Back to Journals » OncoTargets and Therapy » Volume 12
Celastrol inhibits colorectal cancer through TGF-β1/Smad signaling
Authors Jiang Z, Cao Q, Dai G, Wang J, Liu C, Lv L, Pan J
Received 17 September 2018
Accepted for publication 4 December 2018
Published 9 January 2019 Volume 2019:12 Pages 509—518
DOI https://doi.org/10.2147/OTT.S187817
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Zhitao Jiang,1,* Qianyu Cao,2,* Guoliang Dai,3 Jianchun Wang,1 Chundi Liu,1 Lingyan Lv,1 Jinhuo Pan4
1Department of Pharmacy Office, Zhangjiagang Hospital of Traditional Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Zhangjiagang, China; 2The First Clinical College, Nanjing University of Chinese Medicine, Nanjing, China; 3Department of Clinical Pharmacology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China; 4College of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, China
*These authors contributed equally to this work
Background: There are few clinical challenges associated with the treatment of colorectal cancer (CRC). Studies have shown that TGF-β plays a crucial role in CRC. Importantly, celastrol, a major components of the root extract of the traditional Chinese herb Tripterygium wilfordii Hook F, has been shown to inhibit the growth, adhesion, and metastasis of human CRC cells through the inhibition of TGF-β1/Smad signaling.
Materials and methods: Real-time PCR and Western blot tests were proceeded to present TGF-β1, TGF-β receptor type I (TGFβRI), TGF-β receptor type II (TGFβRII), Smad2/3, p-Smad2/3, Smad4, and glyceraldehyde-3-phosphate dehydrogenase expression in human colon cancer cell samples.
Results: Our results indicated that celastrol can reduce the expression levels of TGF-β1, TGFβRI, and TGFβRII in HCT116 and SW620 cells. Furthermore, celastrol could also prevent the increase in Smad4 and p-Smad2/3 in HCT116 and SW620 cells.
Conclusion: Celastrol could inhibit tumor growth through TGF-β1/Smad signaling and might be a promising therapeutic component against CRC.
Keywords: celastrol, colorectal cancer, TGF-β1, Smad
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer with high morbidity and mortality. Surgery is always the first choice if this cancer is diagnosed at the earliest stage. However, since the symptoms of CRC are quite latent and are difficult to discovery at early stage, the majority of the CRC patients are diagnosed later and occasionally with metastasis.1,2 However, an effective treatment for CRC has not been discovered yet. Consequently, a safer and more effective compound against CRC is in high demand.
TGF-β1 has been considered to play a crucial role in the development and progression of CRC. TGF-β1 has two receptors, type I TGF-β receptors (TGFβRI) and type II TGF-β receptors (TGFβRII). Once bound to TGF-β, TGFβRII phosphorylate and activate TGFβRI. The TGF-β1 stimulation triggers the activation of Smads signaling. Previous researches have suggested that TGFβ signaling promoted cancer cell growth, invasion, and metastasis, and inhibition of TGF-β1 prevents metastatic cancer progression.3,4 Hence, the mechanism of TGF-β1 inhibitor that was used for the treatment of CRC has an urgent need to elucidate.
Celastrol is a pharmacologically active compound extracted from the “God of Thunder Vine” or Tripterygium wilfordii Hook F, which has a long history of use in traditional Chinese medicine for the treatment of cancer and other inflammatory diseases.5 Mechanistically, it has proved that celastrol has an capacity to cut down the generation of inflammatory cytokines, such as IL-1, TNF-α, IL-6, and IL-8.6 Recently, celastrol was reported to ameliorate dextran sulfate sodium-induced colitis in mice, by modulating intestinal epithelial homeostasis, oxidative stress in colon, and by lessening inflammatory cytokines.7 However, its mechanism of action remains largely undefined.
Oxaliplatin is commonly used for the effective treatment of CRC. With the use of oxaliplatin, the overall survival of patients with CRC has significantly improved.8 In our experiment, we have used oxaliplatin as a positive control.
Here, we demonstrated the effects of TGF-β1 in CRC cells. Our results indicated that celastrol could inhibit CRC through TGF-β1/Smad signaling and might be a promising therapeutic component against CRC.
Materials and methods
Human samples
The ethics committee of the Nanjing Hospital of Chinese Medicine approved this study. A written informed consent was obtained from each patient, and this study was conducted in accordance with the Declaration of Helsinki. All 50 patients granted full consent for the study. Exclusion criteria were as follow: 1) patients undergoing treatment prior to surgery including chemotherapy, 2) patients with gastrointestinal stromal tumors or lymphoma, 3) patients with other cancers, or 4) patients refusing to participate in this study. After histologically diagnosed, CRC samples and adjacent normal tissues were collected through surgery in Nanjing Hospital of Chinese Medicine.
Reagents
Celastrol was obtained from Sigma-Aldrich (St Louis, MO, USA). The primary antibodies of TGF-β1, Smad2/3, p-Smad2/3, TGFβRI, TGFβRII, and Smad4 were purchased from Abcam (Cambridge, UK). Cell counting kit-8 (CCK-8) was purchased from Biosharp (Hefei, China). Antibody for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and other reagents were purchased from Sigma-Aldrich.
Histomorphological assessment
Human CRC and adjacent normal colon samples were fixed in 4% paraformaldehyde, made into wax blocks, cut into slices. Slices were stained with H&E, as previously described.9
Western blot
Samples were collected, lysed, and then cut into isolated by SDS-PAGE electrophoresis and electrophoretically transferred to polyvinylidene fluoride membranes (Millipore). The membranes were immersed in 5% BSA for not less than 1 hour. After that, the membranes were immersed in primary antibodies (GAPDH, 1:5,000; TGF-β1, 1:1,000; TGFβRI, 1:1,000; TGFβRII, 1:1,000; Smad2/3, 1:500; p-Smad2/3, 1:500; and Smad4, 1:500) at 4°C for more than 8 hours. The membranes were then incubated with secondary antibodies (Chemicon, USA) and developed using enhanced chemiluminescence products (PerkinElmer Inc., Waltham, MA, USA). Photos were obtained using a Molecular Imager System (Gel DocTM XR, 170–8170) and analyzed by the Quantity One-4.6.5 software.
Cell culture
HCT116 and SW620 cells were purchased from the American Type Culture Collection. When transported, the cells grow in a bottle filled with complete DMEM medium containing 10% FBS, 100 mg/mL streptomycin, and 100 U/mL penicillin.
CCK assay for cell proliferation
A gentle centrifugation was done to collect HCT116 or SW620 cells. A total of 1×104 cells (a well) were seeded in 96-well plates. Twenty-four hours later, the cells were cocultured with celastrol in different concentrations for 24 hours. CCK-8 reagent was diluted according to the product manual, and the cells were incubated in the diluted reaction solution for 4 hours. A microplate reader (Bio-Rad, Los Angeles, CA, USA) was used to test the OD values at 450 nm.
Cell adhesion test
The cell adhesion test was conducted as conducted previously.10 In brief, HCT116 and SW620 cells were cultured with or without celastrol for 24 hours. After that, cells were added to 96-well plates, which had been coated with fibronectin, labeled with fluorescein diacetate (5 μg/mL) for 20 minutes, and washed with PBS to remove the unbound cells. DMI3000B inverted microscope (Leica, Germany) was used to get immunofluorescence images.
In vitro migration and invasion tests
The migration and invasion tests were implemented as carried out previously.11 Cells were cultured with or without celastrol for 24 hours for migration and invasion assays.
Real-time PCR
Real-time PCR (RT-PCR) was performed as previously described.11 RNA was extracted using TRIzol reagent and reverse transcribed into cDNA for PCR according to the manufacturer’s instructions. The sense and antisense primers of TGF-β1, TGFβRI, TGFβRII, Smad2/3, p-Smad2/3, Smad4, and GAPDH expression were as follows: TGF-β1: 5′-GTGGAAACCCACAACGAAAT-3′ and 5′-CACGTGCTGCTCCACTTTTA-3′, TGFβRI: 5′-GCAGTAAGACATGATTCAGCCACAG-3′ and 5′-CAATGGAACATCGTCGAGCAA-3′, TGFβRII: 5′-TCGTCCTGTGGACGCGTATC-3′ and 5′-GAAACTTGACTGCACCGTTGTTG-3′, Smad2: 5′-GTCTGCCTTCGGTATTCTGC-3′and 5′-GCTGCTCTTCTGGCTCAGTC-3′, Smad3 5′-AACAACCAGGAGTTCGCTGC-3′ and 5′-GGACCTTGTCAAGCCACTGC-3′, and Smad4: 5′-GGACCGGATTACCCAAGACAGA-3′ and 5′-CTGCAATCGGCATGGTATGAAG-3′.
Statistical analyses
Student’s two-tailed t-test was performed in two groups for comparison. ANOVA test was performed in more than three groups. A Dunnett’s test was used as a method of posttesting. Results are described as mean ± SD. P<0.05 was considered statistically significant. All analyses were performed with GraphPad Prism 7.0 Software.
Results
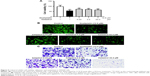
TGF-β/Smad signaling was instigated in human CRC
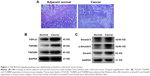
Initially, the H&E analysis was carried out to examine the different pathological state between human colorectal tumors and adjacent normal colon tissues. Our results demonstrated moderately differentiation adenocarcinoma (Figure 1A). We also investigated whether TGF-β/Smad signaling was instigated in these samples. As can be observed in Figure 1B, the expressions of TGF-β1, TGFβRI, and TGFβRII were signally augmented in human colorectal tumors, while in the adjacent normal colon tissues, they are placid. Furthermore, the expressions of Smad2/3, p-Smad2/3, and Smad4 were also signally augmented in CRC tissues (Figure 1C).
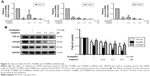
Celastrol inhibited the propagation and metastasis of HCT116 cells
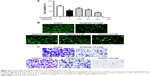
Next, we focus our vision on the influences of celastrol on HCT116 cells. Celastrol could dose dependently suppress human CRC HCT116 cell viability significantly (Figure 2A). Moreover, celastrol dramatically inhibited the adhesion and migratory capacity of HCT116 cells (Figure 2B and C).
Celastrol inhibited the expressions of TGF-β1 and its receptors in HCT116 cells
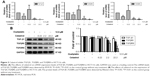
To test the effects of celastrol on TGF-β1 function, we measured the levels of TGF-β1 and its receptors in HCT116 cells. The expression of mRNA levels was determined by PCR. Results showed that the TGF-β1, TGFβRI, and TGFβRII mRNA levels were remarkably decreased by celastrol in HCT116 cells (Figure 3A). Moreover, the TGF-β1, TGFβRI, and TGFβRII protein levels were also significantly inhibited by celastrol (Figure 3B).
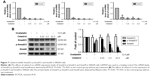
Celastrol inhibited Smad signaling in HCT116 cells
PT-PCR analysis revealed that treatment with celastrol significantly inhibited the mRNA expression of Smad2, Smad3, and Smad4 (Figure 4A). The protein expression levels were then determined by Western blot analysis. Results showed that celastrol significantly inhibited the expression of p-Smad2/3 and Smad4 in HCT116 cells (Figure 4B).
Celastrol inhibited the propagation and metastasis of SW620 cells
SW620 colon carcinoma cell line is a common resource for verifying genetic changes in the late progression of colon cancer, which is derived from secondary tumors. Further analysis was carried out to check out the influence of celastrol in SW620 cells. Celastrol significantly suppressed SW620 cell viability (Figure 5A). Moreover, celastrol significantly inhibited the adhesion and migratory capacity of SW620 cells (Figure 5B and C), which was reconciled with the data in HCT116 cells.
Celastrol the expressions of TGF-β1 and its receptors in SW620 cells
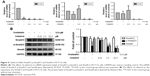
To test the influences of celastrol on TGF-β1 and its receptors, we measured the mRNA levels of TGF-β1 in SW620 cells by RT-PCR. Our data displayed that celastrol signally reduced the mRNA expression of TGF-β1, TGFβRI, and TGFβRII (Figure 6A). Moreover, the TGF-β1, TGFβRI, and TGFβRII protein levels were also significantly inhibited by celastrol in SW620 cells (Figure 6B).
Celastrol inhibited Smad signaling in SW620 cells
Celastrol significantly inhibited the mRNA levels of Smad2, Smad3, and Smad4 (Figure 7A). The protein expression levels were then determined by Western blot analysis. Results showed that celastrol significantly reduced the expression of p-Smad2/3 and Smad4 in SW620 cells (Figure 7B).
Discussion
The present study investigated whether the celastrol has the capacity to treat CRC. We found that celastrol significantly inhibited the growth, adhesion, and metastasis of human CRC cells. Western blot and RT-PCR tests confirmed that both mRNA and protein expression levels of TGF-β1 and its receptors TGFβRI and TGFβRII were reduced by celastrol in HCT116 and SW620 cells. Furthermore, celastrol reduced the levels of Smad4 and p-Smad2/3. Altogether, celastrol may inhibit CRC through TGF-β1, which is a promising treatment for CRC.
CRC is an uppermost cause of cancer-related deaths. During the initial diagnosis, metastasis occurs in 18% of patients with rectal cancer and in 20%–25% of patients with colon cancer.2 Chemotherapy is a routine treatment for advanced cancer. The survival of patients with advanced CRC increases with the effective use of chemotherapy drugs, including fluorouracil–leucovorin, oxaliplatin, and irinotecan.12 However, chemotherapy is associated with serious side effects such as nausea, vomiting, and pain, which can also lead to dose reduction or discontinuation. Therefore, it is important to find new compounds for the treatment of CRC.
Bharat Aggarwal et al summarized that various nutraceuticals such as celastrol from “mother nature” have a huge potential for both prevention and treatment of CRC.13 Rita Cascao systematically reviewed the mechanism of action and the therapeutic properties of celastrol.14 A recent study reported that celastrol could inhibit CRC cell proliferation and migration, and this effect was involved in PI3K/AKT signaling pathway.15 Some researchers have reported the effect of celastrol on other cancers, such as oral squamous cell carcinoma,16 human hepatocellular carcinoma,17 and breast cancer.18 These results show that celastrol has the exact effect on inhibiting other tumors. A recent report exposed that celastrol could effectively ameliorate ulcerative colitis-associated CRC by decreasing the inflammatory reactions and epithelial–mesenchymal transition (EMT).19 They used colonic tumor xenograft models, HCT116 cells, and HT-29 cells to investigate the changes of inflammatory factors and EMT-associated proteins.19 However, studies related to TGF-β1 as an upstream signaling pathway for EMT were ignored.
TGF-β is known to be a tumor suppressor. In the early stages of tumor, it inhibits cell proliferation and induces apoptosis. On the contrary, it acts as a tumor promoter in the later stages of the tumor by inducing invasion and metastasis.20 High levels of TGF-β1 are shown in CRC patients and are associated with tumor progression, recurrence, and low patient survival.21,22 Therefore, inhibition of TGF-β1 may be an aspect of anti-CRC drug development.
There are strong proofs have a access to support that celastrol has the potential therapeutic action in cancer.18,19 It modulates the expression of proinflammatory cytokines6 and suppression of EMT.19 Some of the effects of celastrol are also mediated through TGF-β regulation, since TGF-β signaling has been shown to play a crucial role in EMT. Altogether, these outputs imply that celastrol may be used as a neo-inhibitor of TGF-β1 for CRC treatment.
Currently, we systematically studied the TGF-β1, TGFβRI, and TGFβRII mRNA and protein levels in human CRC. The outputs revealed that their expression in human colon cancer tissues was signally increased (Figure 1B). This is consistent with the other study. Importantly, celastrol could suppress the propagation, adhesion, and metastasis of CRC cells (Figures 2 and 5), and decrease the capacities of TGF-β1 and its receptors (Figures 3 and 6). Meanwhile, the levels of p-Smad2/3 and Smad4 were increased in human colon cancer tissues (Figure 1C). Smad2/3 is the most important signal transduction factor for the transport of TGF-β1 signaling, which is phosphorylated by TGFβRI and forms hetero-oligomers with Smad4.23–25 Here, our outputs show that celastrol could suppress Smad4 and p-Smad2/3 in HCT116 and SW620 cells. Consistent with our outputs, a recent study showed that celastrol decreased TGF-β1-induced EMT and reduced lung cancer invasion and migration.26 Taken together, these results indicate that TGF-β1/Smad signaling is participated in the anti-CRC effect of celastrol. However, the clinical application of celastrol to inhibit CRC requires further research.
In general, high levels of TGF-β1, TGFβRI, and TGFβRII were observed in the CRC specimens. TGF-β1/Smad signaling pathway is involved in the antitumor function of celastrol (Figure 8). Thus, celastrol might be a promising therapeutic component against CRC.
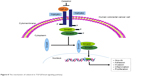  | Figure 8 The mechanism of celastrol in TGF-β/Smad signaling pathway. |
Acknowledgment
This work was supported by the Jiangsu Province Science and Technology Support Program (grant No BE2009682) and Suzhou Science and Technology Project (grant No SYSD2017161).
Disclosure
The authors report no conflicts of interest in this work.
References
Cunningham D, Atkin W, Lenz H-J, et al. Colorectal cancer. Lancet. 2010;375(9719):1030–1047. | ||
de Rosa M, Pace U, Rega D, et al. Genetics, diagnosis and management of colorectal cancer (Review). Oncol Rep. 2015;34(3):1087–1096. | ||
Massagué J. TGFbeta in cancer. Cell. 2008;134(2):215–230. | ||
Biswas S, Guix M, Rinehart C, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117(5):1305–1313. | ||
Calixto JB, Campos MM, Otuki MF, Santos AR. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004;70(2):93–103. | ||
He W, Huang FC, Gavai A, et al. Novel cytokine release inhibitors. Part III: truncated analogs of tripterine. Bioorg Med Chem Lett. 1998;8(24):3659–3664. | ||
Shaker ME, Ashamallah SA, Houssen ME. Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokines and intestinal homeostasis. Chem Biol Interact. 2014;210:26–33. | ||
Becouarn Y, Rougier P. Clinical efficacy of oxaliplatin monotherapy: phase II trials in advanced colorectal cancer. Semin Oncol. 1998;25(2 Suppl 5):23–31. | ||
Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008(6):pdb.prot4986. | ||
Tai YT, Podar K, Catley L, et al. Insulin-like growth factor-1 induces adhesion and migration in human multiple myeloma cells via activation of beta1-integrin and phosphatidylinositol 3′-kinase/AKT signaling. Cancer Res. 2003;63(18):5850–5858. | ||
Dai G, Sun B, Gong T, Pan Z, Meng Q, Ju W. Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of colorectal cancer cells by suppressing TGF-β/Smad signaling. Phytomedicine. 2019;56:126–135. | ||
Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22(7):1209–1214. | ||
Aggarwal B, Prasad S, Sung B, Krishnan S, Guha S. Prevention and treatment of colorectal cancer by natural agents from mother nature. Curr Colorectal Cancer Rep. 2013;9(1):37–56. | ||
Cascão R, Fonseca JE, Moita LF, Moita, Celastrol LF. Celastrol: a spectrum of treatment opportunities in chronic diseases. Front Med. 2017;4:69. | ||
Bufu T, di X, Yilin Z, Gege L, Xi C, Ling W. Celastrol inhibits colorectal cancer cell proliferation and migration through suppression of MMP3 and MMP7 by the PI3K/AKT signaling pathway. Anticancer Drugs. 2018;29(6):530–538. | ||
Fribley AM, Miller JR, Brownell AL, et al. Celastrol induces unfolded protein response-dependent cell death in head and neck cancer. Exp Cell Res. 2015;330(2):412–422. | ||
Zhu H, Yang W, He LJ, et al. Upregulating Noxa by ER stress, celastrol exerts synergistic anti-cancer activity in combination with ABT-737 in human hepatocellular carcinoma cells. PLoS One. 2012;7(12):e52333. | ||
Yadav VR, Sung B, Prasad S, et al. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J Mol Med. 2010;88(12):1243–1253. | ||
Lin L, Sun Y, Wang D, Zheng S, Zhang J, Zheng C. Celastrol ameliorates ulcerative colitis-related colorectal cancer in mice via suppressing inflammatory responses and epithelial-mesenchymal transition. Front Pharmacol. 2015;6:320. | ||
Xu Y, Pasche B. TGF-beta signaling alterations and susceptibility to colorectal cancer. Hum Mol Genet. 2007;1:R14–R20. | ||
Gulubova M, Manolova I, Ananiev J, Julianov A, Yovchev Y, Peeva K. Role of TGF-beta1, its receptor TGFbetaRII, and Smad proteins in the progression of colorectal cancer. Int J Colorectal Dis. 2010;25(5):591–599. | ||
Picon A, Gold LI, Wang J, et al. A subset of metastatic human colon cancers expresses elevated levels of transforming growth factor beta1. Cancer Epidemiol Biomarkers Prev. 1998;7(6):497–504. | ||
Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67(1):753–791. | ||
Hill CS. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2009;19(1):36–46. | ||
Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783–2810. | ||
Kang H, Lee M, Jang SW. Celastrol inhibits TGF-β1-induced epithelial-mesenchymal transition by inhibiting Snail and regulating E-cadherin expression. Biochem Biophys Res Commun. 2013;437(4):550–556. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.