchase the medications, does the government supply freely. Due to pocket expenses, there are conditions where some patients could not afford the cost and fail to purchase the drugs, which leads to non-compliance. Adequate knowledge of the overall burden of diabetes in high-risk populations and countries is a prerequisite for effective diabetes healthcare delivery. This requires urgent targeted interventions to improve glycemic control in this population and prevent further chronic complications.
Limitation Of The Study
The study was a cross-sectional study, where a causal relationship between the independent and dependent variables cannot be established. Medication adherences, dietary intake, blood glucose testing, smoking status, and physical activities were obtained by self-report and may be limited by recall and social desirability bias. None of the patients had HbA1c determination; which is the gold standard to determine patient’s “glycemic level”. Absence of HbA1c determination directly compromise quality service given for the patients; since FBS reflect the glycemic status of the one spot. But measurement of glycated hemoglobin (HBA1c) would show the rate of glycemic control over a 3-month period.
Conclusion
This study revealed that about two thirds of patients had a poor glucose level. Age, exercise, level of education, duration of the treatment, and smoking were significantly associated with poor glycemic control among type 2 diabetes patients. Thus, patients should know rationales of self-care activities and take appropriate intervention accordingly. Health sectors should provide continuous health education that emphasizes behavioral lifestyle modification with importance of encouraging physical activity and cessation of smoking. Patient’s education plays a key role to control the glycemic control, favor treatment success, reduce adverse drug events, and prevent further complication of diabetes. To educate the patient the level of education of the patient, resources, and available materials influence the scope of the education. The area of education should focus on proper utilization of a blood glucose device, foot care hygiene, weight loss for obese and overweight, balanced diet, adherence to medication, and prevention of DM complications. Culturally appropriate educational like fact sheets, toolkits, booklets, CDs, DVDs, pamphlet, webinars and other materials can be used. Additionally, free, colorful, low-literacy patient handouts related to diabetes can be used. More attention should be focused on patients with a longer duration of disease, and patients who have not been educated. The barriers of glycemic control should be explored since this study does not look at barriers. We recommend further large population and longitudinal studies to assess determinants of poor glycemic control over a period of time.
Abbreviations
ADA, American Diabetic Association; AOR, Adjusted odds ratio; BGL, Blood glucose level; BMI, Body mass index; COR, Crude odds ratio; DM, Diabetic mellitus; FBS, Fasting blood sugar; NRH, Nekemte Referral Hospital; OHAs, Oral Hypoglycemic agents; WHO, World Health Organization.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki. Ethical clearance was obtained from the Institutional Review Board (IRB) of the Institute of Health Sciences, Wollega University. Verbal consent was obtained from responsible bodies of the hospital and the DM clinic of the hospital prior to the interview and reviews of the patient data. Written informed consent was obtained from patients to participate in this study after a comprehensive explanation of the purpose and procedure of the study.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgement
We would like to thank Wollega University, Institute of Health Sciences, Department of Pharmacy for logistic support to conduct the research. We are grateful to staff members of DM clinic of NRH, data collectors, and study participants for their cooperation in the success of this study.
Author Contributions
GF, KB, and GB contributed in the design of the study, drafting, analysis, and write up of the manuscript. ET, TT, and HK made the data analysis and interpretation, drafting, and editing of the manuscript. All authors contributed to data analysis, drafting, and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023.
2. Craig M, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2009;10(12):3–12. doi:10.1111/pdi.2009.10.issue-s12
3. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2009;32(1):193–203. doi:10.2337/dc08-9025
4. Center of disease prevention and control. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States. Atlanta: Department of Health and Human Services CDC, and Prevention; 2005.
5. Litwak L, Goh S-Y, Hussein Z, Malek R. Vinay Prusty et al. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational A1chieve study. Diabetol Metab Syndr. 2013;5:57.
6. World Health Organization. Diagnosis and Classification of Diabetes Mellitus and Its Complications Part 1: Diagnosis and Classification of Diabetes Mellitus World; 1999.
7. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi:10.1016/j.diabres.2017.03.024
8. Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269–273. doi:10.5001/omj.2012.68
9. Tadele E, Abrahaley M, Gebretsadik H, Getu K, Dagim A, Yerra R. Factors associated with poor glycemic control in type 2 diabetic patients investigated at Ayder Referral Hospital, Mekelle, Ethiopia. Ijppr Human. 2016;6(3):160–171.
10. Genetics and Diabetes, published in. Reviews in Endocrine and Metabolic Disorders 5.1; 2004:25–36. Available from: http://docplayer.net/5742509-Genetics-and-diabetes.html.
11. Khattab M, Khader YS, Al-Khawaldeh A, Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complication. 2010;24:84–89. doi:10.1016/j.jdiacomp.2008.12.008
12. Koro CE, Bowlin SJ, Bourgeois N, Fedder DO. Glycemic control from 1988 to 2000 among US adults diagnosed with type2 diabetes: a preliminary report. Diabetes Care. 2004;27(1):17−20. doi:10.2337/diacare.27.1.17
13. WHO diabetes fact sheet October, 2013. Report No.: 312.
14. Pamungkas RA, Hadijah S, Mayasari A Nusdin. Factors associated with poor glycemic control among type 2 diabetes mellitus in Indonesia. Belitung Nurs J. 2017;3(3):272–280.
15. Mohammad H, Amir N, Maryam H, Girish T, Surulivelrajan M. Factors that correlate with poor glycemic control in type 2diabetes mellitus patients with complications. Osong Public Health Res Perspect. 2018;9(4):167–174. doi:10.24171/j.phrp.2018.9.4.05
16. Kamuhabwa Appolinary R, Emmanuel C. Predictors of poor glycemic control in type 2 diabetic patients attending public hospitals in Dar es Salaam. Drug Healthc Patient Saf. 2014;6:155–165. doi:10.2147/DHPS.S68786
17. International Diabetes Federation. IDF Diabetes Atlas.
18. Ogurtsova K, Da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi:10.1016/j.diabres.2017.03.024
19. Gonçalves da SD, Alberto SL, Amorim AA. Factors associated with poor glycemic control among patients with type 2 diabetes in the Southeast Region of Brazil. Int J Diabetes Res. 2018;7(2):36–40. doi:10.5923/j.diabetes.20180702.03
20. Kaiser AB, Zhang N, Der Pluijm WV. Global Prevalence of Type 2 Diabetes over the Next Ten Years (2018–2028). Diabetes. 2018; 67(Supplement1):202–LB. doi: 10.2337/db18-202-LB
21. Mbanya JC, Ramiaya K. Diabetes Mellitus. In: Jamison DT, Feachem RG, Makgoba MW, et al., editors. Disease and Mortality in Sub-Saharan Africa.
22. Azevedo M, Alla S. Diabetes in sub-saharan Africa: Kenya, mali, mozambique, Nigeria, South Africa and zambia. Int J Diabetes Dev Ctries. 2008;28(4):101–108. doi:10.4103/0973-3930.45268
23. Onodugo OD, Ezeala-Adikaibe BA, Anyim OB, et al. Glycemic control among medical outpatients in Enugu: a cross sectional survey. J Diabetes Mellitus. 2019;9:50–61. doi:10.4236/jdm.2019.92006
24. Nwafor A, Owhoji A. Prevalence of diabetes mellitus among Nigerians in Port Harcourt correlates with socio-economic status. J Appl Sci Environ Manag. 2001;5(1):75–77.
25. Alberti H, Boudriga N, Nabli M. Primary care management of diabetes in a low/middle income country: a multi-method, qualitative study of barriers and facilitators to care. BMC Fam Pract. 2007;8:63. doi:10.1186/1471-2296-8-63
26. Buowari OY. Diabetes mellitus in developing countries and case series, diabetes mellitus - insights and perspectives, Oluwafemi O. Oguntibeju, IntechOpen; January 23, 2013. Available from: https://www.intechopen.com/books/diabetes-mellitus-insights-and-perspectives/diabetes-mellitus-in-developing-countries-and-case-series.
27. Edo AE, Adediran OS. Carbohydrates in diabetic diet in Nigeria: is it evidence based? Nig J Gen Pract. 2006;7(9):19–23.
28. WHO (World Health Organization). The World Health Report 2000—Health Systems: Improving Performance. Geneva: WHO; 2000.
29. Swai ABM, Lutale J, McLarty DG. Diabetes in Tropical Africa: a prospective study 1981–7: characteristics of newly presenting patients in Dar es Salaam, Tanzania 1981–7. Br Med J. 1990;300:1103–1107. doi:10.1136/bmj.300.6732.1103
30. Stephani V, Opoku D, Beran D. Self-management of diabetes in Sub-Saharan Africa: a systematic review. BMC Public Health. 2018;18(1):1148. doi:10.1186/s12889-018-6050-0
31. Nigatu T. Epidemiology, complications and management of diabetes in Ethiopia: a systematic review. J Diabetes. 2012;4(2):174–180. doi:10.1111/jdb.2012.4.issue-2
32. Desse TA, Eshetie TC, Gudina EK. Predictors and treatment outcome of hyperglycemic emergencies at Jimma UniversitSpecialized Hospital, southwest. BMC Res Notes. 2015;8(553):1–8. doi:10.1186/s13104-015-1495-z
33. Miteku YD, Alemu DT. Glycemic control and associated factors among type 2 diabetic patients at Shanan Gibe Hospital, Southwest Ethiopia. BMC Res Notes. 2017;10:597.
34. Temesgen F, Ermiyas A, Wongelawit K, Aderaw A, Angesom G. Factors associated with glycemic control among diabetic adult out-patients in Northeast Ethiopia. BMC Res Notes. 2018;11:316.
35. Tefera K, Tesfahun E, Hailay G. Factors associated with glycemic control among adult patients with type 2 diabetes mellitus: a cross-sectional survey in Ethiopia. BMC Res Notes. 2016;9:78.
36. Kakade Ashutosh A, Mohanty Ipseeta R, Sandeep R. Assessment of factors associated with poor glycemic control among patients with type II diabetes mellitus. Integr Obesity Diabetes. 2018;4(3):1–6.
37. American Diabetes A. Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):s4–s36.
38. Tekalegn Y, Addissie A, Kebede T, Ayele W. Magnitude of glycemic control and its associated factors among patients with type 2 diabetes at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. PLoS ONE. 2018;13(3):e0193442. doi: 10.1371/journal.pone.0193442
39. Li J, Chattopadhyay K, Xu M, et al. Glycaemic control in type 2 diabetes patients and its predictors: a retrospective database study at a tertiary care diabetes centre in Ningbo, China. BMJ Open. 2018;8(3):e019697. doi:10.1136/bmjopen-2017-019697
40. Abebe SM, Berhane Y, Worku A, Alemu S, Mesfin N. Level of sustained glycemic control and associated factors among patients with diabetes mellitus in Ethiopia: a hospital-based cross-sectional study. Diabetes Metab Syndr Obes. 2015;27(8):65–71. doi:10.2147/DMSO
41. Hailu E, Mariam WH, Belachew T, Birhanu Z. Self-care practice and glycaemic control amongst adults with diabetes at the Jimma University Specialized Hospital in south-west Ethiopia: a cross-sectional study. Afr J Prim Health Care Fam Med. 2012;4(1):311. doi:10.4102/phcfm.v4i1.311
42. Harrabi I, Al Harbi F, Al Ghamdi S. Predictors of glycemic control among patients with type 2 diabetes in Najran Armed Forces Hospital: a pilot study. J Diabetes Mellitus. 2014;4(2):141–147. doi:10.4236/jdm.2014.42021
43. Woldu MA, Wami CD, Lenjisa JL, Tegegne GT, Tesfaye G, Dinsa H. Factors associated with poor glycemic control among patients with type 2 diabetes mellitus in Ambo Hospital, Ambo; Ethiopia. Endocrinol Metab Synd. 2014;3:143.
44. de Pablos-velasco P, Parhofer KG, Bradley C, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf). 2014;80(1):47–56. doi:10.1111/cen.12119
45. Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3(6):110–117. doi:10.4239/wjd.v3.i6.110
46. Chan JCN, Gagliardino JJ, Baik SH, Chantelot J-M, Ferreira SRG, Hancu N et al. Multifaceted Determinants for Achieving Glycemic Control. The International Diabetes Management Practice Study (IDMPS). 2009;32(2):227–233. https://doi.org/10.2337/dc08-0435
47. AJMC Managed markets network. Challenges in Diabetes Management: Glycemic Control, Medication Adherence, and Healthcare Costs. American Journal of Managed Care. Published on: August 21, 2017. Available from: https://www.ajmc.com/journals/supplement/2017/challenges-in-diabetes-management/challenges-in-diabetes-management-article, Accessed July 12, 2019.
48. Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M. Type 2 diabetes in the young: the evolving epidemic. Int Diabetes Federation Consensus Workshop. 2004;27(7):1798–1811.
49. Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4(6):270–281. doi:10.4239/wjd.v4.i6.270
50. Prasad AN. Type 2 diabetes mellitus in young need for early screening. Indian Pediatr. 2011;48(9):683–688. doi:10.1007/s13312-011-0111-0
51. Temneanu OR, Trandafir LM, Purcarea MR. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. 2016;9(3):235–239.
52. Htike ZZ, Webb D, Khunti K, Davies M. Emerging epidemic and challenges of Type 2 diabetes in young adults. Diabetes Manag. 2015;5(6):473–483. doi:10.2217/dmt.15.39
53. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316. doi:10.1210/er.2015-1137
54. Færch K Gender and T2DM [internet]; August 13, 2014. Diapedia 3104972816 rev. no. 10. Available from: https://www.diapedia.org/3104972816/rev/10.
55. Nordström A, Hadrévi J, Olsson T, Franks PW, Nordström P. Higher prevalence of type 2 diabetes in men than in women is associated with differences in visceral fat mass. J Clin Endocrinol Metab. 2016;101(10):3740–3746. doi:10.1210/jc.2016-1915
56. Hilawe EH, Yatsuya H, Kawaguchi L, Aoyama A. Differences by sex in the prevalence of diabetes mellitus, impaired fasting glycaemia and impaired glucose tolerance in sub-Saharan Africa: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(9):671D–682D. doi:10.2471/BLT.12.113415
57. Sasi ST, Kodali M, Burra KC, Muppala BS, Gutta P, Bethanbhatla MK. Self-care activities, diabetic distress and other factors which affected the glycaemic control in a Tertiary Care Teaching Hospital in South India. J Clin Diagn Res. 2013;7(5):857–860. doi:10.7860/JCDR/2013/5726.2958
58. Benoit SR, Fleming R, Philis-Tsimikas A, Ji M. Predictors of glycemic control among patients with type 2 diabetes: a longitudinal study. BMC Public Health. 2005;17(5):36. doi:10.1186/1471-2458-5-36
59. Alramadan Mohammed J, Magliano Dianna J, Almigbal Turky H, et al. Glycemic control for people with type 2 diabetes in Saudi Arabia – an urgent need for a review of management plan. BMC Endocr Disord. 2018;18:62.
60. Peng K, Chen G, Liu C, et al. Association between smoking and glycemic control in diabetic patients: results from the risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2018;10:408–418. doi:10.1111/1753-0407.12625
61. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi:10.1001/jama.298.22.2654
62. Ohkuma T, Iwase M, Fujii H, et al. Dose- and time-dependent association of smoking and its cessation with glycemic control and insulin resistance in male patients with type 2 diabetes mellitus: the fukuoka diabetes registry. PLoS One. 2015;10(3):e0122023. doi:10.1371/journal.pone.0122023
63. Barrett-Connor E, Khaw KT. Cigarette smoking and increased central adiposity. Ann Intern Med. 1989;111:783–787. doi:10.7326/0003-4819-111-10-783
64. Dagogo-Jack S, Results DCCT. Diabetes care in developing countries. Diabetes Care. 1995;18:416–417. doi:10.2337/diacare.18.3.416
65. King H, Aubert RE, Herman WH. Global burden of diabetes prevalence, numerical estimates and projections. Diabetes Care. 1998;21:1414–1431.
66. Yudkin JS. Insulin for the World’s Poorest Countries. Lancet. 2000;355:919–921. doi:10.1016/S0140-6736(99)09225-9
67. Amoah AG, Owusu SK, Saunders JT, et al. Resources for diabetes care at regional health facilities in Southern Ghana. Diabetes Res Clin Pract. 1998;42:123–130. doi:10.1016/S0168-8227(98)00101-6
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
