Back to Journals » Infection and Drug Resistance » Volume 16
Ceftazidime/Avibactam for the Treatment of Carbapenem-Resistant Pseudomonas aeruginosa Infection in Lung Transplant Recipients
Authors Chen J, Liang Q, Ding S, Xu Y, Hu Y, Chen J, Huang M
Received 7 February 2023
Accepted for publication 5 April 2023
Published 15 April 2023 Volume 2023:16 Pages 2237—2246
DOI https://doi.org/10.2147/IDR.S407515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Juan Chen,1,* Qiqiang Liang,1,* Shuo Ding,1 Yongshan Xu,1 Yanting Hu,1 Jingyu Chen,2,3 Man Huang1,3
1Department of General Intensive Care Unit, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China; 2Wuxi Lung Transplant Center, Wuxi People’s Hospital affiliated to Nanjing Medical University, Wuxi, Jiangsu, People’s Republic of China; 3Department of Lung Transplantation, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Man Huang, Department of General Intensive Care Unit, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China, Tel/Fax +86 571 89713427, Email [email protected] Jingyu Chen, Wuxi Lung Transplant Center, Wuxi People’s Hospital affiliated to Nanjing Medical University, Wuxi, Jiangsu, People’s Republic of China, Email [email protected]
Background: Experience of ceftazidime-avibactam (CAZ/AVI) for carbapenem-resistant Pseudomonas aeruginosa (CRPA) infection in recipients after lung transplantation (LT) is relatively limited.
Methods: A retrospective observational study was conducted on lung transplant recipients receiving CAZ/AVI therapy for CRPA infection. The primary outcomes were the 14-day and 30-day mortality. The secondary outcomes were clinical cure and microbiological cure.
Results: Among 183 LT recipients, a total of 15 recipients with CRPA infection who received CAZ/AVI therapy were enrolled in this study. The mean age of recipients was 54 years and 73.3% of recipients were male. The median time from infection onset to initiation of CAZ/AVI treatment was 4 days (IQR, 3– 7) and the mean duration of CAZ/AVI therapy was 10 days. CAZ/AVI was mainly administered as monotherapy in LT recipients (80%). Among these eligible recipients, 14-day and 30-day mortality were 6.7% and 13.3%, respectively. The clinical cure and microbiological cure rates of CAZ/AVI therapy were 53.3% and 60%, respectively. Three recipients (20%) experienced recurrent infection. In addition, the mean lengths of ICU stay and hospital stay were 24 days and 35 days, respectively, among LT recipients.
Conclusion: CAZ/AVI may be an alternative and promising regimen for CRPA eradiation in lung transplant recipients.
Keywords: carbapenem-resistantPseudomonas aeruginosa, ceftazidime/avibactam, lung transplantation, infection, efficacy, mortality
Introduction
Lung transplantation is currently the only therapeutic option for selected patients with end-stage pulmonary disease.1 Although survival among patients with lung transplantation has been improved over time,2 the long-term survival after transplantation is limited.3 It was reported that the 5-year survival after transplantation was about 60% worldwide.3–5 Notably, within the first year after transplantation, infection is one of the two leading causes of death.4,6,7
Unlike other common organ transplants, lung transplant has a high risk of infection because the lung allograft as an essential component of airway is in direct contact with environment outside, leading to the invasion of microorganisms. Besides, transplantation recipients are exposed to risk factors of infection, including the use of immunosuppressive agents, such as tacrolimus, more invasive operations, and prolonged ICU stay.8,9 Therefore, the incidence of infection and colonization after lung transplantation increased, including a variety of multidrug-resistant (MDR) bacterial infections. It was reported that infections were frequent with high incidence among 2761 recipients of solid organ transplant (SOT), and bacteria predominated, including Enterobacteriaceae, Pseudomonas aeruginosa (PA), and Enterococcus spp.10 Importantly, P. aeruginosa was the major bacterial pathogens identified in proven infections of lung transplant.10 Almost 50% of P. aeruginosa bloodstream infections were caused by MDR strains.8 In a prospective observational study of Spain, among P. aeruginosa bacteremia of SOT recipients, 63% of bacteremia caused by extensively drug-resistant (XDR) P. aeruginosa.11 Meanwhile, in transplant recipients, the incidence of infections caused by MDR/XDR P. aeruginosa was higher than in the general population.8,12,13 Notably, MDR P. aeruginosa infection was related with high mortality in case of bacteremia among SOT recipients.11,14,15 Although carbapenems are considered as the drugs of choice for severe P. aeruginosa infections caused by MDR-producing cephalosporinase AmpC or extended-spectrum β-lactamases,16 the prevalence of carbapenem-resistant P. aeruginosa (CRPA) has risen in recent years.17 The World Health Organization (WHO) ranked CRPA as the critical-priority bacterium,18 which posed a global threat to public health. Thus, a therapeutic approach to eradicate CRPA pathogens must be explored and developed.
CAZ/AVI is a new β-lactam/β-lactamase inhibitor, which has been approved by FDA for the treatment of complicated urinary tract infections, complicated intra-abdominal infections, hospital-acquired pneumonia, and infections caused by Gram-negative organisms in patients with limited treatment options.19,20 Available studies had reported the efficacy and safety of CAZ/AVI for refractory P. aeruginosa infection,21–25 but there is rather limited evidence in lung transplant patients. Thus, the purpose of this study was to investigate the therapeutic efficacy of CAZ/AVI against CRPA infection in lung transplant cases.
Materials and Methods
Study Population
This retrospective, single-center, observational study was conducted at a tertiary hospital with 3500 beds in Hangzhou, Zhejiang Province, China. Lung transplant recipients with carbapenem-resistant Pseudomonas aeruginosa infections were enrolled from February 2020 to May 2022. The inclusion criteria of study were as follows: LT recipients with age > 18 years old, a documented CRPA infection, and CAZ/AVI treatment more than 72 hours. The exclusion criteria were: incomplete clinical data, contamination of culture samples, colonization, multiple bacterial infections, and severe fungal infections. During the study period, CAZ/AVI was administered for CRPA infection at recommended dosage according to the guidelines. The lung transplantation recipients received immunosuppressive drugs including tacrolimus (or cyclosporin A), mycophenolate mofetil (MMF), and corticosteroid.5 This study was approved by the Research Ethics Committee of the Second Affiliated Hospital of Zhejiang University (approval number: 2022–0054) and was in accordance with the Declaration of Helsinki. The informed consent was waived due to the anonymous and retrospective nature of this study. All lungs were donated voluntarily with written informed consent, and organ donations and transplantations were conducted in accordance with the Declaration of Istanbul.
Data Collection
Demographic and clinical data of patients were collected from electronic medical record system. Baseline characteristics included age, sex, BMI, primary pulmonary disease, and comorbidities. The degree of comorbidity was quantified using the Charlson Comorbidity Index (CCI). The severity of illness was accessed by Acute Physiology and Chronic Health Evaluation II (APACHE II) and the Sequential Organ Failure Assessment (SOFA). Therapeutic variables included the treatment course, time to initiation of therapy, monotherapy or combined therapy, and antibiotics regimens. Other variables consisted of tracheotomy, continuous renal replacement therapy (CRRT), septic shock, and the length of ICU stay and hospitalization. The primary outcomes were the 14-day and 30-day mortality. The secondary outcomes were clinical cure and microbiological cure. In addition, the results of antimicrobial susceptibility testing were collected.
Definition
The definition of sepsis shock was according to the Third International Consensus Definitions For Sepsis and Sepsis shock (sepsis-3).26 Pulmonary infection was identified on the basis of the standard definitions approved by The International Society for Heart and Lung Transplantation (ISHLT).27 Colonization was defined as positive microbiologic culture in patients with no symptoms and signs associated with infection.27 Combined therapy referred to the treatment of two or more antibiotics for infection. The 14-day and 30-day mortality referred to deaths occurring within 14 and 30 days after the onset of infection, respectively. Clinical cure was defined as the resolution of clinical symptoms and signs as well as the laboratory index related with infection within 14 days from the initiation of CAZ/AVI treatment.28 Microbiological cure was considered as a negative culture of sample following ≥ 7 days of CAZ/AVI treatment.29 Infection relapse was identified when a second microbiologically documented CRPA infection occurred in a patient whose initial infection was categorized as clinical cure.
Microbiology
Microorganism identification was performed with the VITEK system (bioMérieux, Marcy l’Etoile, France), and antimicrobial susceptibility testing was performed and interpreted in accordance with the Clinical and Laboratory Standards Institute (CLSI) standards.30 The minimum inhibitory concentration (MIC) resistance breakpoints for the tested antimicrobial agents to PA isolates were on basis of the criteria of CLSI.
Statistical Analysis
Continuous variables with normal distribution were expressed as the mean and standard deviation, otherwise they were described by median and interquartile range (IQR). Categorical variables were represented by frequency and percentage. The differences of variables between groups were assessed by the Chi-square test for categorical variables, and Mann–Whitney U-test for continuous variables. Statistical analysis was performed in IBM SPSS Statistics v.25.0.
Results
Baseline Characteristics
During the period of study, a total of 15 recipients with CRPA infection treated with CAZ/AVI were enrolled among 183 LT recipients (Figure 1). The baseline and clinical characteristics of eligible patients are shown in Table 1. The mean age of the recipients was 54 years and 73.3% (11/15) were male. The primary pulmonary diseases of the recipients included interstitial lung disease (ILD) (n=9, 60%), chronic obstructive pulmonary disease (COPD) (n=1, 6.7%), bronchiectasis (n=1, 6.7%), and others (n=4, 26.7%). Bilateral LT was performed in 12 recipients (80%), with single LT conducted in 3 recipients (20%). The median CCI score was 2 (IQR, 1–3) and the age-adjusted Charlson Comorbidity Index (aCCI) score was 3.13±1.85. The concurrent underlying diseases in recipients included cardiovascular disease (n=6, 40%), chronic liver disease (n=1, 6.7%), diabetes (n=3, 20%), hypertension (n=2, 13.3%), solid tumor (n=3, 20%), and hematological malignancy (n=1, 6.7%). There were five patients (33.3%) with tracheotomy and six patients (40%) undergoing CRRT. Besides, the source of infection in all recipients was pulmonary infection, meanwhile four recipients (26.7%) were presented with septic shock. The median time from LT to infection occurrence was 4 (IQR, 2–15). The SOFA and APACHE II scores were 9.2±3.88 and 13.47±3.31, respectively.
 |
Table 1 Baseline Characteristics of Lung Transplant Recipients Treated with Ceftazidime/Avibactam for CRPA Infections |
 |
Figure 1 The flowchart of cohort. Abbreviations: CRPA, carbapenem-resistantPseudomonas aeruginosa; CAZ/AVI, ceftazidime/avibactam. |
According to the results of antimicrobial susceptibility testing (Table S1), all isolates were resistant to meropenem and imipenem. Most isolates were in vitro resistant to ceftazidime, cefepime, cefoperazone/sulbactam, ticarcillin/clavulanic acid, piperacillin/tazobactam, ciprofloxacin and levofloxacin, with susceptibility rates of 7.1% to 20%. Furthermore, more isolates showed high in vitro susceptibility to amikacin (80%), tobramycin (92.9%) and colistin (100%).
Treatment Characteristics
The characteristics of antibiotics therapy in CRPA infection recipients are displayed in Table 2. There were several antibiotics prescribed prior to CAZ/AVI treatment, including carbapenems (n=5), cefoperazone/sulbactam (n=4), polymyxins (n=3) and other antibiotics. The median time from infection onset to initiation of CAZ/AVI treatment was 4 days (IQR, 3–7). The mean duration of CAZ/AVI treatment was 10 days. CAZ/AVI was administered as monotherapy in 12 recipients (80%) and as combined therapy in 3 recipients (20%). The antibiotics involved in combination regimens of CAZ/AVI were polymyxins (n=2), quinolones (n=2), carbapenems (n=1) and aminoglycosides (n=1).
 |
Table 2 Treatment Characteristics of Ceftazidime/Avibactam in CRPA Infection Recipients After Lung Transplantation |
Outcomes
Among the 15 CRPA recipients with CAZ/AVI treatment, the primary outcome of 14-day mortality was 6.7%, and 30-day mortality was 13.3% with the fact that one recipient died of septic shock and one recipient died due to cardiogenic shock. The survival curve of 15 recipients was displayed in Figure 2. Besides, the clinical cure and microbiological cure rates of CAZ/AVI therapy were 53.3% and 60%, respectively. The recurrence episode happened in three recipients (20%). In addition, the mean length of ICU stay was 24 days, together with 35 days of mean length of hospital stay. Details of the treatment for CRPA infection in LT recipients are listed in Table 3.
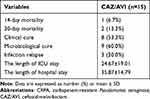 |
Table 3 Outcomes of CAZ/AVI Treatment in Lung Transplant Recipients Infected with CRPA |
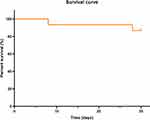 |
Figure 2 Survival curve of lung transplant recipients treated with ceftazidime/avibactam for CRPA infections. Abbreviation: CRPA, carbapenem-resistantPseudomonas aeruginosa. |
Table 4 shows the comparison between microbiological cure and failure in lung transplant recipients treated with ceftazidime/avibactam for CRPA infections. There were statistically significant differences for these variables, including tracheotomy, CRRT, septic shock, SOFA score, monotherapy, the length of ICU stay. Recipients in microbiological failure group experienced a higher proportion of tracheotomy and CRRT, higher SOFA score, a lower proportion of monotherapy, longer length of ICU stay, as well as more septic shock events.
 |
Table 4 Comparison of Characteristics Between Microbiological Cure and Failure in Lung Transplant Recipients Treated with Ceftazidime/Avibactam for CRPA Infections |
Discussion
To our knowledge, this is the first study to evaluate the effectiveness of CAZ/AVI treatment for CRPA infection in lung transplant recipients. Our results reported 14-day mortality, 30-day mortality, clinical cure and microbiological cure rates of 6.7%, 13.3%, 53.3% and 60%, respectively, indicating the promising therapeutic efficacy of CAZ/AVI in the treatment of CRPA pathogen.
In this single-center observational study, we reported that the primary outcomes of 14-day mortality and 30-day mortality were 6.7% and 13.3%, respectively. It was in line with a previous retrospective cohort study of critically ill patients with CRPA infection, which showed a low mortality rate at 14-day (5.9%) and 30-day (13.7%) in the CAZ/AVI treatment group.21 Besides, Jorgensen et al reported that the 30-day mortality was 17.5% among the patients with CAZ/AVI treatment for MDR-PA infection in their cohort,22 which was concordant with the data of our study. Consistently, another study evaluating the potential efficacy of CAZ/AVI in MDR and XDR-PA infection, suggested the 30-day mortality were 12.5%, along with clinical cure rate of 50%.23 It was observed in our cohort that clinical cure rate was 53.3%. In accordance with a retrospective cohort study conducted on MDR/XDR P. aeruginosa infection patients with CAZ/AVI therapy, it reported that clinical cure rate was 54.1% at 14-day, and all-cause mortality was 13.1% at 30-day, which was similar to the results of our study.25 However, a previous multicenter study including MDR Gram-negative bacteria (GNB) (other than Carbapenem-Resistant Enterobacterales (CRE)) patients with CAZ/AVI therapy in Italy, reported the clinical cure rate of 87.8% in MDR-PA patients.24 The discrepancy between previous studies and our study might be due to the distinct research population and different conditions. Here, in our study, the population involved was the recipient who received lung transplantation and was accompanied by the condition of immunocompromise.
Stone et al analyzed the clinical activity of CAZ/AVI against MDR-PA isolates pooled from the adult Phase III clinical trials, and the data suggested that favorable microbiological response rate at test-of-cure (TOC) was 57.1% in 95 patients with MDR-PA infection.31 Similarly, the microbiological cure in LT recipients with CRPA infection of our cohort was 60%. Data from the China Antimicrobial Surveillance Network (CHINET) (www.chinets.com) revealed that most of the 5572 CRPA isolates were susceptible to CAZ/AVI with a resistance rate of 13.9% in 2021. In addition, a multi-center, muti-national surveillance program, named Enhancing Rational Antimicrobials against Carbapenem-resistant P. aeruginosa (ERACE-PA) Global Surveillance Program, was established and had collected 807 CRPA isolates.32 In vitro data indicated that, 72% of isolates was susceptible to CAZ/AVI in all isolates.32 Previous data also supported the in vitro potency of CAZ/AVI against CRPA with 81% of isolates testing susceptible in a multicenter assessment of 34 US hospitals.33 In view of these studies above, CAZ/AVI was demonstrated as active antimicrobials with the potency against CRPA in vivo and vitro.
At present, available evidence regarding the efficacy of CAZ/AVI treatment in solid organ transplantation recipients with MDR-GNB infection was limited. Considering the immunosuppressive drugs used for the prevention and therapy of rejection event, immune function was impaired and the risk of infection was increased in SOT recipients.34 In a single-center cohort of kidney transplantation with carbapenem-resistant Klebsiella pneumoniae (CRKP) infection, recipients in the CAZ/AVI therapy group had better clinical outcomes than those in the other antibiotic regimens group, demonstrating the effectiveness of CAZ/AVI.35 Moreover, another retrospective study evaluated the efficacy of CAZ/AVI in liver transplant recipients with CRKP infection, and showed the clinical benefit of CAZ/AVI with promising results.29 Besides, patients who underwent lung transplantation with XDR-GNB infection were reviewed. The results indicated that CAZ/AVI therapy was related with high rates of survival, clinical success, and safety in 10 recipients (9 CRKP infection and 1 CRPA infection).36 However, in lung transplant recipients, evidence concerning CAZ/AVI treatment for CRPA infection is lacking. Accordingly, our study provided this critical information and revealed the efficacy of CAZ/AVI therapy for CRPA infection in lung transplantation. In view of the current evidence that P. aeruginosa in respiratory samples was correlated with worse outcomes, while P. aeruginosa eradication improved outcomes and maintained pulmonary function in lung transplantation recipients,3 here in our study, CAZ/AVI was considered as a promising choice for CRPA eradication.
There were several limitations in this study. Firstly, the nature of this study was retrospective, observational and non-comparative, hence, it existed limitations including the possible confounding factors in the study without control group. Secondly, the number of patients enrolled in this single-center study is relatively small. Therefore, the study is insufficient to make definitive conclusion about utility of CAZ/AVI treatment in LT recipients with CRPA infection. In addition, there is a limitation that the drug susceptibility result to CAZ/AVI is lacking owing to the retrospective characteristic and limited laboratory techniques. Larger-scale, multi-center, randomized controlled trials are warranted for further validation in future research.
Conclusions
In conclusion, this retrospective observational study provided the evidence regarding effectiveness of CZA/AVI against CRPA infection among lung transplant recipients, indicating that CAZ/AVI might be an alternative and promising option in the fight of CRPA.
Abbreviations
aCCI, age-adjusted Charlson Comorbidity Index; APACHE II, Acute Physiology and Chronic Health Evaluation II; CAZ/AVI, ceftazidime-avibactam; CCI, Charlson Comorbidity Index; CHINET, China Antimicrobial Surveillance Network; CLSI, Clinical and Laboratory Standards Institute; COPD, chronic obstructive pulmonary disease; CRE, Carbapenem-Resistant Enterobacterales; CRKP, carbapenem-resistant Klebsiella pneumoniae; CRPA, carbapenem-resistant Pseudomonas aeruginosa; CRRT, continuous renal replacement therapy; ERACE-PA, Enhancing Rational Antimicrobials against Carbapenem-resistant P. aeruginosa; GNB, Gram-negative bacteria; ILD, interstitial lung disease; IQR, interquartile range; ISHLT, International Society for Heart and Lung Transplantation; LT, lung transplantation; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; PA, Pseudomonas aeruginosa; SOFA, Sequential Organ Failure Assessment; SOT, solid organ transplant; STCS, Swiss Transplant Cohort Study; TOC, test-of-cure; WHO, World Health Organization; XDR, extensively drug-resistant.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Compliance
This study was approved by the Research Ethics Committee of Second Affiliated Hospital of Zhejiang University School of Medicine (approval number: 2022-0054) and was in accordance with the Declaration of Helsinki. For lung transplantation, all lungs were voluntarily donated with written informed consent, and the organ donations and transplantations were conducted in accordance with the Declaration of Istanbul.
Acknowledgments
We thank the staff of the general intensive care unit of the Second Affiliated Hospital of Zhejiang University for their assistance and cooperation in data access and analysis.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82072201).
Disclosure
The authors declare no conflicts of interest.
References
1. Su J, Li C-X, Liu H-Y, et al. The airway microbiota signatures of infection and rejection in lung transplant recipients. Microbiol Spectr. 2022;10(2):e0034421. doi:10.1128/spectrum.00344-21
2. Chambers DC, Perch M, Zuckermann A, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult lung transplantation report - 2021; Focus on recipient characteristics. J Heart Lung Transplant. 2021;40(10):1060–1072. doi:10.1016/j.healun.2021.07.021
3. De Muynck B, Van Herck A, Sacreas A, et al. Successful Pseudomonas aeruginosa eradication improves outcomes after lung transplantation: a retrospective cohort analysis. Eur Respir J. 2020;56(4). doi:10.1183/13993003.01720-2020
4. Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: multiorgan Transplantation. J Heart Lung Transplant. 2018;37(10):1169–1183. doi:10.1016/j.healun.2018.07.020
5. Di Nardo M, Tikkanen J, Husain S, et al. Postoperative management of lung transplant recipients in the intensive care unit. Anesthesiology. 2022;136(3):482–499. doi:10.1097/aln.0000000000004054
6. Perch M, Hayes D, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-ninth adult lung transplantation report-2022; focus on lung transplant recipients with chronic obstructive pulmonary disease. J Heart Lung Transplant. 2022;41(10):1335–1347. doi:10.1016/j.healun.2022.08.007
7. Paglicci L, Borgo V, Lanzarone N, et al. Incidence and risk factors for respiratory tract bacterial colonization and infection in lung transplant recipients. Eur J Clin Microbiol Infect Dis. 2021;40(6):1271–1282. doi:10.1007/s10096-021-04153-1
8. Aguado JM, Silva JT, Fernández-Ruiz M, et al. Management of multidrug resistant Gram-negative bacilli infections in solid organ transplant recipients: SET/GESITRA-SEIMC/REIPI recommendations. Transplant Rev. 2018;32(1):36–57. doi:10.1016/j.trre.2017.07.001
9. Dettori M, Riccardi N, Canetti D, et al. Infections in lung transplanted patients: a review. Pulmonology. 2022. doi:10.1016/j.pulmoe.2022.04.010
10. van Delden C, Stampf S, Hirsch HH, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss transplant cohort study. Clin Infect Dis. 2020;71(7):e159–e169. doi:10.1093/cid/ciz1113
11. Bodro M, Sabé N, Tubau F, et al. Extensively drug-resistant Pseudomonas aeruginosa bacteremia in solid organ transplant recipients. Transplantation. 2015;99(3):616–622. doi:10.1097/tp.0000000000000366
12. Camargo LF, Marra AR, Pignatari AC, et al. Nosocomial bloodstream infections in a nationwide study: comparison between solid organ transplant patients and the general population. Transpl Infect Dis. 2015;17(2):308–313. doi:10.1111/tid.12356
13. van den Bogaart L, Manuel O. Antibiotic therapy for difficult-to-treat infections in lung transplant recipients: a practical approach. Antibiotics. 2022;11(5). doi:10.3390/antibiotics11050612
14. Liu T, Zhang Y, Wan Q. Pseudomonas aeruginosa bacteremia among liver transplant recipients. Infect Drug Resist. 2018;11:2345–2356. doi:10.2147/idr.S180283
15. Zhong ZQ, Luo AJ, Wan QQ, Ye QF. Pseudomonas aeruginosa infection among liver transplant recipients: a clinical analysis of 15 cases. Transplant Proc. 2016;48(6):2130–2134. doi:10.1016/j.transproceed.2016.03.052
16. Zavascki AP, Carvalhaes CG, Picão RC, Gales AC. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy. Expert Rev Anti Infect Ther. 2010;8(1):71–93. doi:10.1586/eri.09.108
17. Hu Y, Qing Y, Chen J, et al. Prevalence, risk factors, and molecular epidemiology of intestinal carbapenem-resistant Pseudomonas aeruginosa. Microbiol Spectr. 2021;9(3):e0134421. doi:10.1128/Spectrum.01344-21
18. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/s1473-3099(17)30753-3
19. Das S, Li J, Riccobene T, et al. Dose selection and validation for ceftazidime-avibactam in adults with complicated intra-abdominal infections, complicated urinary tract infections, and nosocomial pneumonia. Antimicrob Agents Chemother. 2019;63(4). doi:10.1128/aac.02187-18
20. Soriano A, Carmeli Y, Omrani AS, Moore LSP, Tawadrous M, Irani P. Ceftazidime-avibactam for the treatment of serious gram-negative infections with limited treatment options: a systematic literature review. Infect Dis Ther. 2021;10(4):1989–2034. doi:10.1007/s40121-021-00507-6
21. Chen J, Liang Q, Chen X, et al. Ceftazidime/Avibactam versus Polymyxin B in the challenge of carbapenem-resistant Pseudomonas aeruginosa infection. Infect Drug Resist. 2022;15:655–667. doi:10.2147/idr.S350976
22. Jorgensen SCJ, Trinh TD, Zasowski EJ, et al. Real-world experience with ceftazidime-avibactam for multidrug-resistant gram-negative bacterial infections. Open Forum Infect Dis. 2019;6(12):ofz522. doi:10.1093/ofid/ofz522
23. Rodríguez-Núñez O, Ripa M, Morata L, et al. Evaluation of ceftazidime/avibactam for serious infections due to multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. J Glob Antimicrob Resist. 2018;15:136–139. doi:10.1016/j.jgar.2018.07.010
24. Vena A, Giacobbe DR, Castaldo N, et al. Clinical experience with ceftazidime-avibactam for the treatment of infections due to multidrug-resistant gram-negative bacteria other than carbapenem-resistant enterobacterales. Antibiotics. 2020;9(2). doi:10.3390/antibiotics9020071
25. Corbella L, Boán J, San-Juan R, et al. Effectiveness of ceftazidime-avibactam for the treatment of infections due to Pseudomonas aeruginosa. Int J Antimicrob Agents. 2022;59(2):106517. doi:10.1016/j.ijantimicag.2021.106517
26. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
27. Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant. 2011;30(4):361–374. doi:10.1016/j.healun.2011.01.701
28. Gu J, Xu J, Zuo TT, Chen YB. Ceftazidime-avibactam in the treatment of infections from carbapenem-resistant Klebsiella pneumoniae: ceftazidime-avibactam against CR-KP infections. J Glob Antimicrob Resist. 2021;26:20–25. doi:10.1016/j.jgar.2021.04.022
29. Chen F, Zhong H, Yang T, et al. Ceftazidime-avibactam as salvage treatment for infections due to carbapenem-resistant Klebsiella pneumoniae in liver transplantation recipients. Infect Drug Resist. 2021;14:5603–5612. doi:10.2147/idr.S342163
30. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100.
31. Stone GG, Newell P, Gasink LB, et al. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: pooled data from the ceftazidime/avibactam Phase III clinical trial programme. J Antimicrob Chemother. 2018;73(9):2519–2523. doi:10.1093/jac/dky204
32. Gill CM, Aktaþ E, Alfouzan W, et al. The ERACE-PA global surveillance program: ceftolozane/tazobactam and Ceftazidime/avibactam in vitro activity against a global collection of carbapenem-resistant Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2021;40(12):2533–2541. doi:10.1007/s10096-021-04308-0
33. Grupper M, Sutherland C, Nicolau DP. Multicenter evaluation of ceftazidime-avibactam and ceftolozane-tazobactam inhibitory activity against meropenem-nonsusceptible Pseudomonas aeruginosa from Blood, respiratory tract, and wounds. Antimicrob Agents Chemother. 2017;61(10). doi:10.1128/aac.00875-17
34. Møller DL, Sørensen SS, Perch M, et al. Bacterial and fungal bloodstream infections in solid organ transplant recipients: results from a Danish cohort with nationwide follow-up. Clin Microbiol Infect. 2022;28(3):391–397. doi:10.1016/j.cmi.2021.07.021
35. Zhang F, Zhong J, Ding H, Liao G. Efficacy of ceftazidime-avibactam in the treatment of carbapenem-resistant Klebsiella pneumoniae infection after kidney transplantation. Infect Drug Resist. 2021;14:5165–5174. doi:10.2147/idr.S343505
36. Chen W, Sun L, Guo L, et al. Clinical outcomes of ceftazidime-avibactam in lung transplant recipients with infections caused by extensively drug-resistant gram-negative bacilli. Ann Transl Med. 2020;8(3):39. doi:10.21037/atm.2019.10.40
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
