Back to Journals » International Journal of Nanomedicine » Volume 17
Cell Sheet Technology as an Engineering-Based Approach to Bone Regeneration
Authors You Q, Lu M, Li Z, Zhou Y, Tu C
Received 12 July 2022
Accepted for publication 12 November 2022
Published 20 December 2022 Volume 2022:17 Pages 6491—6511
DOI https://doi.org/10.2147/IJN.S382115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Qi You,1,2,* Minxun Lu,1,2,* Zhuangzhuang Li,1,2 Yong Zhou,1,2 Chongqi Tu1,2
1Orthopedic Research Institute, Department of Orthopedics, West China Hospital, Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 2Sichuan Model Worker and Craftsman Talent Innovation Research Studio, Chengdu, Sichuan Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chongqi Tu; Yong Zhou, Department of Orthopedics, West China Hospital, Sichuan University, No. 37, Guoxuexiang, Chengdu, 610041, Sichuan Province, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Bone defects that are congenital or the result of infection, malignancy, or trauma represent a challenge to the global healthcare system. To address this issue, multiple research groups have been developing novel cell sheet technology (CST)-based approaches to promote bone regeneration. These methods hold promise for use in regenerative medicine because they preserve cell-cell contacts, cell-extracellular matrix interactions, and the protein makeup of cell membranes. This review introduces the concept and preparation system of the cell sheet (CS), explores the application of CST in bone regeneration, highlights the current states of the bone regeneration via CST, and offers perspectives on the challenges and future research direction of translating current knowledge from the lab to the clinic.
Keywords: bone defect, cell sheet technology, bone regeneration, bone tissue engineering
Introduction
Bone defects that are congenital or that arise as a consequence of trauma, infection, or cancer have been shown to severely impair patient well-being, yet they are relatively common clinical entities. Approaches that can effectively regenerate or fix these problems are still major medical and economic challenges. Current regenerative strategies rely on the use of autografts,1 allografts,2 combinations of autografts and allografts,3,4 xenografts,5,6 and synthetic grafts7 to facilitate bone reconstruction, but each of these approaches is associated with specific advantages and limitations. Bone tissue engineering strategies have developed substantially over the last two decades, highlighting promising new avenues for regenerative research and the clinical translation of resultant findings.8,9 The majority of conventional tissue engineering techniques involve injecting cell suspensions or transplanting scaffolds that have been seeded with the relevant cells.10,11 However, these delivery strategies generally result in suboptimal cellular survival and limited engraftment, and the injection of cells alone fails to provide the requisite structural support,12 precluding efforts to appropriately regenerate bone tissue morphology and function. Optimal scaffold materials that are biodegradable, exhibit appropriate mechanical properties, and can effectively promote the adhesion, proliferation, and extracellular matrix (ECM) secretory activity of cells have yet to be developed despite extensive research efforts.13 Existing scaffold materials are associated with a few limitations, including poor biological activity, irregular degradation, weak mechanical strength, and immunogenic properties.14,15 Moreover, tissue engineering approaches that utilize trypsin to harvest cells can destroy cell membrane proteins and interfere with interactions among cells or between cells and the ECM, contributing to impaired adhesion and proliferative activity. As a result, cell-seeded scaffolds often exhibit poorly regulate cell-material interactions and high rates of cell death.16
Cell sheet technology (CST) approaches have been developed to overcome many of the abovementioned hurdles to tissue engineering, leading to growing research interest in CST applications. CST can preserve cell-cell junctions, the ECM, and key proteins, including fibronectin, leukemia inhibitory factor receptor (LIFR), integrin-5, stromal cell-derived factor 1 (SDF-1), myosin heavy chain (MHC), vascular endothelial growth factor (VEGF), and β-actin by harvesting cells without the use of trypsin or other proteolytic enzymes.17,18 MHC and β-actin are cytoplasmic, although integrin-α5 and LIFR are membrane-bound. Both fibronectin and integrin α5 play roles in shaping cellular adhesion, while LIFR and MHC serve as differentiation indices, VEGF can promote angiogenic activity, and SDF-1 can recruit a range of progenitor and stem cells.19 Owing to these properties, these proteins are of key clinical importance in cell sheets. Forming cell-cell contacts and secreting ECM prepares cell sheets, they are not subject to scaffold material-related limitations, avoiding the potential for implantation-related inflammatory immune reactions, tissue collapse as a consequence of rapid degradation, or impaired tissue development in the context of slow scaffold degradation.20–23 Many studies to date have explored the value of cell sheets in the context of bone regeneration, demonstrating that these sheets can be used in a scaffold-free manner and that mesenchymal stem cell (MSC) differentiation into bone cells occurs more effectively within the confines of a cell sheet as compared to under monolayer growth conditions.24 To extend their versatility, cell sheets can also be combined with traditional scaffold materials more effectively than combining scaffold materials with free cell suspensions owing to the ability of cell sheets to preserve the ECM and cell-cell interactions.25
Herein, several strategies for preparing cell sheets (CS) and their relative advantages and disadvantages are addressed below. Furthermore, recent advancements in CST-based bone regeneration and the selection of cell sources for use in this therapeutic context were explored. Moreover, the key limitations of CST were reviewed. These are important research directions for the future.
Preparation of Cell Sheets
Several cell sheet preparation strategies have been pioneered to date, including magnetic, mechanical, pH-responsive, electro-responsive, photo-responsive, and temperature-responsive systems. A comprehensive description of the advantages and disadvantages of these approaches is provided in Table 1.
 |
Table 1 The Advantages and Disadvantages of Different Cell Sheet Preparation Systems |
Temperature-Responsive Systems
The initial method of preparing cell sheets was suggested using temperature-responsive systems, which is still considered an effective technique in this field. Through electron-beam irradiation, N-Isopropylacrylamide (NIPAAm) monomers can undergo polymerization and covalent grafting onto tissue culture dish surfaces. Cells can then attach to hydrophobic regions coated by dehydrated poly-NIPAAm (PNIPAAm) when cultured at 37°C. When the temperature of the culture plate is then decreased to 20°C, hydration of the grafted PNIPAAm results in a change in surface wettability and a shift from a hydrophobic to a hydrophilic local microenvironment such that cells detach from the culture surface (Figure 1).26 Several improved versions of this basic temperature-responsive system have been developed or proposed to date.
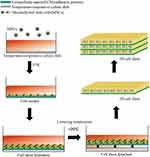 |
Figure 1 Schematic illustration of cell sheet formation and harvesting. |
Various biomolecules have been leveraged to accelerate cell sheet formation through the enhancement of proliferative and/or adhesive activity. For example, the synthetic Arg-Asp-Ser (RGDS) peptide has been immobilized on P(NIPAAm-co-CIPAAm) surfaces to promote enhanced cellular adhesion, thus expediting cell sheet formation.27 More recently, Other research groups have used a heparin-immobilized P(IPAAm-co-CIPAAm) surface to facilitate the binding of proteins such as fibroblast growth factor and heparin-binding epidermal growth factor-like growth factor. Fibroblast growth factor28 or epidermal growth factor29 binding to heparin-immobilized P(NIPAAm-co-CIPAAm) surfaces is sufficient to promote more rapid cellular growth while maintaining cellular activity, thus reducing the overall time needed to generate a confluent cell sheet. Insulin immobilization on these temperature-responsive culture surfaces can also induce rapid cell proliferation, hence accelerating the process.30 The average harvest period of a single CS, such as those formed utilizing human MSCs, human aortic smooth muscle cells, or human dermal fibroblasts, is known to be 7 days.31,32 According to a recently reported study, the application of bulk PNIPAAm substrate nanotopography substantially reduces the time required for cell sheet harvesting to just two days, while also enabling the sheet’s separation from the culture surface.33
To facilitate more rapid cell sheet detachment from culture surfaces, PNIPAAm has been grafted onto a porous membrane (PM) to yield the PNIPAAm-PM substrate, which can decrease the detachment time for prepared cell sheets from 75 min to 30 min relative to unmodified PNIPAAm following a temperature decrease to 20°C.34 To further decrease this cell sheet detachment time, poly(ethylene glycol) (PEG) was co-grafted with PNIPAAm onto the PM, leading to the development of the PNIPAAm(PEG)-PM substrate, which brought the time to 19 minutes after cooling to 20 °C35 Patel et al36 additionally developed thermo-responsive films consisting of a combination of PNIPAAm and 3-aminopropyltriethoxysilane (APTES) that was then deposited onto the surface of glass slides via a spin-coating approach, providing anchoring sites for cells to attach and proliferate. Modulation of the PNIPAAm to APTES ratio enabled these researchers to tune the cell sheet detachment time within the range of 2.5–40 min. More recently, some groups have leveraged thermosensitive Tetronic ®-based hydrogels to detach multiple cell sheets in response to size expansion induced by temperature decreases below 37°C, requiring over 15 min at 25°C but under 10 min at 4°C.37,38
It has also been investigated to manipulate cell sheets in a two-dimensional (2D) format using temperature-sensitive culture plates, with supporting membranes used for cell sheet harvesting often consisting of porous poly (ethylene terephthalate),39 and hydrophilically-modified poly (vinylidene difluoride).40 To facilitate 3D tissue development, plunger-based devices have been designed to aid in the process of cell sheet manipulation.41 This plunger allows for repeated cell sheet layering to generate thicker 3D myoblastic or cardiac tissues,42 hierarchically aligned microstructures,43 and capillary-like networks in 3D tissues.41 Commercial temperature-responsive culture dishes have been employed for cell sheet construction in the context of bone regeneration,44,45 but these systems are subject to two key limitations. Firstly, the required temperature drop can reduce the viability of sensitive cell types.34,46 Secondly, while commercial UpCell®-precoated temperature-responsive culture dishes are available, they are relatively expensive, limiting their more widespread use.17
Electro-Responsive Systems
To immobilize ligands on a gold surface, Yeo et al47 created an electro-responsive system based on electroactive self-assembled monolayers (SAMs). In this setup, the electroactive monolayer-tethered molecules are released upon application of an electrical potential to a gold film, resulting in the oxidation of the film. To create an electro-responsive platform, Inaba et al48 used a gold surface modified with a SAM comprised of alkanethiol and RGD peptides. Within 10 minutes of applying a −1.0 V electrical potential to this surface, the cell sheets that had grown there detached. To decrease the likelihood of harmful chemicals used in the preparation of this system remaining present within prepared cell sheets, which could potentially induce an inflammatory response in vivo, the gold surface was modified with an oligopeptide containing a central RGD adhesion peptide surrounded by terminal cysteine residues. Cell sheets were detached by a negative electrical potential.49 To facilitate cell sheet detachment during electrochemical polarization,50 polyelectrolyte-modified surfaces have also been used in the context of electro-responsive cell sheet preparation,51 Polyelectrolytes adsorb onto oppositely charged surfaces via electrostatic interactions. However, these polyelectrolyte-modified surfaces can introduce local alterations in pH attributable to polyelectrolyte electrochemical dissolution, and these may induce DNA damage or apoptotic cell death.52 A recently reported study has demonstrated the design of an electrochemically switchable approach to micropatterned heterotypic cell sheet preparation utilizing a system consisting of a combination of photolithographic processing and local polyelectrolyte electrochemical dissolution.51 To date, most studies have explored the use of electro-responsive systems to prepare cell sheets in the context of vascularized tissue generation, whereas no reports have specifically leveraged these techniques in the context of bone regeneration.54 The particular platforms and substrates necessary to design these electro-responsive systems may also represent a barrier to their more widespread application.13
Photo-Responsive Systems
Photo-responsive CST preparation strategies have recently emerged as a promising alternative to other techniques given that they do not leave any residues within the resultant cell sheets and they maintain the integrity of the ECM and associated cellular interactions,55,56 preserving cell viability in a non-invasive manner. Importantly, illumination is easily controlled, making this approach to cell sheet harvesting highly efficient and convenient.57 In 2013, researchers discovered that UV radiation might alter the wettability of titanium dioxide (TiO2), allowing for the detachment of cell sheets.58 Several recent studies have demonstrated that a range of surface materials and wavelengths of light can be combined to similarly achieve a robust photo-responsive system.
UV (365–366 nm)-Induced CST
The first study on using UV light (365 nm)58 to trigger CS detachment used quartz coated with TiO2 nanodot-coated quartz serving as a substrate to grow cells for 5 days. In response to subsequent UV (365 nm) irradiation for 20 min, an intact cell sheet that was viable, functional, and capable of reattaching to other surfaces was produced. Importantly, the cells in this sheet were also free of substantial oxidative DNA damage. More recently, many TiO2 film modification approaches have been developed. For example, Cheng et al59 revealed that cells were able to spontaneously detach from nanostructured anatase TiO2 film culture surfaces when exposed to UV (365 nm) irradiation, with such detachment being more rapid than that observed from dense films or nanodot surfaces, suggesting that film nanostructural characteristics are an important consideration in the preparation of cell sheets. Other inorganic compounds have also been incorporated into TiO2 films to improve cell sheet harvesting. In some studies, carbon quantum dots (CQDs) were added to TiO2 films, resulting in faster CS detachment in response to UV (365 nm) irradiation than CQD-free films.60 Other elements incorporated into these TiO2 films include SiO2, Zn, and Gr, resulting in the spontaneous and rapid detachment of functionally intact cell sheets.60–62 Moreover, TiO2 film surfaces decorated using organic materials have been utilized, as in the case of two studies demonstrating that TiO2 films with surface-immobilized RGD peptides can improve the adhesion of cells while allowing for rapid cell sheet detachment within 30 min upon UV (365 nm) irradiation.63,64 A 2017 study explored the use of recombinant human laminin-521 to modify TiO2 films and found that an intact cell sheet characterized by improved proliferation and adhesion could be attained using this strategy. Notably, the result cell sheet could readily reattach to other surfaces, making it well-suited to subsequent transplantation.65 Recently, researchers have leveraged a polydopamine/TiO2 film to construct a cell sheet that could be readily harvested and remained functionally intact.66,67 Researchers have developed light-responsive multilayer cell sheets using a TiO2 coating and photo-cross-linkable gelatin methacrylate. The micropatterning of this film was achieved via photomask-assisted UV254 illumination (Figure 2A), while cell sheet harvesting was achieved via UV (365 nm) irradiation (Figure 2B). This strategy was demonstrated to be effective as a means of stacking cell sheets in a multilayered manner.53 This new CST approach has facilitated the design of other versatile cell sheets as in the case of a pre-vascularized cell sheet amenable to subsequent transfer.68 In a recent report, One group of scientists recently published a paper detailing their efforts to create a silicon surface that responds to both light and temperature by employing mixed polymer brushes.69 The combination of light and temperature stimuli enhanced cell sheet collecting effectiveness when cells were cultivated on this surface and released in response to temperature decreases and UV (366 nm) irradiation. This strategy highlights a promising range of approaches that can be used to facilitate cell sheet collection in response to various stimuli.
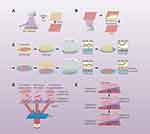 |
Figure 2 Schematic illustration of light-induced cell sheet (CS) preparation and harvest. (A) Ultraviolet 254 (UV254)-induced cell sheet patterning on titanium oxide (TiO2) nanodots film (TNF). (B) UV365-induced anisotropic cell sheet (ACS) detachment on TNF. (C) The procedure for reactive oxygen species (ROS) -induced CS transfer from Hp-PK film to fibrin gel, and then the stacking process under green light. (D) A schematic illustration for the harvest of multiple CSs by near-infrared (NIR) light. (E) A schematic illustration for the precisely directed CS detachment from the gradient photothermal surface. Notes: Reproduced from Liu C, Zhou Y, Sun M, et al. Light-induced cell alignment and harvest for anisotropic cell sheet technology. ACS Appl Mater Interfaces. 2017;9(42):36513–36524. Copyright 2017, American Chemistry Society. 67 Reproduced from Koo MA, Hee Hong S, Hee Lee M, et al. Effective stacking and transplantation of stem cell sheets using exogenous ROS-producing film for accelerated wound healing. Acta Biomater. 2019;95:418–426. Copyright 2019, with permission from Elsevier.72 Reproduced from Na JH, Seok J, Han M, Lim H, Kim HO, Kim E. Harvesting of living cell sheets by the dynamic generation of diffractive photothermal pattern on PEDOT. Adv Funct Mater. 2017;27:10. Copyright 2017, John Wiley and Sons.73 |
Visible Light (400–800) Induced CST
Wang et al55 originally documented the fabrication of cell sheets utilizing a visible light-responsive technique, in which the sheets were detached from p/n junction-containing silicon wafer substrates (Si(p/n)) in response to illumination with visible light. Another study has reported a potent ROS-responsive cell sheet preparation strategy using a haematoporphyrin-incorporated polyketone (Hp-Pk) film that generates ROS in response to green light (510 nm).70 This technique allows for the efficient spatiotemporal regulation of cellular detachment.71 In more recent studies, researchers have achieved success in preparing a three-layered cell sheet via this approach that was subsequently used to repair wound defects in nude mice (Figure 2C).85 While this strategy can effectively release cell sheets for downstream utilization, the high levels of ROS that are generated have the potential to harm cells and damage DNA, inducing apoptotic cell death as the duration or intensity of exposure is increased.71
NIR Light (808 nm)-Induced CST
Na et al99 constructed cell sheets using a near-infrared (NIR) light-based approach that is promising owing to the ability of NIR light to penetrate tissues while remaining safer than visible or ultraviolet light at a given intensity level (Figure 2D). These researchers were able to separate cell sheets in 5 minutes owing to the poly(3,4-ethylenedioxythiophene) (PEDOT) substrate’s strong photothermal efficiency in response to NIR light diffraction through a micropatterned optical lens. More recently, a PEDOT substrate prepared with a thickness gradient via electrodeposition was utilized to facilitate cell sheet preparation. As the temperature on the thicker side of this substrate rose more rapidly in response to uniform NIR irradiation, collagen dissociation occurred more rapidly such that cell sheets detached in a controlled manner along the thickness gradient72 (Figure 2E).
Mechanisms of Light-Induced CST
The formation and separation of light-induced CS have related to the following factors:1) Surface wettability changes under light illumination. There is evidence that some materials (such as organic material substrates and TiO2) change their hydrophobic to hydrophilic properties when exposed to light.73,74 Hydrophilic surfaces are generated when oxygen vacancies are created by UV irradiation, changing the Ti4+ sites to Ti3+ sites that interact with water molecules in the surrounding solution or air.75 Once the oxygen vacancies are filled by water, terminal hydroxyl groups (TiOHT) are generated, which can react with the amino groups (-NH3+) of surface-bound proteins to cause a change in the shape of the proteins.76 Once the CS has detached, the release of external sticky proteins may be monitored. Second, the surface accumulates electrons as a result of exposure to light. Cell adhesion is enhanced on positively charged surfaces because the cell membrane is negatively charged. TiO2 is excited by UV light to generate electron-hole (e-/h+) pairs, which are then efficiently separated and transferred to create a surface with varying potentials.60 The negative potential of the film surface may be augmented by the buildup of electrons thereon. The CSs detached the film surfaces because of the light-triggered, negative charge.77 Surface charge is regulated by surface voltage and potential78 and it has the potential to change the conformation of sticky proteins79 and their release. Protein conformational changes (from -helix to -sheet transition) have been linked to a negative charge and reactive oxygen species (ROS) in UV- and visible-light-induced CS detachment, leading to protein release and CS detachment.80 3) it might cause a structural shift in collagens. Adsorbed collagen molecules are heated locally as the photothermal effects heat the PEDOT surface rather than the cell medium. As the temperature rises, collagen loses its water content, and the triple helices unfurl into a polypeptide chain in a random-coil form. As a result of this structural modification, collagens disintegrate and dissociate into the medium.81 It follows that the CSs and their intermediates break apart. Specifically, the additive effects of surface characteristics82 can be used to control cell adhesion and detachment. When light is introduced, each of the aforementioned processes works together and happens simultaneously. To put it simply, CSs will detach from surfaces on their own under these conditions.
Given that light-mediated cell sheet harvesting is a relatively recent technique, it has primarily been utilized in the context of cutaneous wound healing and osseointegration to date.70,83 While these experiments have yielded promising outcomes, further work is necessary to optimize this technique given that prolonged NIR irradiation can adversely impact the viability and activity of cells within the resultant cell sheet,72 with ROS generated in the context of resultant cell sheet detachment having the potential to damage the cell membrane.84 Clear standards regarding the biosafety of light in these photo-responsive systems are currently lacking.
Magnetic Systems
Ito et al87 were the first to discuss magnetic techniques for the fabrication of cell sheets. The magnetic attraction was used to construct multilayered MCL-labeled cell sheets in an ultralow-attachment plate after cells took up positively charged magnetite cationic liposomes (MCLs). When the magnetic field was removed, these cell sheets were readily harvested using a magnet. More recently, researchers proposed the utilization of RGD peptide-conjugated MCLs (RGD-MCLs) to facilitate magnet-based cell sheet preparation, with RGD-MCLs facilitating robust cellular adhesion while retaining the ability for cell sheets to be harvested when the magnetic field was removed.88 Fe3O4 magnetic nanoparticles (MNPs) coated with nanoscale graphene oxide (nGO@Fe3O4) have also been proposed for use in the context of cell sheet generation, with cells that take up these particles being highly amenable to multilayered cell sheet preparation in a system in which the thickness of the cell sheet could be regulated via repeated cell addition.89 Several cell sheet types have been reportedly prepared using magnet-based approaches, including sheets consisting of MSCs, hepatocytes, endothelial cells, and cardiomyocytes86,87,90 (Figure 3). While magnetic nanoparticles have shown great promise as an efficient, inexpensive, and straightforward approach to cell sheet preparation, they do not allow for the harvesting of unmodified cell sheets. In addition, the resultant cell sheets do not detach in the form of cellular monolayers, instead forming cellular aggregate clumps.71,86
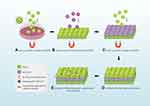 |
Figure 3 Schematic illustration of the fabricated 3D vascularized heterotypic cell sheet by magnetic responsive system. MSCs, mesenchymal stem cells. MNPs, magnetite nanoparticles. HUVECs, human umbilical vein endothelial cells. Notes: Reproduced from Silva AS, Santos LF, Mendes MC, et al. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials. 2020;231:119664. Copyright 2020, with permission from Elsevier.89 |
pH-Responsive Systems
Guillaume-Gentil et al91 suggested a pH-responsive culturing technique for the manufacture of cell sheets. Layer-by-layer deposition of cationic poly (allylamine hydrochloride) and anionic poly (styrene sulfonate) onto conductive electrodes made of indium tin oxide was used in this method, and cells were then seeded onto the resulting surface. When the pH was decreased to 4.0, an intact cell sheet monolayer could readily be obtained. While this technique was effective as a means of facilitating cell sheet harvesting, it induced unavoidable cellular damage owing to the pH-sensitive nature of cells.92 Separation of cells in response to pH shifts was facilitated by Chen et al93 using a novel chitosan-based method. In this case, chitosan was used for cell culture. Cell adhesion to the chitosan substrate was facilitated by the release of fibronectin from these cells, which was adsorbed on the substrate when the pH of the culture fluid was maintained at 7.2. However, an increase in the culture media pH to 7.65 resulted in chitosan surface deprotonation, yielding this surface a positive charge that leads to fibronectin desorption and cellular detachment.94 This strategy, however, has not yet been used to successfully promote complete cell sheet detachment. These pH-responsive systems have only been evaluated in a handful of studies to date, primarily owing to the limited pH range (6.8–7.4) required for normal cellular function.
Mechanical Systems
Mechanical systems offer a means of preparing cell sheets without the need for particular culture substrates or techniques, but these systems can require difficult-to-implement manipulation strategies. There have been relatively few studies specifically exploring simple mechanical cell sheet preparation techniques, yet these techniques are often used in the context of CST applications. At the most basic level, cells grown in an appropriate cell sheet induction medium for days or weeks can be detached using a cell scraper and forceps following the formation of a viable cell sheet.95 In one report, Imashiro et al17 utilized ultrasonic vibration to detach cell sheets from standard cell culture vessels without adversely impacting cellular viability (Figure 4). Alternatively, some research groups have utilized cell sheet induction medium containing gelatin to produce cell sheets that were more proliferative and associated with a robust ECM, yielding stronger, thicker sheets that could be more easily collected.96 To date, cell sheets prepared using this strategy have frequently been employed in the context of bone and cartilage regeneration.97,98,100
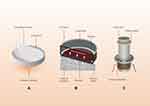 |
Figure 4 Cell sheet (CS)-detaching process by ultrasonic vibration. (A) Schematic illustration of CS detachment. (B) The CS was detached from the bottom of the dish by ultrasonic vibration. (C) CS-detaching system in an incubator. Notes: Adapted from Imashiro C, Hirano M, Morikura T, et al. Detachment of cell sheets from clinically ubiquitous cell culture vessels by ultrasonic vibration. Sci Rep. 2020;10(1):9468. Copyright © 2020, The Author(s), Creative Commons CC BY license.17 |
To improve cell sheet maneuverability when utilizing a mechanical approach, amniotic membranes (AMs), which consist of a thin tissue layer that covers the outermost placental surface, can be used as a cell culture substrate. Given that AMs are often discarded after delivery, there are no major ethical concerns regarding their use, and they are easily accessible. Importantly, AMs exhibit antifibrotic, antiangiogenic, and antimicrobial properties together with acceptable mechanical properties. In addition, AMs are largely not immunogenic and suppress pain and inflammation, making them well-suited to use as a tissue culture scaffold.101–103 Nam et al104 seeded canine corneal epithelial cells on canine AMs, leading to successful corneal epithelial sheet preparation. More recently, human periodontal ligament-derived cells (PDLCs) sheets and human dental pulp stem cells (DPSCs) sheets have been generated on AM surfaced by different research teams.105,106 Lindenmair et al107 successfully achieved intact human AM (hAM)-mediated osteodifferentiation in vitro and patented their approach.108 Mohr et al109 combined chorionic membrane-derived cells and hAM to improve the osteogenic differentiation of these cells, while Starecki et al110 combined autologous bone and AMs to successfully repair critical femoral bone defects in rats. Takizawa et al111 subcutaneously implanted AM-associated DPSC sheets into nude mice’s maxillary bone defect sites, and within 4 weeks, they observed mineralization and bone defect regeneration. Importantly, AM-cell sheet composites have been explored in clinical contexts by Amemiya et al,112 who cultured autologous oral mucosal epithelial cell sheets on AM substrate surfaces prior to use for the intraoral repair of mucosal defects in 5 patients. Following transplantation, these patients did not exhibit any signs of rejection, bleeding, infection, or sheet detachment, and new oral mucous membrane tissue ultimately developed at the treated site.
The Application of CST to Promote Bone Regeneration
The Use of Cell Sheets Alone
The use of CST to hasten bone healing, alleviate chronic pain and infection, and lessen the risk of immunologic responses associated with fresh frozen allogeneic bone transplants has gained popularity in recent years (Table 2).23 Rapid advances in the CST field have led to the application of the techniques discussed above in the context of bone tissue regeneration (Figure 5). For example, in 2008 Akahane et al113 subcutaneously transplanted osteogenic cell sheets in rats, and found that after 6 weeks these transplanted sheets exhibited evidence of new bone formation characterized by the presence of osteocytes, a mineralized matrix, and an osteoblast lining. In a separate report, researchers implanted layered cell sheets prepared from rat dental pulp (DP) cells into the subrenal capsule of nude mice, and observed new bone development at 8 weeks post-implantation.114 Ueyama et al115 further performed the transplantation of osteogenic bone marrow stem cell (BMSC) sheets into maxillofacial bone defects in rats, thereby promoting extensive new bone development in the implanted region at 8 weeks post-surgery. A number of studies have also highlighted the beneficial effects of osteogenic cell sheet application in the context of delayed bone union or nonunion, which is a complex process that can be shaped by a range of mechanical and/or biological factors.116–118
 |
Table 2 The Application of Cell Sheet Technology in Bone Regeneration in vivo |
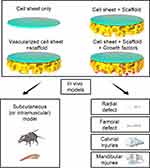 |
Figure 5 The types of constructions and in vivo models used in bone regeneration. |
The Use of Cell Sheets in Combination with Scaffolds
While cell sheet formation is primarily dependent upon interactions among cells and a robust ECM, these sheets lack any intrinsic mechanical strength, limiting their utility in the context of bone defect repair. However, combining these cell sheets with scaffolds can provide the requisite spatial and mechanical strength to make such repair strategies significantly more feasible. In addition, a novel osteoinductive material is also necessary to enhance bone regenerative outcomes.119–122 For example, Akahane et al113 subcutaneously implanted rats with hydroxyapatite (HA) scaffolds wrapped in osteogenic cell sheets, with subsequent histological analyses revealing that new bone growth was detectable at 4 weeks post-implantation within HA pores. In their study, Ueha et al123 utilized BMSC-containing beta-tricalcium phosphate (β-TCP) scaffolds wrapped in osteogenic BMSC sheets that were subcutaneously implanted, thereby promoting new bone formation within femoral bone defects. Moreover, Zhang et al124 leveraged biphasic tricalcium phosphate (BCP) ceramics wrapped with gold nanoparticle (AuNP)-treated PDLSC sheets to prepare a composite material that was then subcutaneously implanted into tissue pockets in nude mice, with the incorporated AuNPs significantly expediting ectopic bone growth in this model system at 8 weeks post-implantation.
As an alternative to ceramic scaffolds, polymeric scaffolds combined with cell sheets have been explored as tools to promote bone regeneration. Xie et al45 for example, generated composites consisting of BMCs contained within porous poly(sebacoyl diglyceride) (PSeD) that were wrapped using human ethmoid sinus mucosal-derived MSC (hESMSC) sheets, and they found that these composites effectively promoted new bone growth at 8 weeks post-implantation into rat calvarial defects (8 mm). Poly lactic-co-glycolic acid (PLGA) scaffolds wrapped using osteogenic cell sheets have also been utilized to promote new bone growth in the context of canine mandibular bone defects,125 while Zhao et al126 seeded PDLSCs on a polycaprolactone (PCL)-simvastatin (SIM) membrane scaffold, with the resultant cell sheet-scaffold construct being subcutaneously implanted into nude athymic mice, resulting in enhanced ectopic mineralization at 8 weeks post-implantation. Calcined bovine bones and allografts are only two examples of natural bone-derived scaffolds that have been utilized to promote faster bone regeneration.127,128 In their recent study, Yu et al129 transplanted angle-ply collagen membrane-supported cell sheets into murine calvarial defects, and found that these sheets were associated with more bone formation than that observed in other treatment groups at both 4 and 8 weeks post-implantation.
Combined Use of Growth Factors and Cell Sheets
Growth factors are important mediators of bone regeneration and have been combined with cell sheets to improve regenerative outcomes in several recent reports. For example, Qi et al130 observed significant increases in bone formation at 4 and 8 weeks post-surgery in a rabbit ulnar segmental defect model system when animals were implanted with recombinant human bone morphogenetic protein-2 (rhBMP-2)-loaded calcium sulfate wrapped in BMSC sheets as compared to the outcomes observed for rabbits treated with cell sheets or rhBMP-2/CS alone, consistent with the ability of the utilized bioactive microparticles to facilitate sustained BMP-2 delivery. In another study, Dang et al131 detected improved bone defect healing in a rat calvarial bone defect model when human BMSC sheets were combined with such microparticles. Moreover, Chen et al132 found that the transplantation of MSC sheets in combination with local stromal cell-derived factor-1 (SDF-1) injection was associated with accelerated bone tissue healing. Similarly, Hu et al133 generated a complex formed from concentrated growth factor (CGF) and adipose-derived stem cell (ADSC) sheets, with the resultant complex being used to repair rat skull defects, effectively promoting new bone formation.
The Importance of Vascularized Cell Sheets in Bone Regeneration Applications
Pre-vascularization is critical to ensuring that engineered bone tissue remains viable and successfully integrates with host bone tissue following implantation. Osteogenic differentiated pre-vascularized MSC sheets preserve their immunomodulatory and microvascular characteristics upon implantation.134 In some recent studies, research teams have sought to enhance vascularization in tissue-engineered bone. For example, Panduwawala et al135 employed cell sheets consisting of human PDLC and human umbilical vein endothelial cells (HUVEC) sheets wrapped within human tooth roots and subcutaneously implanted within immunodeficient mice, leading to robust bone and vascular lumen development at 8 weeks post-implantation. In a separate report, multilayered cell sheets consisting of human ADSC and HUVEC sheets were implanted in a chick embryo model system, revealing that human vascular structures were preserved and human cells were capable of migrating and integrating with the chick vasculature within 3 weeks post-implantation.86 Xu et al136 seeded an undifferentiated BMSC cell sheet with BMSC-derived endothelial cells (ECs) to create a pre-vascularized cell sheet; they then implanted this sheet and an osteogenic BMSC sheet into rat calvarial defects, where the pre-vascularized group showed superior bone tissue formation and a greater number of functional perfused blood vessels compared to the control group. To further investigate the synergistic promotion of angiogenesis and osteogenesis in a subcutaneous heterotopic transplantation experiment, Zhang et al137 generated a double-cell sheet complex consisting of a combination of osteogenic and vascular endothelial cell sheets and combined this complex with coral HA. In another novel report, researchers encircled a porous β-TCP scaffold with an arteriovenous loop via insertion onto the lateral groove, with the resultant complex being wrapped in a BMSC sheet before transplantation into a rabbit thigh muscle pocket, leading to the accelerated development of vascular and bone tissue.138
A thin membrane called the periosteum covers the surface of bone tissue and serves a critical function in controlling the growth and repair of bone.13 Through the deposition of HUVECs on undifferentiated hMSC sheets, Kang et al95 were able to recreate the fibrous layer of native periosteal tissue, and the osteogenic periosteal tissue layer was achieved through the activation of osteogenesis in hMSC sheets. When these cell sheets were combined and then wrapped in a porous β-TCP scaffold, the authors were able to create a biomimetic periosteum that could be effectively implanted subcutaneously in nude mice, whereupon it was sufficient to drive angiogenic activity and anastomose with the local host vasculature and to promote osteogenic activity. Zhang et al.139 Similarly, these biomimetic periosteal scaffolds were shown to stimulate faster bone and vascular formation when they were constructed from rat BMSCs, wrapped in a porous β-TCP scaffold, and transplanted into calvarial defects in rats.
Cells Type Used for the Generation of Cell Sheets
Since its initial emergence in the 1980s–1990s,23 As time has progressed, tissue engineering has matured, and critical success criteria have been identified for various tissue engineering strategies, including sufficient blood supply, an appropriate number of progenitor cells, appropriate quantities of signals necessary to induce the differentiation of those cells in the appropriate order, and an appropriate ECM or scaffold capable of supporting engineered tissue development.140 Therefore, the success of tissue engineering aimed at bone regeneration relies heavily on the selection of seed cells with strong osteogenic capacity. A range of cell types has been used to develop cell sheets for use in the context of bone regeneration, including BMSCs,141 ADSCs,133 hESMSCs,45 dental follicle cells,142 PDLCs,143 DPSCs,44 and genetically modified cells.144 BMSCs exhibit greater osteogenic potential than ADSCs,145 while DPSCs are thought to exhibit greater proliferative and clonogenic potential than BMSCs.44 Human amniotic MSCs (hAMSCs) and human umbilical cord MSCs (HUCMSCs) have also been previously leveraged to promote osteochondral defect repair.100,146 Currently, hAMSCs have attracted substantial research interest owing to their higher yields and superior osteogenic potential relative to ADSCs,147 with these cells offering similar advantages over hBMSCs.148 Moreover, hAMSCs exhibit greater immunosuppressive activity relative to HUCMSCs.149 Importantly, Since hAMSCs are produced from amniotic membranes, which are often discarded as medical waste, hAMSC-based therapy does not raise the same ethical difficulties as other kinds of stem cell-based treatment.100 To date, hAMSCs have been utilized to treat diabetes.150 Due to their impressive proliferative capacity, multipotency, immunomodulatory capabilities, and powerful paracrine effects, urine-derived stem cells (USCs) have lately attracted substantial attention in the context of employing them in cell-based treatments.151 For instance, Guan et al,152 developed a construct comprised of USCs and a β-TCP scaffold that successfully promoted new bone formation when implanted into femoral segmental bone defects. Xing et al153 have also reported similar findings. Genetically modified cell sheets have also been explored as potential tools for use in the context of bone regeneration,144,154–156 although this strategy remains to be tested in human patients in a clinical setting, and further efforts are necessary to optimize this approach through the design of novel gene transfection approaches, efforts to control and prolong transgene expression, and other safety-related improvements.157
Clinical Applications and Challenges
However, several therapeutic applications have lately been documented in the context of regeneration of cornea,158 lungs,159 heart,160,161 esophagi,162,163 middle ears,164,165 periodontal tissue,143 blood vessels,166 skin,167 and knee cartilage168,169 regeneration (Table 3). At present, the use of CST has shown great promise as a method for fostering effective bone tissue repair. Despite major advances in this field, the therapeutic potential of this technology has yet to be completely exploited since numerous issues and constraints remain unsolved. For one, CST is limited by the immunogenicity and viability of cell sheets following implantation, necessitating efforts to identify additional cell types with satisfactory osteogenic characteristics and to leverage autologous cell sources wherever possible to mitigate potential immune reactivity. The possibility of in vitro cell multiplication, with its accompanying danger of contamination or adverse cell alterations, is another major obstacle to the clinical implementation of CST. The cells used for cell sheet production must be free of bacteria, mycoplasma, viruses, or endotoxin in order to be safely utilized. As such, cell sheet production requires formally defined processes and highly skilled operators at present. Notably, it is difficult to regenerate bone tissue with CST because of the lack of a reliable blood supply. In this view, CST-based initiatives to produce vascularized 3D tissues are an effective way of studying this field. Lastly, cell sheets generally lack durable mechanical properties, and the ECM and cellular components within these sheets differ significantly from those in native bone tissue. The use of cell sheets in isolation to recapitulate bone tissue may thus be challenging. To overcome these challenges, further improvements to existing CST preparation systems and/or new systems will be essential. Moreover, research focused on the development of a novel osteoinductive material is also warranted to enhance these regenerative outcomes.
 |
Table 3 Clinical Applications of the Cell Sheet Technology |
Conclusions
The use of CST has shown great promise as a method for promoting effective bone tissue repair. There are several options for creating CSs. Preparing CSs for bone regeneration is most commonly done through temperature-responsive and mechanical approaches. This is likely because temperature-responsive systems are the most traditional system and mechanical approaches are simple because they do not require any special culture substrates or techniques. Multilayered CSs are also manufactured via a magnetic method, with the form being carefully controlled by varying the amount of magnets or the magnet pattern. There have been significant advancements in this area, but many uncertainties and limits remain before the therapeutic promise of this technology can be completely realized. Complicated structures and morphologies in hard tissue are challenging to recreate with CSs alone. Improving current preparation methods or proposing a new, effective preparation system is necessary to answer these problems. To further improve these regeneration results, research into the creation of a new osteoinductive substance is required.
Author Contributions
Qi You and Minxun Lu contributed equally to this work and wrote the manuscript. Qi You, Minxun Lu and Zhuangzhuang Li participated in searching for literature and edited the paper. Chongqi Tu and Yong Zhou contributed to the study design and critically reviewed the manuscript.
Funding
The work was supported by the Science and Technology Research Program of Sichuan Province (2020YFS0036), QingDao research institutes of SiChuan University, Research of biomedical materials and 3D printing related products (20GZ30301), China postdoctoral Science Foundation (2021M702342) and 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18017).
Disclosure
There is no conflict of interest.
References
1. Houdek MT, Wagner ER, Bishop AT, et al. Complications and long-term outcomes of free fibula reconstruction following resection of a malignant tumor in the extremities. Plast Reconstr Surg. 2017;139(2):510e–519e. doi:10.1097/PRS.0000000000003004
2. Aponte-Tinao LA, Albergo JI, Ayerza MA, et al. What are the complications of allograft reconstructions for sarcoma resection in children younger than 10 years at long-term followup? Clin Orthop Relat Res. 2018;476(3):548–555. doi:10.1007/s11999.0000000000000055
3. Manfrini CEM. Microsurgical reconstruction with vascularized fibula and massive bone allograft for bone tumors. Eur J Orthop Surg Traumatol. 2019;29:307–311.
4. Muscolo DL. Does the addition of a vascularized fibula improve the results of a massive bone allograft alone for intercalary femur reconstruction of malignant bone tumors in children? Clin Orthop Relat Res. 2021;479:1309–1310. doi:10.1097/CORR.0000000000001701
5. Ghanaati S, Barbeck M, Booms P, et al. Potential lack of “standardized” processing techniques for production of allogeneic and xenogeneic bone blocks for application in humans. Acta Biomater. 2014;10(8):3557–3562. doi:10.1016/j.actbio.2014.04.017
6. Kubosch EJ, Bernstein A, Wolf L, et al. Clinical trial and in-vitro study comparing the efficacy of treating bony lesions with allografts versus synthetic or highly-processed xenogeneic bone grafts. BMC Musculoskelet Disord. 2016;17:77. doi:10.1186/s12891-016-0930-1
7. Buser Z, Brodke DS, Youssef JA, et al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neurosurg Spine. 2016;25(4):509–516. doi:10.3171/2016.1.SPINE151005
8. Shahabipour F, Ashammakhi N, Oskuee RK, et al. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl Res. 2020;216:57–76. doi:10.1016/j.trsl.2019.08.010
9. Perez JR, Kouroupis D, Li DJ, et al. Tissue engineering and cell-based therapies for fractures and bone defects. Front Bioeng Biotechnol. 2018;6:105. doi:10.3389/fbioe.2018.00105
10. Hidalgo-Bastida LA, Cartmell SH. Mesenchymal stem cells, osteoblasts and extracellular matrix proteins: enhancing cell adhesion and differentiation for bone tissue engineering. Tissue Eng Part B Rev. 2010;16(4):405–412. doi:10.1089/ten.teb.2009.0714
11. Gao C, Peng S, Feng P, et al. Bone biomaterials and interactions with stem cells. Bone Res. 2017;5:17059. doi:10.1038/boneres.2017.59
12. Tang Y, Tong X, Conrad B, et al. Injectable and in situ crosslinkable gelatin microribbon hydrogels for stem cell delivery and bone regeneration in vivo. Theranostics. 2020;10(13):6035–6047. doi:10.7150/thno.41096
13. Lu Y, Zhang W, Wang J, Yang G. Recent advances in cell sheet technology for bone and cartilage regeneration: from preparation to application. Int J Oral Sci. 2019;11(2). doi:10.1038/s41368-019-0050-5
14. Zhao L, Burguera EF, Xu HH, et al. Fatigue and human umbilical cord stem cell seeding characteristics of calcium phosphate-chitosan-biodegradable fiber scaffolds. Biomaterials. 2010;31(5):840–847. doi:10.1016/j.biomaterials.2009.09.106
15. Castro AGB, Polini A, Azami Z, et al. Incorporation of PLLA micro-fillers for mechanical reinforcement of calcium-phosphate cement. J Mech Behav Biomed Mater. 2017;71:286–294. doi:10.1016/j.jmbbm.2017.03.027
16. Yang J, Yamato M, Kohno C, et al. Cell sheet engineering: recreating tissues without biodegradable scaffolds. Biomaterials. 2005;26(33):6415–6422. doi:10.1016/j.biomaterials.2005.04.061
17. Imashiro C, Hirano M, Morikura T, et al. Detachment of cell sheets from clinically ubiquitous cell culture vessels by ultrasonic vibration. Sci Rep. 2020;10(1):9468. doi:10.1038/s41598-020-66375-1
18. Kurashina Y, Hirano M, Imashiro C, et al. Enzyme-free cell detachment mediated by resonance vibration with temperature modulation. Biotechnol Bioeng. 2017;114(10):2279–2288. doi:10.1002/bit.26361
19. Tadakuma K, Tanaka N, Haraguchi Y, et al. A device for the rapid transfer/transplantation of living cell sheets with the absence of cell damage. Biomaterials. 2013;34(36):9018–9025. doi:10.1016/j.biomaterials.2013.08.006
20. Chen H, Liu Y, Wang C, et al. Design and properties of biomimetic irregular scaffolds for bone tissue engineering. Comput Biol Med. 2021;130:104241. doi:10.1016/j.compbiomed.2021.104241
21. Roseti L, Parisi V, Petretta M, et al. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 2017;78:1246–1262. doi:10.1016/j.msec.2017.05.017
22. Kawecki F, Clafshenkel WP, Fortin M, Auger FA, Fradette J. Biomimetic Tissue‐engineered bone substitutes for maxillofacial and craniofacial repair: the potential of cell sheet technologies. Adv Healthcare Mater. 2018;7:1700919. doi:10.1002/adhm.201700919
23. Cagdas YA, Esat KA, Semih A, Lale STN, Cevik TA. A concise review on the use of mesenchymal stem cells in cell sheet-based tissue engineering with special emphasis on bone tissue regeneration. Stem Cells Int. 2017;2017. doi:10.1155/2017/2374161
24. Nimiritsky P, Novoseletskaya E, Eremichev R, et al. Self-organization provides cell fate commitment in MSC sheet condensed areas via ROCK-dependent mechanism. Biomedicines. 2021;9(9):1192. doi:10.3390/biomedicines9091192
25. Kim Y, Lee SH, Kang BJ, et al. Comparison of osteogenesis between adipose-derived mesenchymal stem cells and their sheets on Poly-ε-Caprolactone/β -tricalcium phosphate composite scaffolds in canine bone defects. Stem Cells Int. 2016;2016:8414715. doi:10.1155/2016/8414715
26. Tang Z, Okano T. Recent development of temperature-responsive surfaces and their application for cell sheet engineering. Regen Biomater. 2014;1(1):91–102. doi:10.1093/rb/rbu011
27. Mitsuhiro Ebara MY, Yamato M, Aoyagi T, Kikuchi A, Sakai K, Okano T. Immobilization of cell-adhesive peptides to temperature-responsive surfaces facilitates both serum-free cell adhesion and noninvasive cell harvest. Tissue Eng. 2004;10(7–8):1125–1135. doi:10.1089/ten.2004.10.1125
28. Arisaka Y, Kobayashi J, Yamato M, et al. Switching of cell growth/detachment on heparin-functionalized thermoresponsive surface for rapid cell sheet fabrication and manipulation. Biomaterials. 2013;34(17):4214–4222. doi:10.1016/j.biomaterials.2013.02.056
29. Arisaka Y, Kobayashi J, Ohashi K, et al. A heparin-modified thermoresponsive surface with heparin-binding epidermal growth factor-like growth factor for maintaining hepatic functions in vitro and harvesting hepatocyte sheets. Regen Ther. 2016;3:97–106. doi:10.1016/j.reth.2016.03.003
30. Hatakeyama H, Kikuchi A, Yamato M, et al. Influence of insulin immobilization to thermoresponsive culture surfaces on cell proliferation and thermally induced cell detachment. Biomaterials. 2005;26(25):5167–5176. doi:10.1016/j.biomaterials.2004.11.061
31. Hobo K, Shimizu T, Sekine H, et al. Therapeutic angiogenesis using tissue engineered human smooth muscle cell sheets. Arterioscler Thromb Vasc Biol. 2008;28(4):637–643. doi:10.1161/ATVBAHA.107.151829
32. Qian Z, Ross D, Jia W, et al. Bioactive polydimethylsiloxane surface for optimal human mesenchymal stem cell sheet culture. Bioact Mater. 2018;3(2):167–173. doi:10.1016/j.bioactmat.2018.01.005
33. Choi A, Yoon H, Han SJ, et al. Rapid harvesting of stem cell sheets by thermoresponsive bulk poly (N -isopropylacrylamide) (PNIPAAm) nanotopography. Biomater Sci. 2020;8(19):5260–5270. doi:10.1039/D0BM01338B
34. Hyeong kwon AK, Yamato M, Sakurai Y, Okano T, Okano T. Rapid cell sheet detachment from poly(N-isopropylacrylamide)-grafted porous cell culture membranes. J Biomed Mater Res. 2020;50(1):82–89. doi:10.1002/(SICI)1097-4636(200004)50:1<82::AID-JBM12>3.0.CO;2-7
35. Oh Hyeong Kwon AK, Yamato M, Okano T, Okano T. Accelerated cell sheet recovery by co-grafting of PEG with PIPAAm onto porous cell culture membranes. Biomaterials. 2003;2003(24):1223–1232. doi:10.1016/S0142-9612(02)00469-6
36. Patel NG, Cavicchia JP, Zhang G, et al. Rapid cell sheet detachment using spin-coated pNIPAAm films retained on surfaces by an aminopropyltriethoxysilane network. Acta Biomater. 2012;8(7):2559–2567. doi:10.1016/j.actbio.2012.03.031
37. Jun I, Lee YB, Choi YS, et al. Transfer stamping of human mesenchymal stem cell patches using thermally expandable hydrogels with tunable cell-adhesive properties. Biomaterials. 2015;54:44–54. doi:10.1016/j.biomaterials.2015.03.016
38. Lee YB, Shin YM, Kim EM, et al. Facile cell sheet harvest and translocation mediated by a thermally expandable hydrogel with controlled cell adhesion. Adv Healthcare Mater. 2016;5(18):2320–2324. doi:10.1002/adhm.201600210
39. Kikuchi A, Okuhara M, Karikusa F, et al. Two-dimensional manipulation of confluently cultured vascular endothelial cells using temperature-responsive poly(N-isopropylacrylamide)-grafted surfaces. J Biomater Sci Polym Ed. 1998;9(12):1331–1348. doi:10.1163/156856298X00424
40. Ohk MH, Yamato M, Kikuchi A, Okano T. Creation of designed shape cell sheets that are noninvasively harvested and moved onto another surface. Biomacromolecules. 2000;2000(1):377–381.
41. Sasagawa T, Shimizu T, Sekiya S, et al. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials. 2010;31(7):1646–1654. doi:10.1016/j.biomaterials.2009.11.036
42. Haraguchi Y, Shimizu T, Sasagawa T, et al. Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat Protoc. 2012;7(5):850–858. doi:10.1038/nprot.2012.027
43. Takahashi H, Shimizu T, Nakayama M, et al. The use of anisotropic cell sheets to control orientation during the self-organization of 3D muscle tissue. Biomaterials. 2013;34(30):7372–7380. doi:10.1016/j.biomaterials.2013.06.033
44. Fujii Y, Kawase-Koga Y, Hojo H, et al. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res Ther. 2018;9(1):24. doi:10.1186/s13287-018-0783-7
45. Xie Q, Wang Z, Huang Y, et al. Characterization of human ethmoid sinus mucosa derived mesenchymal stem cells (hESMSCs) and the application of hESMSCs cell sheets in bone regeneration. Biomaterials. 2015;66:67–82. doi:10.1016/j.biomaterials.2015.07.013
46. Giuliodori AM, Di Pietro F, Marzi S, et al. The cspA mRNA is a thermosensor that modulates translation of the cold-shock protein CspA. Mol Cell. 2010;37(1):21–33. doi:10.1016/j.molcel.2009.11.033
47. Yeo WS, Hodneland CD, Mrksich M. Electroactive monolayer substrates that selectively release adherent cells. Chembiochem. 2001;2(7):590–593. doi:10.1002/1439-7633(20010803)2:7/8<590::AID-CBIC590>3.0.CO;2-D
48. Inaba R, Khademhosseini A, Suzuki H, et al. Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials. 2009;30(21):3573–3579. doi:10.1016/j.biomaterials.2009.03.045
49. Seto Y, Inaba R, Okuyama T, et al. Engineering of capillary-like structures in tissue constructs by electrochemical detachment of cells. Biomaterials. 2010;31(8):2209–2215. doi:10.1016/j.biomaterials.2009.11.104
50. Guillaume-Gentil O, Akiyama Y, Schuler M, et al. Polyelectrolyte coatings with a potential for electronic control and cell sheet engineering. Adv Mater. 2008;20(3):560–565. doi:10.1002/adma.200700758
51. Guillaume-Gentil O, Gabi M, Zenobi-Wong M, et al. Electrochemically switchable platform for the micro-patterning and release of heterotypic cell sheets. Biomed Microdevices. 2010;13(1):221–230. doi:10.1007/s10544-010-9487-1
52. T-kl HX, Yang J-M, Liu LF, Liu LF. Acidic pH induces topoisomerase II-mediated DNA damage. Proc Natl Acad Sci. 2003;100(9):5205–5210. doi:10.1073/pnas.0935978100
53. Liu C, Zhou Y, Sun M, et al. Light-induced cell alignment and harvest for anisotropic cell sheet technology. ACS Appl Mater Interfaces. 2017;9(42):36513–36524. doi:10.1021/acsami.7b07202
54. Mochizuki N, Kakegawa T, Osaki T, et al. Tissue engineering based on electrochemical desorption of an RGD-containing oligopeptide. J Tissue Eng Regen Med. 2013;7(3):236–243. doi:10.1002/term.519
55. Wang X, Yao C, Weng W, et al. Visible-Light-responsive surfaces for efficient, noninvasive cell sheet harvesting. ACS Appl Mater Interfaces. 2017;9(34):28250–28259. doi:10.1021/acsami.7b08868
56. Na J, Song SY, Kim JD, et al. Protein-engineered large area adipose-derived stem cell sheets for wound healing. Sci Rep. 2018;8(1):15869. doi:10.1038/s41598-018-34119-x
57. Cheng Z, Cheng K, Weng W. SiO2/TiO2 nanocomposite films on polystyrene for light-induced cell detachment application. ACS Appl Mater Interfaces. 2017;9(3):2130–2137. doi:10.1021/acsami.6b14182
58. Hong Y, Yu M, Weng W, et al. Light-induced cell detachment for cell sheet technology. Biomaterials. 2013;34(1):11–18. doi:10.1016/j.biomaterials.2012.09.043
59. Cheng K, Wan HP, Weng WJ. A facile approach to improve light induced cell sheet harvesting through nanostructure optimization. RSC Adv. 2015;5(108):88965–88972. doi:10.1039/C5RA17116D
60. Wang X, Cheng K, Weng W, et al. Light-Induced Cell-Sheet Harvest on TiO 2 Films Sensitized with Carbon Quantum Dots. ChemPlusChem. 2016;81(11):1166–1173. doi:10.1002/cplu.201600202
61. Yao LL, Weng WJ, Cheng K, Cheng K, Cheng K. TiO/ZnO composite nanodots films and their cellular responses. Sol-Gel Sci. 2018;86(2):459–467. doi:10.1007/s10971-018-4643-9
62. Long X, Yi Y, Wang X, et al. Gr/TiO2 films with light-controlled positive/negative charge for cell harvesting application. ACS Biomater Sci Eng. 2020;6(4):2020–2028. doi:10.1021/acsbiomaterials.9b01946
63. Yu M-L, Yu M-F, Zhu L-Q, et al. The effects of TiO2Nanodot films with RGD immobilization on light-induced cell sheet technology. Biomed Res Int. 2015;2015:1–10.
64. Cheng K, Wang T, Yu M, et al. Effects of RGD immobilization on light-induced cell sheet detachment from TiO2 nanodots films. Mater Sci Eng C. 2016;63:240–246. doi:10.1016/j.msec.2016.02.072
65. Jiang Z, Xi Y, Lai K, et al. Laminin-521 promotes rat bone marrow mesenchymal stem cell sheet formation on light-induced cell sheet technology. Biomed Res Int. 2017;2017:1–11.
66. Zhu Y, Cheng Z, Weng W, et al. A facile synthesis of polydopamine/TiO2 composite films for cell sheet harvest application. Colloids Surf B Biointerfaces. 2018;172:355–361. doi:10.1016/j.colsurfb.2018.08.058
67. Jiang Z, Zhu D, Yu K, et al. Recent advances in light-induced cell sheet technology. Acta Biomater. 2021;119:30–41. doi:10.1016/j.actbio.2020.10.044
68. Zhou Y, Dong L, Liu C, et al. Engineering prevascularized composite cell sheet by light-induced cell sheet technology. RSC Adv. 2017;7(52):32468–32477. doi:10.1039/C7RA05333A
69. Evci M, Aydin HM, Caykara T, Caykara T. Synthesis of temperature and light sensitive mixed polymer brushes via combination of surface-initiated PET–A- TRP and interface-mediated RAFT polymerization for cell sheet application. Appl Surf Sci. 2020;2020:145572. doi:10.1016/j.apsusc.2020.145572
70. Koo MA, Lee MH, Kwon BJ, et al. Exogenous ROS-induced cell sheet transfer based on hematoporphyrin-polyketone film via a one-step process. Biomaterials. 2018;161:47–56. doi:10.1016/j.biomaterials.2018.01.030
71. Koo MA, Lee MH, Park JC. Recent advances in ROS-responsive cell sheet techniques for tissue engineering. Int J Mol Sci. 2019;20:22. doi:10.3390/ijms20225656
72. Jing J, Chen S, Lu Q. Gradient photothermal field for precisely directing cell sheet detachment. Adv Biosyst. 2019;3(5):e1800334. doi:10.1002/adbi.201800334
73. Yu ML, Yu MF, Zhu LQ, et al. The effects of TiO 2 nanodot films with RGD immobilization on light-induced cell sheet technology. Biomed Res Int. 2015;2015:582359. doi:10.1155/2015/582359
74. Me A, At B, Hmab C, et al. Synthesis of temperature and light sensitive mixed polymer brushes via combination of surface-initiated PET–ATRP and interface-mediated RAFT polymerization for cell sheet application. Appl Surf Sci. 2020;511:145572.
75. Yu M, Gong J, Zhou Y, et al. Surface hydroxyl groups regulate the osteogenic differentiation of mesenchymal stem cells on titanium and tantalum metals. J Mater Chem B. 2017;5(21):3955–3963. doi:10.1039/C7TB00111H
76. Hong Y, Yu M, Lin J, et al. Surface hydroxyl groups direct cellular response on amorphous and anatase TiO2 nanodots. Colloids Surf B Biointerfaces. 2014;123:68–74. doi:10.1016/j.colsurfb.2014.08.030
77. Zhao X, Jin L, Shi H, et al. Recent advances of designing dynamic surfaces to regulate cell adhesion. Colloids Interface Sci Commun. 2020;35(14):100249.
78. Aramesh M, Shimoni O, Ostrikov K, et al. Surface charge effects in protein adsorption on nanodiamonds. Nanoscale. 2015;7(13):5726–5736. doi:10.1039/C5NR00250H
79. Zhu Y, Yao L, Liu Z, et al. Electrical potential specified release of BSA/Hep/polypyrrole composite film and its cellular responses. ACS Appl Mater Interfaces. 2019;11(28):25457–25464. doi:10.1021/acsami.9b09333
80. Appadu A, Jelokhani-Niaraki M, DeBruin L. Conformational changes and association of membrane-interacting peptides in myelin membrane models: a case of the c-terminal peptide of proteolipid protein and the antimicrobial peptide melittin. J Phys Chem B. 2015;119(47):14821–14830. doi:10.1021/acs.jpcb.5b07375
81. Kim JD, Heo JS, Park T, et al. Photothermally induced local dissociation of collagens for harvesting of cell sheets. Angewandte Chemie. 2015;54(20):5869–5873. doi:10.1002/anie.201411386
82. Guo S, Zhu X, Li M, et al. Parallel control over surface charge and wettability using polyelectrolyte architecture: effect on protein adsorption and cell adhesion. ACS Appl Mater Interfaces. 2016;8(44):30552–30563. doi:10.1021/acsami.6b09481
83. Jiang Z, Wang H, Yu K, et al. Light-controlled BMSC sheet-implant complexes with improved osteogenesis via an LRP5/beta-Catenin/Runx2 regulatory loop. ACS Appl Mater Interfaces. 2017;9(40):34674–34686. doi:10.1021/acsami.7b10184
84. Tatiana A, Kolesnikova DK, Skirtach AG, Mohwald H. Laser-induced cell detachment, patterning, and regrowth on gold nanoparticle functionalized surfaces. ACS Nano. 2012;6:9585–9595.
85. Koo MA, Hee Hong S, Hee Lee M, et al. Effective stacking and transplantation of stem cell sheets using exogenous ROS-producing film for accelerated wound healing. Acta Biomater. 2019;95:418–426. doi:10.1016/j.actbio.2019.01.019
86. Silva AS, Santos LF, Mendes MC, et al. Multi-layer pre-vascularized magnetic cell sheets for bone regeneration. Biomaterials. 2020;231:119664. doi:10.1016/j.biomaterials.2019.119664
87. Akira Ito MH, Honda H, Hata K-I, Kagami H, Ueda M, Kobayashi T. Construction and harvest of multilayered keratinocyte sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 2004;10(5–6):873–880. doi:10.1089/1076327041348446
88. Ito A, Ino K, Kobayashi T, et al. The effect of RGD peptide-conjugated magnetite cationic liposomes on cell growth and cell sheet harvesting. Biomaterials. 2005;26(31):6185–6193. doi:10.1016/j.biomaterials.2005.03.039
89. Zhang W, Yang G, Wang X, et al. Magnetically controlled growth-factor-immobilized multilayer cell sheets for complex tissue regeneration. Adv Mater. 2017;29:43. doi:10.1002/adma.201703795
90. Shimizu K, Ito A, Lee JK, et al. Construction of multi-layered cardiomyocyte sheets using magnetite nanoparticles and magnetic force. Biotechnol Bioeng. 2007;96(4):803–809. doi:10.1002/bit.21094
91. Guillaume-Gentil O, Semenov OV, Zisch AH, et al. pH-controlled recovery of placenta-derived mesenchymal stem cell sheets. Biomaterials. 2011;32(19):4376–4384. doi:10.1016/j.biomaterials.2011.02.058
92. Jahn K, Kelkar S, Zhao H, et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J Bone Miner Res. 2017;32(8):1761–1772. doi:10.1002/jbmr.3167
93. Chen YH, Chung YC, Wang IJ, et al. Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials. 2012;33(5):1336–1342. doi:10.1016/j.biomaterials.2011.10.048
94. Patel NG, Zhang G. Responsive systems for cell sheet detachment. Organogenesis. 2013;9(2):93–100. doi:10.4161/org.25149
95. Kang Y, Ren L, Yang Y. Engineering vascularized bone grafts by integrating a biomimetic periosteum and beta-TCP scaffold. ACS Appl Mater Interfaces. 2014;6(12):9622–9633. doi:10.1021/am502056q
96. Kim AY, Kim Y, Lee SH, et al. Effect of gelatin on osteogenic cell sheet formation using canine adipose-derived mesenchymal stem cells. Cell Transplant. 2017;26(1):115–123. doi:10.3727/096368916X693338
97. Ding L, Tang S, Liang P, et al. Bone regeneration of canine peri-implant defects using cell sheets of adipose-derived mesenchymal stem cells and platelet-rich fibrin membranes. J Oral Maxillofac Surg. 2019;77(3):499–514. doi:10.1016/j.joms.2018.10.018
98. Zhang H, Zhou Y, Yu N, et al. Construction of vascularized tissue-engineered bone with polylysine-modified coral hydroxyapatite and a double cell-sheet complex to repair a large radius bone defect in rabbits. Acta Biomater. 2019;91:82–98. doi:10.1016/j.actbio.2019.04.024
99. Na JH, Seok J, Han M, Lim H, Kim HO, Kim E. Harvesting of living cell sheets by the dynamic generation of diffractive photothermal pattern on PEDOT. Adv Funct Mater. 2017;27:10. doi:10.1002/adfm.201604260
100. You Q, Liu Z, Zhang J, et al. Human amniotic mesenchymal stem cell sheets encapsulating cartilage particles facilitate repair of rabbit osteochondral defects. Am J Sports Med. 2020;48(3):599–611. doi:10.1177/0363546519897912
101. Shigeto Shimmura JS, Ohashi Y, Tsubota K. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders.pdf. Cornea. 2001;20(4):408–413. doi:10.1097/00003226-200105000-00015
102. Diaz-Prado S, Muinos-Lopez E, Hermida-Gomez T, et al. Human amniotic membrane as an alternative source of stem cells for regenerative medicine. Differentiation. 2011;81(3):162–171. doi:10.1016/j.diff.2011.01.005
103. Dh-k YH, David G, Kim W-S, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348–352. doi:10.1097/00003226-200005000-00018
104. Nam E, Takahashi A, Fujita N, et al. Cultivation of corneal epithelial cell sheets on canine amniotic membrane. Vet Ophthalmol. 2013;16(4):263–268. doi:10.1111/j.1463-5224.2012.01070.x
105. Adachi K, Amemiya T, Nakamura T, et al. Human periodontal ligament cell sheets cultured on amniotic membrane substrate. Oral Dis. 2014;20(6):582–590. doi:10.1111/odi.12176
106. Honjo K, Yamamoto T, Adachi T, et al. Evaluation of a dental pulp-derived cell sheet cultured on amniotic membrane substrate. Biomed Mater Eng. 2015;25(2):203–212. doi:10.3233/BME-151270
107. Lindenmair A, Wolbank S, Stadler G, et al. Osteogenic differentiation of intact human amniotic membrane. Biomaterials. 2010;31(33):8659–8665. doi:10.1016/j.biomaterials.2010.07.090
108. Eibl J, Redl H. Process for differentiating stem cells of the amniotic membrane; 2013.
109. Mohr S, Portmann-Lanz CB, Schoeberlein A, et al. Generation of an osteogenic graft from human placenta and placenta-derived mesenchymal stem cells. Reprod Sci. 2010;17(11):1006–1015. doi:10.1177/1933719110377471
110. Starecki M, Schwartz JA, Grande DA. Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Adv Orthop Surg. 2014;2014:1–4. doi:10.1155/2014/572586
111. Takizawa S, Yamamoto T, Honjo KI, et al. Transplantation of dental pulp-derived cell sheets cultured on human amniotic membrane induced to differentiate into bone. Oral Dis. 2019;25(5):1352–1362. doi:10.1111/odi.13096
112. Amemiya T, Nakamura T, Yamamoto T, et al. Autologous transplantation of oral mucosal epithelial cell sheets cultured on an amniotic membrane substrate for intraoral mucosal defects. PLoS One. 2015;10(4):e0125391. doi:10.1371/journal.pone.0125391
113. Akahane M, Nakamura A, Ohgushi H, et al. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J Tissue Eng Regen Med. 2008;2(4):196–201. doi:10.1002/term.81
114. Yan H, Oshima M, Raju R, et al. Dentin-pulp complex tissue regeneration via three-dimensional cell sheet layering. Tissue Eng Part C Methods. 2021;27(10):559–570. doi:10.1089/ten.tec.2021.0171
115. Ueyama Y, Yagyuu T, Maeda M, et al. Maxillofacial bone regeneration with osteogenic matrix cell sheets: an experimental study in rats. Arch Oral Biol. 2016;72:138–145. doi:10.1016/j.archoralbio.2016.08.017
116. Nakamura A, Akahane M, Shigematsu H, et al. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010;46(2):418–424. doi:10.1016/j.bone.2009.08.048
117. Shimizu T, Akahane M, Morita Y, et al. The regeneration and augmentation of bone with injectable osteogenic cell sheet in a rat critical fracture healing model. Injury. 2015;46(8):1457–1464. doi:10.1016/j.injury.2015.04.031
118. Yoon Y, Jung T, Afan Shahid M, et al. Frozen-thawed gelatin-induced osteogenic cell sheets of canine adipose-derived mesenchymal stromal cells improved fracture healing in canine model. J Vet Sci. 2019;20(6):e63. doi:10.4142/jvs.2019.20.e63
119. Song P, Li M, Zhang B, et al. DLP fabricating of precision GelMA/HAp porous composite scaffold for bone tissue engineering application. Composites Part B. 2022;244:110163. doi:10.1016/j.compositesb.2022.110163
120. Chen Q, Li J, Han F, et al. A multifunctional composite hydrogel that rescues the ROS microenvironment and guides the immune response for repair of osteoporotic bone defects. Adv Funct Mater. 2022;32(27):2201067.
121. Zhang Y, Li C, Zhang W, et al. 3D-printed NIR-responsive shape memory polyurethane/magnesium scaffolds with tight-contact for robust bone regeneration. Bioact Mater. 2022;16:218–231. doi:10.1016/j.bioactmat.2021.12.032
122. Pei X, Wang L, Zhou C, et al. Ti6Al4V orthopedic implant with biomimetic heterogeneous structure via 3D printing for improving osteogenesis. Mater Des. 2022;221:110964.
123. Ueha T, Akahane M, Shimizu T, et al. Utility of tricalcium phosphate and osteogenic matrix cell sheet constructs for bone defect reconstruction. World J Stem Cells. 2015;7(5):873–882. doi:10.4252/wjsc.v7.i5.873
124. Zhang Y, Wang P, Wang Y, et al. Gold nanoparticles promote the bone regeneration of periodontal ligament stem cell sheets through activation of autophagy. Int J Nanomedicine. 2021;16:61–73. doi:10.2147/IJN.S282246
125. Xiuli Shan DH. Bone engineering by cell sheet technology to repair mandibular defects. Exp Ther Med. 2017;14:5007–5017.
126. Zhao B, Chen J, Zhao L, et al. A simvastatin-releasing scaffold with periodontal ligament stem cell sheets for periodontal regeneration. J Appl Biomater Funct Mater. 2020;18:2280800019900094. doi:10.1177/2280800019900094
127. Liu Y, Ming L, Luo H, et al. Integration of a calcined bovine bone and BMSC-sheet 3D scaffold and the promotion of bone regeneration in large defects. Biomaterials. 2013;34(38):9998–10006. doi:10.1016/j.biomaterials.2013.09.040
128. Shang X, Shu B, Wang Y, et al. Human mesenchymal stromal cell sheet enhances allograft repair in a mouse model. Sci Rep. 2017;7(1):7982. doi:10.1038/s41598-017-08804-2
129. Yu L, Cai Y, Wang H, et al. Biomimetic bone regeneration using angle-ply collagen membrane-supported cell sheets subjected to mechanical conditioning. Acta Biomater. 2020;112:75–86. doi:10.1016/j.actbio.2020.05.041
130. Qi Y, Wang Y, Yan W, et al. Combined mesenchymal stem cell sheets and rhBMP-2-releasing calcium Sulfate–rhBMP-2 scaffolds for segmental bone tissue engineering. Cell Transplant. 2012;21(4):693–705. doi:10.3727/096368911X623844
131. Dang PN, Herberg S, Varghai D, et al. Endochondral ossification in critical-sized bone defects via readily implantable scaffold-free stem cell constructs. Stem Cells Transl Med. 2017;6(7):1644–1659. doi:10.1002/sctm.16-0222
132. Chen G, Fang T, Qi Y, et al. Combined use of mesenchymal stromal cell sheet transplantation and local injection of SDF-1 for bone repair in a rat nonunion model. Cell Transplant. 2016;25(10):1801–1817. doi:10.3727/096368916X690980
133. Hu T, Zhang H, Yu W, et al. The combination of concentrated growth factor and adipose-derived stem cell sheet repairs skull defects in rats. Tissue Eng Regen Med. 2021;18(5):905–913. doi:10.1007/s13770-021-00371-y
134. Jia W, Sharma D, He W, et al. Preservation of microvascular integrity and immunomodulatory property of prevascularized human mesenchymal stem cell sheets. J Tissue Eng Regen Med. 2021;15(3):207–218. doi:10.1002/term.3167
135. Panduwawala CP, Zhan X, Dissanayaka WL, et al. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J Periodontal Res. 2017;52(3):408–418. doi:10.1111/jre.12405
136. Xu M, Li J, Liu X, et al. Fabrication of vascularized and scaffold-free bone tissue using endothelial and osteogenic cells differentiated from bone marrow derived mesenchymal stem cells. Tissue Cell. 2019;61:21–29. doi:10.1016/j.tice.2019.08.003
137. Zhang H, Zhou Y, Zhang W, et al. Construction of vascularized tissue-engineered bone with a double-cell sheet complex. Acta Biomater. 2018;77:212–227. doi:10.1016/j.actbio.2018.07.024
138. Ma D, Ren L, Cao Z, et al. Prefabrication of axially vascularized bone by combining beta-tricalciumphosphate, arteriovenous loop, and cell sheet technique. Tissue Eng Regen Med. 2016;13(5):579–584. doi:10.1007/s13770-016-9095-0
139. Zhang D, Gao P, Li Q, et al. Engineering biomimetic periosteum with beta-TCP scaffolds to promote bone formation in calvarial defects of rats. Stem Cell Res Ther. 2017;8(1):134. doi:10.1186/s13287-017-0592-4
140. Bartold PM, Gronthos S, Ivanovski S, et al. Tissue engineered periodontal products. J Periodontal Res. 2016;51(1):1–15. doi:10.1111/jre.12275
141. Liu Y, Wang H, Dou H, et al. Bone regeneration capacities of alveolar bone mesenchymal stem cells sheet in rabbit calvarial bone defect. J Tissue Eng. 2020;11:2041731420930379. doi:10.1177/2041731420930379
142. Yang H, Li J, Hu Y, et al. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent Mater. 2019;35(9):1238–1253. doi:10.1016/j.dental.2019.05.016
143. Iwata T, Yamato M, Washio K, et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets - A safety and efficacy study in ten patients. Regen Ther. 2018;9:38–44. doi:10.1016/j.reth.2018.07.002
144. Jin H, Zhang K, Qiao C, et al. Efficiently engineered cell sheet using a complex of polyethylenimine-alginate nanocomposites plus bone morphogenetic protein 2 gene to promote new bone formation. Int J Nanomedicine. 2014;9:2179–2190.
145. Mohamed-Ahmed S, Yassin MA, Rashad A, et al. Comparison of bone regenerative capacity of donor-matched human adipose-derived and bone marrow mesenchymal stem cells. Cell Tissue Res. 2021;383(3):1061–1075. doi:10.1007/s00441-020-03315-5
146. Le TM, Vu NB, Huynh PD, et al. Treatment of osteochondral femoral head defect by human umbilical cord mesenchymal stem cell sheet transplantation: an experimental study in rats. Adv Exp Med Biol. 2021;2021:1–5.
147. Mercuri NT. Amniotic mesenchymal stromal cells exhibit preferential osteogenic and chondrogenic differentiation and enhanced matrix production compared with adipose mesenchymal stromal cells. Am J Sports Med. 2017;45:2637–2647.
148. Hong JQ, Gao Y, Song J, et al. Comparison of biological characteristics and immunosuppressive activity between human amniotic mesenchymal stem cells and human bone marrow mesenchymal stem cells. Zhongguo shi yan xue ye xue za zhi. 2016;24(3):858–864. doi:10.7534/j.issn.1009-2137.2016.03.041
149. Mu Y, Wu X, Hao Z. Comparative evaluation of mesenchymal stromal cells from umbilical cord and amniotic membrane in xeno-free conditions. BMC Cell Biol. 2018;19(1):27. doi:10.1186/s12860-018-0178-8
150. Liu Y, Cao DL, Guo LB, et al. Amniotic stem cell transplantation therapy for type 1 diabetes: a case report. J Int Med Res. 2013;41(4):1370–1377. doi:10.1177/0300060513487640
151. Huang YZ, He T, Cui J, et al. Urine-derived stem cells for regenerative medicine: basic biology, applications, and challenges. Tissue Eng Part B Rev. 2022;28(5):978–994. doi:10.1089/ten.teb.2021.0142
152. Guan J, Zhang J, Li H, et al. Human urine derived stem cells in combination with β-TCP can be applied for bone regeneration. PLoS One. 2015;10(5):e0125253. doi:10.1371/journal.pone.0125253
153. Xing F, Li L, Sun J, et al. Surface mineralized biphasic calcium phosphate ceramics loaded with urine-derived stem cells are effective in bone regeneration. J Orthop Surg Res. 2019;14(1):419. doi:10.1186/s13018-019-1500-7
154. Wang L, Wei X, Duan C, et al. Bone marrow mesenchymal stem cell sheets with high expression of hBD3 and CTGF promote periodontal regeneration. Mater Sci Eng C Mater Biol Appl. 2022;10:112657.
155. Kim Y, Kang BJ, Kim WH, et al. Evaluation of mesenchymal stem cell sheets overexpressing BMP-7 in canine critical-sized bone defects. Int J Mol Sci. 2018;19:7.
156. Su ZP, Tian L, Shang HT, et al. Experimental study on the bone morphogenetic protein 1-modified bone marrow mesenchymal stem cell sheets to promote mandibular distraction osteogenesis. Front Surg. 2021;8:786351. doi:10.3389/fsurg.2021.786351
157. Jiang Z, Li N, Zhu D, Ren L, Yang G. Genetically modified cell sheets in regenerative medicine and tissue engineering. Biomaterials. 2021;275:120908. doi:10.1016/j.biomaterials.2021.120908
158. Kohji Nishida MY, Hayashida Y, Watanabe, K, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;2004(351):1187–1196. doi:10.1056/NEJMoa040455
159. Kanzaki M, Takagi R, Washio K, et al. Bio-artificial pleura using an autologous dermal fibroblast sheet. NPJ Regen Med. 2017;2:26. doi:10.1038/s41536-017-0031-2
160. Sawa Y, Miyagawa S, Sakaguchi T, et al. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: report of a case. Surg Today. 2012;42(2):181–184. doi:10.1007/s00595-011-0106-4
161. Sawa Y, Yoshikawa Y, Toda K, et al. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ J. 2015;79(5):991–999. doi:10.1253/circj.CJ-15-0243
162. Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143(3):582–588 e582. doi:10.1053/j.gastro.2012.04.050
163. Yamaguchi N, Isomoto H, Kobayashi S, et al. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7(1):17460. doi:10.1038/s41598-017-17663-w
164. Yamamoto K, Yamato M, Morino T, et al. Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen Med. 2017;2:6. doi:10.1038/s41536-017-0010-7
165. Yamamoto K, Morino T, Kasai Y, et al. Cell sheet transplantation prevents inflammatory adhesions: a new treatment for adhesive otitis media. Regen Ther. 2021;18:457–463. doi:10.1016/j.reth.2021.10.001
166. L’Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357(14):1451–1453. doi:10.1056/NEJMc071536
167. Jiang YN, Wang YX, Zheng YJ, et al. 含异体角质形成细胞和成纤维细胞的细胞膜片治疗II度烧伤创面的临床研究 [Clinical study of cell sheets containing allogeneic keratinocytes and fibroblasts for the treatment of partial-thickness burn wounds]. Zhonghua shao shang za zhi. 2020;36(3):171–178. Chinese. doi:10.3760/cma.j.cn501120-20191113-00426
168. Sato M, Yamato M, Mitani G, et al. Combined surgery and chondrocyte cell-sheet transplantation improves clinical and structural outcomes in knee osteoarthritis. NPJ Regen Med. 2019;4:4. doi:10.1038/s41536-019-0069-4
169. Sato M, Yamato M, Hamahashi K, et al. Articular cartilage regeneration using cell sheet technology. Anatomical Record. 2014;297(1):36–43. doi:10.1002/ar.22829
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
