Back to Journals » Cancer Management and Research » Volume 15
Cell Cycle-Related lncRNAs as Innovative Targets to Advance Cancer Management
Authors Liang XR, Liu YF, Chen F, Zhou ZX, Zhang LJ, Lin ZJ
Received 6 February 2023
Accepted for publication 13 June 2023
Published 3 July 2023 Volume 2023:15 Pages 547—561
DOI https://doi.org/10.2147/CMAR.S407371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Xiao-Ru Liang,1,* Yan-Fei Liu,1,* Feng Chen,2 Zhi-Xia Zhou,3 Li-Jie Zhang,1 Zhi-Juan Lin1
1Key Laboratory of Immune Microenvironment and Inflammatory Disease Research in Universities of Shandong Province, School of Basic Medical Sciences, Weifang Medical University, Weifang, People’s Republic of China; 2Department of General Surgery, Weifang Traditional Chinese Hospital, Weifang, Shandong, People’s Republic of China; 3Institute for Translational Medicine, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhi-Juan Lin; Li-Jie Zhang, Key Laboratory of Immune Microenvironment and Inflammatory Disease Research in Universities of Shandong Province, School of Basic Medical Sciences, Weifang Medical University, Weifang, People’s Republic of China, Tel +86-536-8462530, Email [email protected]; [email protected]
Abstract: Long non-coding RNAs (lncRNAs) are non-coding RNAs (ncRNAs) longer than 200nt. They have complex biological functions and take part in multiple fundamental biological processes, such as cell proliferation, differentiation, survival and apoptosis. Recent studies suggest that lncRNAs modulate critical regulatory proteins involved in cancer cell cycle, such as cyclin, cell cycle protein-dependent kinases (CDK) and cell cycle protein-dependent kinase inhibitors (CKI) through different mechanisms. To clarify the role of lncRNAs in the regulation of cell cycle will provide new ideas for design of antitumor therapies which intervene with the cell cycle progression. In this paper, we review the recent studies about the controlling of lncRNAs on cell cycle related proteins such as cyclin, CDK and CKI in different cancers. We further outline the different mechanisms involved in this regulation and describe the emerging role of cell cycle-related lncRNAs in cancer diagnosis and therapy.
Keywords: cyclin, cyclin-CDK, CDK inhibitors, lncRNA
Introduction
The cell cycle means the process that a cell undergoes from the end of one mitosis to the completion of the next mitosis, including two periods, interphase and cytokinesis (M phase).1 Interphase is further divided into prophase of DNA synthesis (G1 phase), S phase of DNA synthesis (S phase) and late phase of DNA synthesis (G2 phase).1,2 Mammalian cell cycle is controlled by cyclin-dependent kinases (CDKs) and their related pathways.3 Activation of CDK4 and CDK6 can affect G1 phase progression at an early stage. They bind to cyclin D (CCND) to phosphorylate the retinoblastoma protein pRb, prevent its binding, and inhibit E2F transcription factors to promote cells to the next phase.4,5 However, recent studies have found that cyclin D-CDK is not involved in the initial inactivation of Rb, but rather “primes” cells to enter the cell cycle by preventing them from exiting or facilitating their entry into the G1 phase.2 CDK associates with its regulatory proteins to control the different cell cycle phases.6 Cyclin E (CCNE) binds to CDK2 and regulates the progression of S phase, while cyclin B (CCNB) binds to CDK1 and regulates the G2/M phase transition.7 The cyclin dependent kinase inhibitors (CKIs) inhibit the activity of CDK and cyclin, and block cells cycle at different stages.4 Apart from its roles in cell cycle regulation, cell cycle regulators are involved in cell invasion, migration, apoptosis and DNA repairing. Thus, the abnormal expression or deletion of cyclin, CDK and CKI will cause the cell cycle disorder and closely related to the occurrence and development of cancers.
Some evidence points to the important role of long non-coding RNAs (lncRNAs) in the regulation of the aforesaid proteins’ expression or activity. Several studies have indicated that these regulators of cell cycle are targets of lncRNAs. LncRNAs are transcripts more than 200 nucleotides and commonly not involved in encoding proteins.8 Previous studies have reported aberrant lncRNA expression in various cancers where they function as oncogenes or tumor suppressors (Figure 1). LncRNAs influence cancer progression through multiple mechanisms including epigenetic regulation, DNA damage, cell cycle and chromosomal instability.3,9–11 The interaction between lncRNAs and cell cycle regulators can affect cell cycle progression in many tumor cells. Therefore, to clarify the mechanisms of cell cycle controlling by lncRNAs will provide a new perspective for early diagnosis and treatment of tumors. In this review, we summarize the regulatory function and mechanism of lncRNAs on multiple malignant behaviors by modulating cell cycle regulators, such as cyclins, CDKs and CKIs, in different cancers and point out the emerging role of cell cycle-related lncRNAs in the diagnosis and treatment of tumors.
 |
Figure 1 LncRNAs associated with various types of cancer. |
LncRNAs are Involved in Cancer Cell Cycle
Cell cycle is regulated by cycle-related proteins and cycle aberrancy can cause many diseases including cancers. Recent studies have suggested that some lncRNAs are involved in cancer cell cycle regulation. Understanding the role of lncRNAs in regulating cell cycle factors will not only provide new insights into the regulation of fundamental cellular processes, but reveal new pathogenic mechanisms and develop rational anti-cancer therapeutic targets in the future.
Cyclin-Targeted Cell Malignancy Regulation Mediated by lncRNAs in Tumors
Cyclin is an important regulator and plays a crucial role in cell cycle of cancers. The cyclin family mainly contains four members, that is cyclin A, B, C and D. Cyclin D, a proto-oncogene in cancer cells, is a key regulator of the G1/S phase.12 Cyclin A plays an established role in S phase and promotes specific cell cycle events and transitions.13 Cyclin B is an important regulator of G2 to M phase transition.14 Cyclin E, G1-S phase transition regulator, has a crucial role especially in the early stages of cancer.15 Recently, some lncRNAs are reported to interact with cyclins and influence various malignant behaviors of cancer cells. Here, we describe the regulatory roles involved in lncRNA mediating cyclin in different types of tumors (Table 1).
 |
Table 1 The Regulation of lncRNAs on Cyclin in Tumors |
Digestive System Tumors
HOTAIR knockdown increased miR-454-3p expression, thereby inhibiting gastric cancer (GC) cell proliferation through downregulation of STAT3/cyclin D1 (CCND1) activity.16 Inhibition of lncRNA TTTY15 could reduce GC progression by competitively binding miR-98-5p to suppress CCND2 expression, and TTTY15 might become a new target for GC therapy.17 PVT1 positively regulated GC cell proliferation, migration and cell cycle progression through the regulation of miR-16 and CCND1.18 The downregulation of LINC01667 was involved in the inhibition of GC cell proliferation through miR-138-5p/cyclin E1 (CCNE1) axis.19
In hepatocellular carcinoma (HCC), LINC00152 facilitated HCC cell proliferation by controlling cell cycle progression through regulation of CCND1, thus, it was identified as a potential therapeutic target for HCC.20 The knockdown of PR11-295G20.2 partially inhibited HCC cell proliferation, invasion and migration through the miR-6884-3p/cyclin B1 (CCNB1) pathway.21 LINC01488 actively promoted miR-124-3p and miR-138-5p biogenesis to reduce vimentin expression and negatively regulated CCNE, thereby inhibiting the progression of G1 phase and suppressing the entry of S phase.22
LncRNA CASC9 was found to be upregulated in pancreatic tissues and it could upregulate CCND1 expression by targeting miR-497-5p, then contribute to pancreatic cancer (PC) progression.23 LncRNA XIST promoted CCND1 expression by competitively binding with miR-129-5p in esophageal squamous cell carcinoma (ESCC).24 In esophageal carcinoma (EC), LINC01234 bound to miR-193a-5p to induce CCNE1, thus promoted EC cell proliferation and inhibited cell apoptosis.25
Similarly, HOXD-AS1 upregulated CCND1 by targeting miR-526b-3p and MCF2L-AS1 partially modulated the expression of CCNE1 by targeting miR-874-3p to promote colorectal cancer (CRC) cell proliferation, migration and invasion, providing a theoretical basis for developing anticancer therapy for CRC.26,27 In addition, HNF1A-AS1 might also be a promising target for CRC due to its interaction with CCND1, including stabilizing CCND1 mRNA by interacting with IGF2BP2 through m6A modification, regulating CCND1 expression by targeting miR-93-5p or suppressing PDCD4 to activate the PI3K/AKT pathway.28
Urogenital Tumors
In renal cell carcinoma (RCC), CDKN2B-AS1 promoted the expression of CCND1 and CCND2 and enhanced the invasion of RCC.29 Similarly, both LOXL1-AS1/miR-541-3p and ADAMTS9-AS1/miR-142-5p targeted CCND1, and aberrant expression of CCND1 eventually led to cell cycle progression and carcinogenesis in prostate cancer (PC).30,31 Upregulation of MALAT1 promoted the proliferation of bladder cancer cells through the miR-34a/CCND1 axis.32
In breast cancer (BC), LINC01355 blocked BC cells in G0/G1 phase and inhibited BC growth through FOXO3-mediated CCND1 transcriptional repression.33 GACAT3 competitively bound to miR-497 and enhanced CCND2 expression to promote BC progression.34 The knockdown of LINC00473 inhibited the expression of CCNE1, while the inhibition of miR-424-5p eliminated the inhibitory effect of LINC00473 knockdown on CCNE1 protein expression. The ceRNA network formed by LINC00473, miR-424-5p, and CCNE1 provided a new clue for cancer diagnosis and treatment.35 In ovarian cancer (OC), HOTAIR stimulated CCND1 and CCND2 expression by negatively regulating miR-206 expression, thereby promoting OC cell proliferation, migration and invasion.36 Knockdown of PCAT-1 suppressed cell proliferation, invasion and migration, and also reduced cyclin D1/CDK4 expression to arrest the cell cycle in G0/G1 phase.37 CRNDE controlled CCNB1 expression through miR-183 to affect cervical cancer (CC) cell proliferation, migration and invasion.38 Lnc_000231 promoted CC cell proliferation and tumor formation by interacting with miR-497-5p and maintaining CCNE1 expression.39 In addition, TINCR upregulated CCND1 expression by uptake of miR-302 to promote cervical squamous cell carcinoma (CSCC) cell proliferation.40
Respiratory System Tumors
In non-small cell lung cancer (NSCLC), PCNA-AS1 upregulated CCND1 to promote cell proliferation.41 Reduced CCND1 and CDK4 expression after overexpression of Linc00703 inhibited NSCLC progression.42 MCF2L-AS1 and CCND1 expression was upregulated in gefitinib-resistant NSCLC patients. MCF2L-AS1 promoted CCND1 mRNA stability by combining with ELAVL1, thereby facilitating NSCLC cell growth and gefitinib resistance.43 In lung adenocarcinoma (LUAD), Linc00467 and PTPRG-AS1, respectively, bound to miR-20b-5p and miR-124-3p, to promote CCND1 expression and proliferation of LUAD cells.44,45 RP11-805J14.5 suppressed the growth, invasion and migration of LUAD cells by sponging miR-34b-3p and miR-139-5p and down-regulating CCND2, which made RP11805J14.5 a potential target for LUAD therapy.46
Other Tumors
In osteosarcoma (OS), FLVCR-AS1 bound to miR-381-3p to promote CCND1 expression and OS cell proliferation.47 Similarly, NR2F1-AS1 competitively bound to miR-338-3p to facilitate CCND1 expression and promote thyroid cancer (TC) cell proliferation and migration.48 LINC00887 also interacted with CCND1 to regulate cell cycle progression and exacerbate the malignant progression of glioma.49 RUNX1-IT1 competitively bound to miR-195 to upregulate CCND1 expression, thereby contributing to the proliferation of glioblastoma (GBM) cells.50 In multiple myeloma (MM), competitive inhibition of miR-128-3p by lncRNA HCP5 promoted PLAGL2 activation of wnt/β-catenin/CCND1 signaling.51 Moreover, PVT1 activated the expression of β-catenin, c-myc and CCND1 to promote the proliferation and migration of pituitary adenoma cells.52
CDK-Targeted Cell Malignancy Regulation Mediated by lncRNAs in Tumors
Different CDKs are involved in the regulation of different cell cycle phases. For example, CDK4, CDK6 mainly acts in G1 phase and CDK2 mainly acts in S phase.53 CDK4, CDK6 and CDK2 are often overexpressed and promotes cancer cell cycle progression, which in turn accelerates cell division and tumor growth.54 Recent studies have shown that CDK expression could be regulated by certain lncRNAs and further affect tumorigenesis and progression (Table 2). Therefore, to clarify the interaction between lncRNAs and CDKs is essential for developing targeted strategy in the future.
 |
Table 2 The Regulation of lncRNAs on CDK in Tumors |
Digestive System Tumors
LncRNA GCRL1 promoted GC cells proliferation and metastasis by targeting CDK4 and LINC00974 upregulated CDK6 to accelerate the G1-S phase transition to promote GC cell proliferation, indicating their oncogenic roles and promising blocking targets for GC.55,56
LINC00630 could recruit E2F1 to the promoter region of CDK2 to promote CDK2 transcription, thereby promoting HCC cell proliferation, inhibiting apoptosis and accelerating the cell cycle.57 VPS9D-AS1 was found to bind with HuR protein, thus affecting the post-transcriptional expression of CDK4 mRNA and driving HCC cell proliferation.58 Transcription factor YY1 triggered transcription of LINC00205, and YY1-regulated LINC00205 promoted cell cycle and cancer cell proliferation via miR-26a-5p/CDK6-mediated ceRNA axis.59 Knockdown of CRNDE could inhibit CDK6 expression by targeting miR-33a-5p, blocking the cell cycle at G0/G1 phase. These oncogenic lncRNAs provide potential therapeutic targets for HCC cancer treatment despite their variable actions.60
In CRC, MCM3AP-AS1 facilitated cell cycle progression by targeting miR-545/CDK4 and NR2F2-AS1 knockdown induced cell cycle block by downregulating CDK6.61,62 Transcription factor FOXP1 provoked transcription of CASC21, which interacted with miR-539-5p and increased CDK6 expression to promote cancer cell proliferation.63 HAGLR promoted CRC progression through miR-185-5p/CDK4/CDK6 axis.64 These lncRNAs could be promising targets for CRC diagnosis due to their abnormal expression in CRC tissues and blocking targets for treatment due to their significant carcinogenic effect.
Urogenital Tumors
In adrenocortical carcinoma (ACC), UCA1 promoted ACC growth by regulating the miR-298/CDK6 axis.65 LncRNA HOST2/let-7b/CDK6 axis was involved in the regulation of triple-negative breast cancer (TNBC) cell proliferation.66 SNHG15 promoted cancer cell proliferation by inhibiting miR-370-3p to upregulate CDK6, which provide a new theoretical basis for new clinical treatment strategies.67 In CC, LINC00313 promoted CDK6 expression through competitive binding of miR-4677-3p, thus promoting cancer progression.68 Similarly, MACC1-AS1 promoted cell cycle progression and cell proliferation by competitively inhibiting miR-34a to enhance CDK6.69 LncRNA NR2F2-AS1 positively regulated CDK4 expression to promote PC cell proliferation.70
Respiratory System Tumors
LINC01194 and FOXD3-AS1 were both highly expressed in NSCLC tissues and cell lines. LINC01194 regulated the miR-486-5p/CDK4 axis and FOXD3-AS1 interacted with miR-135a-5p/CDK6 to promote the proliferation of NSCLC cells.71,72 As an oncogenic lncRNA, HNF1A-AS1 was notably correlated with advanced TNM stage, tumor size and lymph node metastasis.73 Mechanically, HNF1A-AS1 upregulated CDK6 expression by targeting miR-149-5p and provided new evidence for NSCLC potential therapeutic target.74 Similarly, MEG8 had a great potential to facilitate proliferation, invasion and migration of NSCLC by sponging miR-107 and targeting CDK6 to regulate the Rb/E2F3 signaling pathway.75 In addition, the level of AWPPH was negatively correlated with the overall survival rate of NSCLC patients and AWPPH/miRNA-204/CDK6 regulatory loop was identified to aggravate the malignant progression of NSCLC.76 Similarly, TFAP2A-AS1 contributed to cancer progression by regulating the miR-584-3p/CDK4 axis77 (Figure 2).
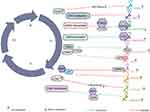
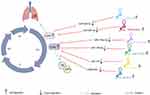 |
Figure 2 CDK-targeted cell cycle regulation in NSCLC mediated by lncRNA. LINC01194 regulated the miR-486-5p/CDK4 axis and FOXD3-AS1 interacted with miR-135a-5p/CDK6 to promote the proliferation of NSCLC cells. HNF1A-AS1 regulated the miR-149-5p/CDK6 axis and MEG8 interacted with miR-107/CDK6 to facilitate proliferation, invasion and migration of NSCLC. AWPPH/miRNA-204/CDK6 regulatory loop was identified to aggravate the malignant progression of NSCLC and TFAP2A-AS1 contributed to cancer progression by regulating the miR-584-3p/CDK4 axis.71–77 |
Other Tumors
In OS, 91H could serve as new targets for cancer therapy due to their influence on cell proliferation, through inducing CDK4 promoter methylation, or LOC100129620/miR-335-3p/CDK6 signaling pathway.78,79 In papillary thyroid cancer (PTC), SNHG6 promoted CDK6 expression by regulating miR-186.80 Similarly, PCGEM1 and H19 promoted or inhibited glioma development by competitively binding the corresponding miRNAs to regulate CDK development.81,82 On the contrary, HAND2-AS1 was lowly expressed in glioma cells and tissues, and CDK was negatively correlated with HAND2-AS1. HAND2-AS1 regulated cell proliferation, invasion and migration through regulation of CDK6 and provided new insights into the diagnosis and treatment of cancer.83 In nasopharyngeal carcinoma (NPC), LINC00704 could recruit ETS1 to the promoter region of CDK6 to promote CDK6 transcription, facilitating cell cycle progression and promising as a diagnostic marker and molecular therapeutic target for cancer.84
CDK Inhibitor-Targeted Cell Malignancy Regulation Mediated by lncRNAs in Tumors
Many lncRNAs regulate cell cycle by participating in the regulation of CDK inhibitors that delay or stop the cell cycle progression. There are two families of CKI proteins, including inhibitors of INK4 and CDK interacting protein/kinase inhibitory protein (CIP/KIP).10
INK4 Family Inhibitors
The INK4 family members contain p16INK4a, p15INK4b, p18INK4c and p19INK4d (hereafter referred to as p16, p15, p18, p19),85 among which, p16, p15 and p19 bind to CDK4 and CDK6, preventing them from binding to cyclin D, thereby inhibiting CDK4/6-mediated phosphorylation of pRb and exiting G1 phase.86 The INK4 locus encodes three important genes: p15 (CDKN2B), p16 (CDKN2A) genes and ARF, which are tumor suppressor genes.87
LncRNA ANRIL binds to the INK4 locus and interacts specifically with SUZ12 in the PRC2 complex and with CBX7 in the PRC1 complex. ANRIL suppresses the INK4 locus by recognizing H3K27me3 and CBX7, and inhibits p15 transcription with SUZ12.88 E2F1 transcriptionally activates ANRIL in an ATM-dependent manner and participates in DNA repair.89 After DNA repair, ANRIL promotes cell growth and re-enters the cell cycle by repressing the INK4 locus (Figure 3). In addition, knockdown of lncRNA ANROC increases p16, p15 and ARF levels and ANROC acts as a negative regulator of cell cycle progression by suppressing CCNB1 expression, thereby inhibiting cell proliferation.87 LINC01012 has also been found to be negatively correlated with p19. LINC01012 expression is upregulated in CC and stimulates the proliferation and migration of cancer cells, thereby promoting CC progression by downregulating p19.90
 |
Figure 3 Mechanism of lncRNA ANRIL-mediated regulation of the INK4 locus. LncRNA ANRIL binds to the INK4 locus and interacts specifically with SUZ12 in the PRC2 complex and with CBX7 in the PRC1 complex. ANRIL suppresses the INK4 locus by recognizing H3K27me3 and CBX7, and inhibits p15 transcription with SUZ12. E2F1 transcriptionally activates ANRIL in an ATM-dependent manner and participates in DNA repair. After DNA repair, ANRIL promoted cell growth and re-enters the cell cycle by repressing the INK4 locus.88,89 |
Cip/Kip Family Inhibitors
Cip/Kip family inhibitors include p21cip1, p27kip1, and p57kip2, which are involved in cell cycle regulation, transcriptional regulation, and cell migration.91 ZXF1 mediates cancer cell cycle progression by competitively binding to miR-378-3p to regulate the expression of PCDHA3. ZXF1 binds directly to p21 and blocks CDC20 (E3 ligase of p21) mediated ubiquitin degradation. Thus, ZXF1 becomes a promising diagnostic and prognostic indicator.92 HCG11 exerts tumor suppressive effects by increasing the expression of p27. On the one hand, HCG11 increases p27 expression by inhibiting the activity of miR-942-5p, thereby inhibiting cancer progression. On the other hand, HCG11 stabilizes p27 mRNA by recruiting IGF2BP2 (an RNA-binding protein).93 SNHG17 is involved in GC genesis and development as a member of PRC2-mediated epigenetic regulation. P15 and p57 are downstream regulators involved in SNHG17-mediated GC cell cycle and proliferation processes. SNHG17 regulates p15 and p57 by binding to EZH2 to mediate epigenetic p15 and p57 transcriptional inhibition. Activation of PRC2 and induction of GC cell proliferation by SNHG17 suggest that SNHG17 can directly bind to EZH2 and repress p15 and p57 expression in GC.94
Emerging evidences about lncRNAs on cell cycle regulatory proteins indicate that lncRNAs can influence various malignant behaviors of cancer cells including not only cell proliferation but apoptosis, migration and invasion, even drug resistance. To further clarify the interaction mechanisms between aforesaid regulators will surely pave the way for design of anticancer therapies which intervene with the cell malignancy progression.
The Regulatory Mechanism of lncRNAs Involved in Cancer Cell Cycle
The regulatory roles of lncRNAs on cancer cell cycle involve different mechanisms, including sponging by the ceRNA network, epigenetic modifications, transcription regulations and other regulatory pathways (Figure 4).
The ceRNA Network
LncRNAs can regulate gene expression by acting as endogenous target mimics, which compete with miRNAs. This mode of action is called “miRNA sponge”, and lncRNAs with this function are called competitive endogenous RNAs (ceRNAs).8 Several lncRNAs are involved in the regulation of cancer cell cycle by this way. For instance, knockdown of LINC00473 inhibited CCNE1 expression, while inhibition of miR-424-5P eliminated the inhibitory effect of LINC00473 knockdown on CCNE1 protein expression.35 Inhibition of CRNDE enhanced the level of miR-33a-5p to suppress CDK6 expression thereby blocking the G0/G1 phase of colorectal cancer.60
The Epigenetic Modifications
Epigenetics refers to genetic alterations in gene expression without alterations in DNA sequence. The molecular mechanisms mainly include DNA methylation, histone modifications, chromatin remodeling and non-coding RNA.8 LncRNAs, as epigenetic regulators, could adjust epigenetic modifications in the nucleus and regulate gene transcription by regulating histone or DNA modifications, mainly methylation and acetylation. For example, knockdown of lncRNA 91H suppressed the progression of osteosarcoma and inhibited tumorigenesis by inducing methylation of the CDK4 promoter.78 SNHG6 could modulate the G1-S phase transition by recruiting EZH2 to the p27 promoter region, and increase the enrichment of H3K27Me3, inhibit the expression of p27, ultimately regulate the cell cycle, and promote cancer progression. Thus, it provided a new target for NSCLC treatment.95
The Transcription Regulations
Certain lncRNAs also modulate the cancer cell cycle via the transcription regulations. This process occurs mainly in the nucleus. LncRNAs bind directly to DNA sequences and repress gene transcription. In addition, they interacted directly with proteins (mainly transcription factors) to repress or activate the expression of downstream genes.8 For example, overexpression of LINC01355 selectively enhanced the binding of the CCND1 promoter region to FOXO3 protein that was a transcriptional repressor of CCND1. The FOXO3/CCND1 axis plays an important role in LINC01355-mediated tumor suppression.33 LINC00704 promoted CDK6 transcription by recruiting ETS1 to the promoter region of CDK6, promoting the cancer cell cycle progression.84 LINC00630 was a novel regulator of E2F1 transcriptional activity. Overexpression of LINC00630 promoted the binding of E2F1 to the CDK2 promoter region, which in turn promoted CDK2 expression and finally led to HCC cell proliferation, inhibition of apoptosis and acceleration of the cell cycle.57
Other Regulatory Mechanisms
LncRNA VPS9D1-AS1 could directly bind to the HuR protein and thus impact the stability and expression of the CDK4 mRNA, thereby impacting HCC cell proliferation.58 H19 was able to bind to eIF4A3, a core exon junction complex (EJC) component that was loaded onto mRNAs by pre-mRNA splicing, thus modulating the expression of CCND1, CCNE1 and CDK4, finally promoting CRC cell proliferation.96 UCA1 could suppress the tumor suppressor p27 through interaction with heterogeneous nuclear ribonucleoprotein I (hnRNP I). UCA1 mediated tumor growth by interaction with hnRNP I, thus leading to the suppression of p27 protein expression and causing G1 phase cell cycle arrest.97
Although lncRNAs play a crucial role in cancer development and progression through complex interaction with cycle regulatory proteins, the molecular mechanisms underlying their function are not fully understood partly due to the tissue-specific modes of action of lncRNAs. Further insight into the biological significance and function of lncRNAs and cycle regulators requires additional studies, which may not only reveal additional mechanisms of action but develop promising anticancer strategies.
Emerging Role of Cell Cycle-Related lncRNAs in Cancer Diagnosis and Therapy
More and more studies have shown that lncRNAs can be used as new tools for early diagnosis and treatment of cancer. Their abnormal expression is related to different cancer types and has obvious specificity. The specific expression of lncRNAs and their detection in patients’ serum, plasma, saliva or urine make them effective diagnostic markers for different cancers.98–100 LncRNA DRAIR is low expressed, and the plasma DRAIR level of patients is decreased. The downregulation of DRAIR is sufficient to distinguish stage I and II patients from healthy controls and thus can be used as an early diagnostic marker for cancer.101 Another example is that the expression levels of LNCR565 and LNCR641 in the serum of glioblastoma multiforme patients are significantly higher than those of healthy controls. LNCR565 and LNCR641 can be used as diagnostic markers in BC patients.102 In addition, one lncRNA can also be abnormally expressed in a variety of cancers, which is called non-specific. For example, the expression level of LUCAT1 is increased in GC, lung cancer, CC and other cancers.103–105 The non-specificity of lncRNA enables them to diagnose different cancers, and can also make one cancer to be associated with multiple lncRNAs. For example, elevated HULC, H19, HOTAIR, GACAT2 in a patient’s serum can be used as a diagnostic marker for GC.106 Tantai et al reported that XIST and HIF1A-AS1 in serum are elevated, which can be used as diagnostic markers of non-small cell lung cancer, and that the combined detection of XIST and HIF1A-AS1 has a higher diagnostic rate for NSCLC than the detection of a single marker.107 In addition, some lncRNAs can be used to predict cancer prognosis. For example, LINC01488 is associated with the prognosis of HCC, and HOXD-AS1 is associated with the prognosis of CRC.22,26
Chemotherapy, radiotherapy and surgical resection of tumors are the traditional treatment methods of cancer, which can lead to cancer recurrence in many cases. Studies have shown that interfering with the dysregulated lncRNA level can effectively normalize cancer-related cellular changes. Zhang et al found that LINC00628 interacts with EZH2 and regulates gene expression by regulating H3K27me3. In this study, LINC00628 inhibits the proliferation, migration and colony formation of GC cells and acts as an inhibitor of GC.108 Similarly, it has been reported that lncRNAs HULC and AK126698 act as tumor suppressors by inhibiting cell proliferation and migration.109,110 These studies indicate that lncRNA can be a new therapeutic target for cancer therapy.
Conclusion and Future Perspective
LncRNAs were aberrantly expressed in many types of cancer and their dysregulation plays an important role in cancer cell development, progression and survival. LncRNAs are related to a variety of clinicopathological features, such as tumor TNM staging, lymph node metastasis, distant metastasis and short overall survival time and involved in various cellular processes including cell proliferation, apoptosis, invasion and migration. In this review, we pay close attention to the regulation of the lncRNAs on the cell cycle regulators of cancers. LncRNAs regulate the cell cycle factors, such as CDK and CDK inhibitors through various mechanisms, including sponging by the ceRNA network, epigenetic modifications, transcription regulations and other regulatory pathways. Although the regulation of cell cycle by lncRNAs was closely related to tumorigenesis and development, many lncRNAs with functional roles and the mechanisms by which they regulate the tumor cell malignancy have not been fully clarified due to the complex regulatory relationships among molecules. Therefore, an in-depth understanding of the roles and ways of lncRNAs in cell cycle regulation will be helpful for clarifying the interaction between lncRNAs and the cell cycle molecules of cancers.
The differential expression of cell cycle-related lncRNAs in normal and cancer tissues and cell lines makes them a promising and powerful selection tool for use in cancer diagnosis and provide new insights into drug candidates for cancer therapy. However, the expression of lncRNAs in certain cancers remains unclear due to limited research. Therefore, further studies are needed to confirm the exact expression of lncRNAs in various cancers. In addition, lncRNAs were currently less studied in human plasma, serum, urine and other body fluids, which hinders the application of lncRNAs in diagnosis. More studies on the expression and stability of lncRNAs in non-invasive body fluids are needed to make lncRNAs an ideal tool for disease diagnosis. Also, there is limited information on the use of lncRNAs for therapeutic applications, and further preclinical and clinical studies on the efficacy, stability, and safety of lncRNAs-targeted drugs are needed. In addition to the efficacy, stability, and safety of lncRNAs-targeted drugs for cancer therapy, further studies are needed to investigate the expression and stability of lncRNAs in non-invasive body fluids used for cancer diagnosis.
In conclusion, we highlight the regulation of lncRNAs-modulated cancer progression through various cell cycle regulators in tumors. LncRNAs can be used as diagnostic biomarkers and therapeutic targets for cancers, but this part of the research is still under-developed and needs to be further explored to provide new insights in the mechanisms of action and the development of new biomarkers and therapeutic targets.
Author Contributions
All authors made significant contributions to the reported work, including the conceptualization, literature collection, figure, and tabulation, or in all of these areas; participated in drafting, revising, or critically reviewing the article; gave final approval to the version to be published; have agreed on the journal to which the article has been submitted; and have agreed to take responsibility for all aspects of the work.
Funding
The study was funded by the National Natural Science Foundation of China (32000495), National Natural Science Foundation of Shandong Province (ZR2020MH202 and ZR2020MH250).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang Z. Regulation of cell cycle progression by growth factor-induced cell signaling. Cells. 2021;10(12):3327. doi:10.3390/cells10123327
2. Matthews HK, Bertoli C, de Bruin RAM. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2022;23(1):74–88. doi:10.1038/s41580-021-00404-3
3. Kitagawa M, Kitagawa K, Kotake Y, Niida H, Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell Mol Life Sci. 2013;70(24):4785–4794. doi:10.1007/s00018-013-1423-0
4. Zhang M, Zhang L, Hei R, et al. CDK inhibitors in cancer therapy, an overview of recent development. Am J Cancer Res. 2021;11(5):1913–1935.
5. Wenzel ES, Singh ATK. Cell-cycle checkpoints and aneuploidy on the path to cancer. In Vivo. 2018;32(1):1–5. doi:10.21873/invivo.11197
6. Ghafouri-Fard S, Shoorei H, Anamag FT, Taheri M. The role of non-coding RNAs in controlling cell cycle related proteins in cancer cells. Front Oncol. 2020;10:608975. doi:10.3389/fonc.2020.608975
7. Mens MMJ, Ghanbari M. Cell cycle regulation of stem cells by MicroRNAs. Stem Cell Rev Rep. 2018;14(3):309–322. doi:10.1007/s12015-018-9808-y
8. Zhang X, Wang W, Zhu W, et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. 2019;20(22). doi:10.3390/ijms20225573
9. Yin X, Lin H, Lin L, Miao L, He J, Zhuo Z. LncRNAs and CircRNAs in cancer. Med Comm. 2022;3(2):e141. doi:10.1002/mco2.141
10. Heydarnezhad Asl M, Pasban Khelejani F, Bahojb Mahdavi SZ, Emrahi L, Jebelli A, Mokhtarzadeh A. The various regulatory functions of long noncoding RNAs in apoptosis, cell cycle, and cellular senescence. J Cell Biochem. 2022;123(6):995–1024. doi:10.1002/jcb.30221
11. Chen B, Dragomir MP, Fabris L, et al. The long noncoding RNA CCAT2 induces chromosomal instability through BOP1-AURKB signaling. Gastroenterology. 2020;159(6):2146–2162.e33. doi:10.1053/j.gastro.2020.08.018
12. Gennaro VJ, Stanek TJ, Peck AR, et al. Control of CCND1 ubiquitylation by the catalytic SAGA subunit USP22 is essential for cell cycle progression through G1 in cancer cells. Proc Natl Acad Sci U S A. 2018;115(40):E9298–E9307. doi:10.1073/pnas.1807704115
13. Dumitru AMG, Compton DA. Identifying cyclin A/Cdk1 substrates in mitosis in human cells. Methods Mol Biol. 2022;2415:175–182. doi:10.1007/978-1-0716-1904-9_13
14. Pan M, Hong K, Chen X, et al. BmCyclin B and BmCyclin B3 are required for cell cycle progression in the silkworm, Bombyx mori. Sci China Life Sci. 2013;56(4):360–365. doi:10.1007/s11427-013-4459-3
15. Fagundes R, Teixeira LK. Cyclin E/CDK2: DNA replication, replication stress and genomic instability. Front Cell Dev Biol. 2021;9:774845. doi:10.3389/fcell.2021.774845
16. Jiang D, Li H, Xiang H, et al. Long Chain non-coding RNA (lncRNA) HOTAIR knockdown increases miR-454-3p to suppress gastric cancer growth by targeting STAT3/Cyclin D1. Med Sci Monit. 2019;25:1537–1548. doi:10.12659/MSM.913087
17. Wen X, Han W, Liu C. Long non-coding RNA TTTY15 silencing inhibits gastric cancer progression by sponging microRNA-98-5p to down-regulate cyclin D2 expression. Bioengineered. 2022;13(3):7380–7391. doi:10.1080/21655979.2022.2047398
18. Lv H, Zhou D, Liu G. PVT1/miR-16/CCND1 axis regulates gastric cancer progression. Open Med. 2023;18(1):20220550. doi:10.1515/med-2022-0550
19. Li L, Dong Z, Shi P, et al. Bruceine D inhibits cell proliferation through downregulating LINC01667/MicroRNA-138-5p/Cyclin E1 axis in gastric cancer. Front Pharmacol. 2020;11:584960. doi:10.3389/fphar.2020.584960
20. Ma P, Wang H, Sun J, et al. LINC00152 promotes cell cycle progression in hepatocellular carcinoma via miR-193a/b-3p/CCND1 axis. Cell Cycle. 2018;17(8):974–984. doi:10.1080/15384101.2018.1464834
21. Li J, Xia T, Cao J, et al. RP11-295G20.2 facilitates hepatocellular carcinoma progression via the miR-6884-3p/CCNB1 pathway. Aging. 2020;12(14):14918–14932. doi:10.18632/aging.103552
22. Lin SL, Lin YH, Chi HC, et al. A novel long non-coding RNA-01488 suppressed metastasis and tumorigenesis by inducing miRNAs that reduce vimentin expression and ubiquitination of cyclin E. Cells. 2020;9(6). doi:10.3390/cells9061504
23. Zhou J, Song G, Su M, Zhang H, Yang T, Song Z. Long noncoding RNA CASC9 promotes pancreatic cancer progression by acting as a ceRNA of miR-497-5p to upregulate expression of CCND1. Environ Toxicol. 2023. doi:10.1002/tox.23761
24. Wang H, Li H, Yu Y, et al. Long non-coding RNA XIST promotes the progression of esophageal squamous cell carcinoma through sponging miR-129-5p and upregulating CCND1 expression. Cell Cycle. 2021;20(1):39–53. doi:10.1080/15384101.2020.1856497
25. Ma J, Han LN, Song JR, et al. Long noncoding RNA LINC01234 silencing exerts an anti-oncogenic effect in esophageal cancer cells through microRNA-193a-5p-mediated CCNE1 downregulation. Cell Oncol. 2020;43(3):377–394. doi:10.1007/s13402-019-00493-5
26. Yan F, Ma Y, Liu L, Li L, Deng J, Sun J. Long noncoding RNA HOXD-AS1 promotes the proliferation, migration, and invasion of colorectal cancer via the miR-526b-3p/CCND1 axis. J Surg Res. 2020;255:525–535. doi:10.1016/j.jss.2020.05.078
27. Huang FK, Zheng CY, Huang LK, Lin CQ, Zhou JF, Wang JX. Long non-coding RNA MCF2L-AS1 promotes the aggressiveness of colorectal cancer by sponging miR-874-3p and thereby up-regulating CCNE1. J Gene Med. 2021;23(1):e3285. doi:10.1002/jgm.3285
28. Bian Y, Wang Y, Xu S, et al. m(6)A modification of long non-coding RNA HNF1A-AS1 facilitates cell cycle progression in colorectal cancer via IGF2BP2-mediated CCND1 mRNA stabilization. Cells. 2022;11(19). doi:10.3390/cells11193008
29. Dasgupta P, Kulkarni P, Majid S, et al. LncRNA CDKN2B-AS1/miR-141/cyclin D network regulates tumor progression and metastasis of renal cell carcinoma. Cell Death Dis. 2020;11(8):660. doi:10.1038/s41419-020-02877-0
30. Long B, Li N, Xu XX, et al. Long noncoding RNA LOXL1-AS1 regulates prostate cancer cell proliferation and cell cycle progression through miR-541-3p and CCND1. Biochem Biophys Res Commun. 2018;505(2):561–568. doi:10.1016/j.bbrc.2018.09.160
31. Zhou Z, Wu X, Zhou Y, Yan W. Long non-coding RNA ADAMTS9-AS1 inhibits the progression of prostate cancer by modulating the miR-142-5p/CCND1 axis. J Gene Med. 2021;23(5):e3331. doi:10.1002/jgm.3331
32. Liu Y, Gao S, Du Q, Zhao Q. Knockdown of long non-coding RNA metastasis associated lung adenocarcinoma transcript 1 inhibits the proliferation and migration of bladder cancer cells by modulating the microRNA-34a/cyclin D1 axis. Int J Mol Med. 2019;43(1):547–556. doi:10.3892/ijmm.2018.3959
33. Ai B, Kong X, Wang X, et al. LINC01355 suppresses breast cancer growth through FOXO3-mediated transcriptional repression of CCND1. Cell Death Dis. 2019;10(7):502. doi:10.1038/s41419-019-1741-8
34. Zhong H, Yang J, Zhang B, et al. LncRNA GACAT3 predicts poor prognosis and promotes cell proliferation in breast cancer through regulation of miR-497/CCND2. Cancer Biomark. 2018;22(4):787–797. doi:10.3233/CBM-181354
35. Zhang C, Yang T. Long non-coding RNA LINC00473 promotes breast cancer progression via miR-424-5p/CCNE1 pathway. Protein Pept Lett. 2023;30(1):72–84. doi:10.2174/0929866530666221026164454
36. Chang L, Guo R, Yuan Z, Shi H, Zhang D. LncRNA HOTAIR regulates CCND1 and CCND2 expression by sponging mir-206 in ovarian cancer. Cell Physiol Biochem. 2018;49(4):1289–1303. doi:10.1159/000493408
37. Ding C, Wei R, Rodríguez RA, Del Mar Requena Mullor M. LncRNA PCAT-1 plays an oncogenic role in epithelial ovarian cancer by modulating cyclinD1/CDK4 expression. Int J Clin Exp Pathol. 2019;12(6):2148–2156.
38. Bai X, Wang W, Zhao P, et al. LncRNA CRNDE acts as an oncogene in cervical cancer through sponging miR-183 to regulate CCNB1 expression. Carcinogenesis. 2020;41(1):111–121. doi:10.1093/carcin/bgz166
39. Zhang Y, Li X, Zhang J, Mao L. E6 hijacks KDM5C/lnc_000231/miR-497-5p/CCNE1 axis to promote cervical cancer progression. J Cell Mol Med. 2020;24(19):11422–11433. doi:10.1111/jcmm.15746
40. Hou A, Zhang Y, Zheng Y, Fan Y, Liu H, Zhou X. LncRNA terminal differentiation-induced ncRNA (TINCR) sponges miR-302 to upregulate cyclin D1 in cervical squamous cell carcinoma (CSCC). Hum Cell. 2019;32(4):515–521. doi:10.1007/s13577-019-00268-y
41. Wu C, Zhu XT, Xia L, et al. High expression of long noncoding RNA PCNA-AS1 promotes non-small-cell lung cancer cell proliferation and oncogenic activity via upregulating CCND1. J Cancer. 2020;11(7):1959–1967. doi:10.7150/jca.39087
42. Sun HB, Han XL, Zhong M, Yu DJ. Linc00703 suppresses non-small cell lung cancer progression by modulating CyclinD1/CDK4 expression. Eur Rev Med Pharmacol Sci. 2020;24(11):6131–6138. doi:10.26355/eurrev_202006_21508
43. Shan KZ, Yang SF, Deng YJ, Yue PY, Du ZQ. E2F1-induced long non-coding RNA MCF2L-AS1 modulates Cyclin D1 mRNA stability through ELAVL1 to induce Gefitinib resistance in non-small cell lung cancer. Acta Biochim Pol. 2022;69(4):795–804. doi:10.18388/abp.2020_6118
44. Ding H, Luo Y, Hu K, Liu P, Xiong M. Linc00467 promotes lung adenocarcinoma proliferation via sponging miR-20b-5p to activate CCND1 expression. Onco Targets Ther. 2019;12:6733–6743. doi:10.2147/OTT.S207748
45. Xue Y, Diao M, Lyu J, et al. Long noncoding RNAs PTPRG antisense RNA 1 targets cyclin D1 to facilitate cell proliferation in lung adenocarcinoma. Cancer Biother Radiopharm. 2021. doi:10.1089/cbr.2021.0168
46. Zhu H, Xu X, Zheng E, et al. LncRNA RP11‑805J14.5 functions as a ceRNA to regulate CCND2 by sponging miR‑34b‑3p and miR‑139‑5p in lung adenocarcinoma. Oncol Rep. 2022;48(3). doi:10.3892/or.2022.8376
47. Yang G, He F, Duan H, Shen J, Dong Q. lncRNA FLVCR-AS1 promotes osteosarcoma growth by targeting miR381-3p/CCND1. Onco Targets Ther. 2020;13:163–172. doi:10.2147/OTT.S214813
48. Guo F, Fu Q, Wang Y, Sui G. Long non-coding RNA NR2F1-AS1 promoted proliferation and migration yet suppressed apoptosis of thyroid cancer cells through regulating miRNA-338-3p/CCND1 axis. J Cell Mol Med. 2019;23(9):5907–5919. doi:10.1111/jcmm.14386
49. Shen XM, Han S, Liu N, Xu HQ, Yan CX, Yu CJ. LINC00887 aggravates the malignant progression of glioma via upregulating CCND1. Eur Rev Med Pharmacol Sci. 2021;25(4):1928–1935. doi:10.26355/eurrev_202102_25091
50. Wu Z. MiR-195 connects lncRNA RUNX1-IT1 and cyclin D1 to regulate the proliferation of glioblastoma cells. Int J Neurosci. 2023;133(1):13–18. doi:10.1080/00207454.2021.1881090
51. Liu Q, Ran R, Song M, et al. LncRNA HCP5 acts as a miR-128-3p sponge to promote the progression of multiple myeloma through activating Wnt/beta-catenin/cyclin D1 signaling via PLAGL2. Cell Biol Toxicol. 2021. doi:10.1007/s10565-021-09628-7
52. Zhang Y, Tan Y, Wang H, Xu M, Xu L. Long non-coding RNA Plasmacytoma Variant Translocation 1 (PVT1) enhances proliferation, migration, and Epithelial-Mesenchymal Transition (EMT) of pituitary adenoma cells by activating beta-Catenin, c-Myc, and Cyclin D1 expression. Med Sci Monit. 2019;25:7652–7659. doi:10.12659/MSM.917110
53. van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262(5142):2050–2054. doi:10.1126/science.8266103
54. Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130–146. doi:10.1038/nrd4504
55. Lin Z, Zhou Z, Guo H, et al. Long noncoding RNA gastric cancer-related lncRNA1 mediates gastric malignancy through miRNA-885-3p and cyclin-dependent kinase 4. Cell Death Dis. 2018;9(6):607. doi:10.1038/s41419-018-0643-5
56. Gao H, Yin Y, Qian A, Guo R, Qi J. LncRNA LINC00974 upregulates CDK6 to promote cell cycle progression in gastric carcinoma. Cancer Biother Radiopharm. 2019;34(10):666–670. doi:10.1089/cbr.2019.2904
57. Kang J, Huang X, Dong W, Zhu X, Li M, Cui N. Long non-coding RNA LINC00630 facilitates hepatocellular carcinoma progression through recruiting transcription factor E2F1 to up-regulate cyclin-dependent kinase 2 expression. Hum Exp Toxicol. 2021;40(12_suppl):S257–S268. doi:10.1177/09603271211038744
58. Zhou N, Li S, Wu D, Zhang F, Tang F, Li Y. The lncRNA VPS9D1-AS1 promotes hepatocellular carcinoma cell cycle progression by regulating the HuR/CDK4 axis. DNA Cell Biol. 2021;40(10):1278–1289. doi:10.1089/dna.2021.0235
59. Cheng T, Yao Y, Zhang S, et al. LINC00205, a YY1-modulated lncRNA, serves as a sponge for miR-26a-5p facilitating the proliferation of hepatocellular carcinoma cells by elevating CDK6. Eur Rev Med Pharmacol Sci. 2021;25(20):6208–6219. doi:10.26355/eurrev_202110_26991
60. Lin C, Xiang Y, Sheng J, Liu S, Cui M, Zhang X. Long non-coding RNA CRNDE promotes malignant progression of hepatocellular carcinoma through the miR-33a-5p/CDK6 axis. J Physiol Biochem. 2020;76(3):469–481. doi:10.1007/s13105-020-00754-0
61. Ma X, Luo J, Zhang Y, Sun D, Lin Y. LncRNA MCM3AP-AS1 upregulates CDK4 by sponging miR-545 to suppress G1 arrest in colorectal cancer. Cancer Manag Res. 2020;12:8117–8124. doi:10.2147/cmar.S247330
62. Li F, Jiang Z, Shao X, Zou N. Downregulation of lncRNA NR2F2 antisense RNA 1 induces G1 arrest of colorectal cancer cells by downregulating cyclin-dependent kinase 6. Dig Dis Sci. 2020;65(2):464–469. doi:10.1007/s10620-019-05782-5
63. Gong T, Li Y, Feng L, et al. CASC21, a FOXP1 induced long non-coding RNA, promotes colorectal cancer growth by regulating CDK6. Aging. 2020;12(12):12086–12106. doi:10.18632/aging.103376
64. Sun W, Nie W, Wang Z, Zhang H, Li Y, Fang X. Lnc HAGLR promotes colon cancer progression through sponging miR-185-5p and activating CDK4 and CDK6 in vitro and in vivo. Onco Targets Ther. 2020;13:5913–5925. doi:10.2147/OTT.S246092
65. Guo N, Sun Q, Fu D, Zhang Y. Long non-coding RNA UCA1 promoted the growth of adrenocortical cancer cells via modulating the miR-298-CDK6 axis. Gene. 2019;703:26–34. doi:10.1016/j.gene.2019.03.066
66. Zhang Y, Zhang H, Kang H, Huo W, Zhou Y, Zhang Y. Knockdown of long non-coding RNA HOST2 inhibits the proliferation of triple negative breast cancer via regulation of the let-7b/CDK6 axis. Int J Mol Med. 2019;43(2):1049–1057. doi:10.3892/ijmm.2018.3995
67. Wang Y, Ding M, Yuan X, et al. lncRNA SNHG15 promotes ovarian cancer progression through regulated CDK6 via sponging miR-370-3p. Biomed Res Int. 2021;2021:9394563. doi:10.1155/2021/9394563
68. Zhai Y, Liu Y, Wang Z, Wang W, Zhou J, Lu J. Long non-coding RNA LINC00313 accelerates cervical carcinoma progression by miR-4677-3p/CDK6 axis. Onco Targets Ther. 2021;14:2213–2226. doi:10.2147/OTT.S265007
69. Jin J, Chen X, Chen J, Geng X. Long noncoding RNA MACC1-AS1 is a potential sponge of microRNA-34a in cervical squamous cell carcinoma and upregulates cyclin-dependent kinase 6. Oncol Lett. 2020;19(3):2339–2345. doi:10.3892/ol.2020.11346
70. Fu X, Wang D, Shu T, Cui D, Fu Q. LncRNA NR2F2-AS1 positively regulates CDK4 to promote cancer cell proliferation in prostate carcinoma. Aging Male. 2020;23(5):1073–1079. doi:10.1080/13685538.2019.1670157
71. Xing Z, Zhang Z, Gao Y, et al. The lncRNA LINC01194/miR-486-5p axis facilitates malignancy in non-small cell lung cancer via regulating CDK4. Onco Targets Ther. 2020;13:3151–3163. doi:10.2147/ott.S235037
72. Guo H, Lin S, Gan Z, Xie J, Zhou J, Hu M. lncRNA FOXD3-AS1 promotes the progression of non-small cell lung cancer by regulating the miR-135a-5p/CDK6 axis. Oncol Lett. 2021;22(6):853. doi:10.3892/ol.2021.13114
73. Ma YF, Liang T, Li CR, Li YJ, Jin S, Liu Y. Long non-coding RNA HNF1A-AS1 up-regulation in non-small cell lung cancer correlates to poor survival. Eur Rev Med Pharmacol Sci. 2016;20(23):4858–4863.
74. Liu L, Chen Y, Li Q, Duan P. lncRNA HNF1A-AS1 modulates non-small cell lung cancer progression by targeting miR-149-5p/Cdk6. J Cell Biochem. 2019;120(11):18736–18750. doi:10.1002/jcb.29186
75. Liu Y, Li L, Shang P, Song X. LncRNA MEG8 promotes tumor progression of non-small cell lung cancer via regulating miR-107/CDK6 axis. Anticancer Drugs. 2020;31(10):1065–1073. doi:10.1097/cad.0000000000000970
76. Wu D, Qin BY, Qi XG, Hong LL, Zhong HB, Huang JY. LncRNA AWPPH accelerates the progression of non-small cell lung cancer by sponging miRNA-204 to upregulate CDK6. Eur Rev Med Pharmacol Sci. 2020;24(8):4281–4287. doi:10.26355/eurrev_202004_21008
77. Zhang Y, Ma L, Zhang T, Li P, Xu J, Wang Z. Long noncoding RNA TFAP2A-AS1 exerts promotive effects in non-small cell lung cancer progression via controlling the microRNA-548a-3p/CDK4 axis as a competitive endogenous RNA. Oncol Res. 2021;29(2):129–139. doi:10.32604/or.2022.03563
78. Cheng S, Zheng J, Liu X, et al. Knockdown of 91 H suppresses the tumorigenesis of osteosarcoma via inducing methylation of CDK4 promoter. Technol Cancer Res Treat. 2021;20:1533033821990006. doi:10.1177/1533033821990006
79. Chen Y, Tang G, Qian H, et al. LncRNA LOC100129620 promotes osteosarcoma progression through regulating CDK6 expression, tumor angiogenesis, and macrophage polarization. Aging. 2021;13(10):14258–14276. doi:10.18632/aging.203042
80. Xu J, Liao M. Long noncoding RNA SNHG6 promotes papillary thyroid cancer cells proliferation via regulating miR-186/CDK6 axis. Gland Surg. 2021;10(10):2935–2944. doi:10.21037/gs-21-586
81. Liu SL, Chen MH, Wang XB, et al. LncRNA PCGEM1 contributes to malignant behaviors of glioma by regulating miR-539-5p/CDK6 axis. Aging. 2021;13(4):5475–5484. doi:10.18632/aging.202476
82. Chen X, Li Y, Zuo C, et al. Long non-coding RNA H19 regulates glioma cell growth and metastasis via miR-200a-Mediated CDK6 and ZEB1 expression. Front Oncol. 2021;11:757650. doi:10.3389/fonc.2021.757650
83. Liu S, Li Y. LncRNA HAND2-AS1 attenuates glioma cell proliferation, invasion and migration by targeting CDK6. Neurol Res. 2022;44(8):677–683. doi:10.1080/01616412.2022.2035620
84. Wang Y, Tan QY, Shen Y, et al. LINC00704 contributes to the proliferation and accelerates the cell cycle of nasopharyngeal carcinoma cells via regulating ETS1/CDK6 axis. Kaohsiung J Med Sci. 2022. doi:10.1002/kjm2.12491
85. Hume S, Dianov GL, Ramadan K. A unified model for the G1/S cell cycle transition. Nucleic Acids Res. 2020;48(22):12483–12501. doi:10.1093/nar/gkaa1002
86. Guiducci G, Stojic L. Long noncoding RNAs at the crossroads of cell cycle and genome integrity. Trends Genet. 2021;37(6):528–546. doi:10.1016/j.tig.2021.01.006
87. Kotake Y, Tsuruda T. Long noncoding RNA ANROC on the INK4 locus functions to suppress cell proliferation. Cancer Genomics Proteomics. 2020;17(4):425–430. doi:10.21873/cgp.20201
88. Lou N, Liu G, Pan Y. Long noncoding RNA ANRIL as a novel biomarker in human cancer. Future Oncol. 2020;16(35):2981–2995. doi:10.2217/fon-2020-0470
89. Wan G, Mathur R, Hu X, et al. Long non-coding RNA ANRIL (CDKN2B-AS) is induced by the ATM-E2F1 signaling pathway. Cell Signal. 2013;25(5):1086–1095. doi:10.1016/j.cellsig.2013.02.006
90. Zhang K, Ni X, Ma X, Sun R, Qiu J, Luo C. LINC01012 upregulation promotes cervical cancer proliferation and migration via downregulation of CDKN2D. Oncol Lett. 2023;25(3):124. doi:10.3892/ol.2023.13710
91. Bury M, Le Calve B, Ferbeyre G, Blank V, Lessard F. New insights into CDK regulators: novel opportunities for cancer therapy. Trends Cell Biol. 2021;31(5):331–344. doi:10.1016/j.tcb.2021.01.010
92. Kong D, Hou Y, Li W, Ma X, Jiang J. LncRNA-ZXF1 stabilizes P21 expression in endometrioid endometrial carcinoma by inhibiting ubiquitination-mediated degradation and regulating the miR-378a-3p/PCDHA3 axis. Mol Oncol. 2022;16(3):813–829. doi:10.1002/1878-0261.12940
93. Gu J, Dai B, Shi X, et al. lncRNA HCG11 suppresses human osteosarcoma growth through upregulating p27 Kip1. Aging. 2021;13(17):21743–21757. doi:10.18632/aging.203517
94. Zhang G, Xu Y, Wang S, et al. LncRNA SNHG17 promotes gastric cancer progression by epigenetically silencing of p15 and p57. J Cell Physiol. 2019;234(4):5163–5174. doi:10.1002/jcp.27320
95. Wang Q, Zhang W, Yin D, Tang Z, Zhang E, Wu W. Gene amplification-driven lncRNA SNHG6 promotes tumorigenesis via epigenetically suppressing p27 expression and regulating cell cycle in non-small cell lung cancer. Cell Death Discov. 2022;8(1):485. doi:10.1038/s41420-022-01276-y
96. Han D, Gao X, Wang M, et al. Long noncoding RNA H19 indicates a poor prognosis of colorectal cancer and promotes tumor growth by recruiting and binding to eIF4A3. Oncotarget. 2016;7(16):22159–22173. doi:10.18632/oncotarget.8063
97. Huang J, Zhou N, Watabe K, et al. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1). Cell Death Dis. 2014;5(1):e1008. doi:10.1038/cddis.2013.541
98. Zhang Y, Tang L. The application of lncRNAs in cancer treatment and diagnosis. Recent Pat Anticancer Drug Discov. 2018;13(3):292–301. doi:10.2174/1574892813666180226121819
99. Nandwani A, Rathore S, Datta M. LncRNAs in cancer: regulatory and therapeutic implications. Cancer Lett. 2021;501:162–171. doi:10.1016/j.canlet.2020.11.048
100. Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. doi:10.1038/nm.3981
101. Jin T. LncRNA DRAIR is a novel prognostic and diagnostic biomarker for gastric cancer. Mamm Genome. 2021;32(6):503–507. doi:10.1007/s00335-021-09911-2
102. Amer RG, El Arab LR E, Abd El Ghany D, Saad AS, Bahie-Eldin N, Swellam M. Prognostic utility of lncRNAs (LINC00565 and LINC00641) as molecular markers in glioblastoma multiforme (GBM). J Neurooncol. 2022;158(3):435–444. doi:10.1007/s11060-022-04030-7
103. Chi J, Liu T, Shi C, et al. Long non-coding RNA LUCAT1 promotes proliferation and invasion in gastric cancer by regulating miR-134-5p/YWHAZ axis. Biomed Pharmacother. 2019;118:109201. doi:10.1016/j.biopha.2019.109201
104. Zhou Q, Hou Z, Zuo S, et al. LUCAT1 promotes colorectal cancer tumorigenesis by targeting the ribosomal protein L40-MDM2-p53 pathway through binding with UBA52. Cancer Sci. 2019;110(4):1194–1207. doi:10.1111/cas.13951
105. Wang W, Dong ML, Zhang W, Liu T. Long noncoding LUCAT1 promotes cisplatin resistance of non-small cell lung cancer by promoting IGF-2. Eur Rev Med Pharmacol Sci. 2019;23(12):5229–5234. doi:10.26355/eurrev_201906_18188
106. Badowski C, He B, Garmire LX. Blood-derived lncRNAs as biomarkers for cancer diagnosis: the good, the bad and the beauty. NPJ Precis Oncol. 2022;6(1):40. doi:10.1038/s41698-022-00283-7
107. Tantai J, Hu D, Yang Y, Geng J. Combined identification of long non-coding RNA XIST and HIF1A-AS1 in serum as an effective screening for non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(7):7887–7895.
108. Zhang ZZ, Zhao G, Zhuang C, et al. Long non-coding RNA LINC00628 functions as a gastric cancer suppressor via long-range modulating the expression of cell cycle related genes. Sci Rep. 2016;6:27435. doi:10.1038/srep27435
109. Li SP, Xu HX, Yu Y, et al. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget. 2016;7(27):42431–42446. doi:10.18632/oncotarget.9883
110. Fu X, Li H, Liu C, Hu B, Li T, Wang Y. Long noncoding RNA AK126698 inhibits proliferation and migration of non-small cell lung cancer cells by targeting Frizzled-8 and suppressing Wnt/β-catenin signaling pathway. Onco Targets Ther. 2016;9:3815–3827. doi:10.2147/ott.S100633
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.