Back to Journals » Infection and Drug Resistance » Volume 16
Ceftazidime-Avibactam Treatment for Severe Post-Neurosurgical Meningitis and Abscess Caused by Extended-Spectrum β-Lactamase Escherichia coli in a Pediatric Patient: A Case Report
Authors Ren J , Wang Q, Liu L, Xiao Y, Ji P, Du H, Wang S, Zheng Y, Yang Q
Received 4 January 2023
Accepted for publication 24 March 2023
Published 30 March 2023 Volume 2023:16 Pages 1905—1911
DOI https://doi.org/10.2147/IDR.S403527
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jing Ren,1,* Qinhui Wang,1,* Linna Liu,1 Yunfeng Xiao,1 Peigang Ji,2 Hui Du,3 Shan Wang,4 Yao Zheng,1 Qi Yang1
1Department of Pharmacy, the Second Affiliated Hospital of Air Force Medical University, Xi’an, Shaanxi, People’s Republic of China; 2Department of Neurosurgery, the Second Affiliated Hospital of Air Force Medical University, Xi’an, Shaanxi, People’s Republic of China; 3Xi’an Institute for Food and Drug Control, Xi’an, Shaanxi, People’s Republic of China; 4Department of Pharmacy, NYU Langone Hospital – Long Island, Mineola, NY, USA
*These authors contributed equally to this work
Correspondence: Qi Yang, Tel +86 15829673096, Fax +86-029-84777154, Email [email protected]
Abstract: Post-neurosurgical infections caused by multidrug-resistant Enterobacterales are difficult to treat due to limited therapeutic options. Ceftazidime-avibactam (CAZ-AVI), a combination of cephalosporin and a novel β-lactamase inhibitor, has exhibited potential activity against multi/extensive drug-resistant (MDR/XDR) gram-negative bacilli. Several reports have described the successful treatment of central infections caused by MDR/XDR Pseudomonas aeruginosa or Enterobacterales. However, data on the efficacy and effective drug distribution of CAZ-AVI in the central nervous system (CNS), particularly in children, are lacking. We report a case of a 4-year-old girl with post-neurosurgical meningitis and abscess caused by extended-spectrum β-lactamase-producing Escherichia coli successfully treated with CAZ-AVI. CAZ-AVI therapeutic drug monitoring was performed to evaluate its efficacy and effective drug distribution in the CNS. We measured CAZ (15.6, 7.1, and 3.5 μg/mL) and AVI (4.0, 2.1, and 1.2 μg/mL) in cerebrospinal fluid (CSF) samples obtained 3, 5, and 7 h after the administration of the 15th CAZ-AVI dose (2.5 g, q8h, iv), respectively. We also measured CAZ (57.0 and 25.8 μg/mL) and AVI (11.3 and 4.5 μg/mL) in serum samples obtained 3 and 5 h after the administration, respectively. CAZ-AVI achieved an adequate CSF concentration throughout the drug interval. Our case provides evidence for using CAZ-AVI to treat CNS infections.
Keywords: therapeutic drug monitoring, cerebrospinal fluid, central nervous system, infection
Introduction
Post-neurosurgical infection caused by multidrug-resistant (MDR) Enterobacterales is a severe disease associated with high mortality.1–3 Therapeutic options are limited due to high microbial resistance and poor blood-brain penetration of most agents used for MDR bacteria. Carbapenems are the preferred therapeutic option for severe infections caused by extended-spectrum β-lactamase (ESBL)-producing organisms. Despite being sensitive, some ESBL-producing organisms may have high minimum inhibitory concentrations (MICs) to carbapenems. However, the clinical outcome may be poor with adequate doses of carbapenems in some patients. Therefore, searching for better options to treat central nervous system (CNS) infections is occasionally necessary.
Ceftazidime-avibactam (CAZ-AVI) is a parenteral cephalosporin combined with a novel non-β-lactam β-lactamase inhibitor. AVI protects CAZ against degradation by Klebsiella pneumoniae carbapenemases, AmpC cephalosporinases, OXA-48, and ESBLs (eg, TEM, SHV, and CTX-M) to expand the antibacterial activity spectrum.4 However, to the best of our knowledge, only nine published reports have described CNS infections treated with CAZ-AVI.4–12 Only two studies have measured blood and cerebrospinal fluid (CSF) concentrations in two patients, and both were adults.7,8 Therefore, this is the first case that measured a pediatric patient’s blood and CSF concentrations of CAZ-AVI. Furthermore, we assessed the penetration of the drug in pediatric CNS infection. This may provide a theoretical basis for using CAZ-AVI in CNS infections in children.
Case Presentation
A 4-year-old girl with a body weight of 17.5 kg presented to our hospital with left-hand numbness. No recognizable information has been included herein to protect the child’s anonymity and private health information. Written informed consent was obtained. Enhanced nuclear magnetic resonance imaging indicated a space occupying the right frontal, parietal lobe lesion. Central neurocytoma was considered; however, she was healthy and had no inherited diseases. The patient underwent excision of the intracranial space-occupying lesion and lumbar cistern catheterization 5 days after admission. Cefuroxime, omeprazole, vitamin C, and potassium chloride were administered perioperatively for a few days. Five days after the operation, the patient developed a CSF incision leakage, followed by a high fever (40°C); therefore, cefoperazone-sulbactam (0.5 g) was administered intravenously (IV) twice a day. As the patient’s body temperature did not change, vancomycin 0.25 g every 6 h and meropenem 0.7 g, q8h were administered IV 2 days later based on the patient’s weight and after multidisciplinary consultation. Growth of ESBL-producing Escherichia coli (E. coli) was identified in a CSF specimen collected 15 days after admission (Table 1).
 |
Table 1 Susceptibility Results for Escherichia Coli in the Cerebrospinal Fluid Sample |
Initial bacterial identification and antimicrobial susceptibility testing (AST) were conducted using an automated mass spectrometry system (VITEKMS, bioMérieux, Inc., Durham, NC). AST result interpretation was based on the Clinical and Laboratory Standards Institute guidelines. Next-generation sequencing (NGS) of the CSF confirmed the presence of CTX-M enzyme-producing E. Coli and its drug resistance. The CSF drainage velocity was 8.3 mL/h. After discharging residual CSF, its samples extracted from the lumbar drainage catheter had a white blood cell count of 140×106/L, glucose level of <1.10 mmol/L, and protein level of 1406.87 mg/L. The patient was diagnosed with CNS infection. Vancomycin was discontinued, and meropenem was continued. After 9 days of meropenem treatment, repeated CSF cultures were still positive, with a CSF glucose level of 1.32 mmol/L. The patient’s body temperature fluctuated at approximately 39°C.
MDR E. coli was identified by bacterial subculture on day 15. AST revealed resistance to aztreonam, cefepime, cefotaxime, and ampicillin. The MICs of CAZ-AVI and meropenem were 0.125 μg/mL and 1.000 μg/mL, respectively. Considering the excellent activity of the bacteria, CAZ-AVI (1.25 g; IV; and every 8 h) was administered from day 20 of admission. CSF sample collected 3 days later was negative for E. coli, the color of the CSF became clear, and the CFS glucose level increased (Table 2). The lumbar drainage catheter was removed on day 28. CSF examination on day 32 revealed increased protein and decreased glucose levels. The patient’s body temperature remained at 39°C. Furthermore, enhanced magnetic resonance imaging revealed an abscess around the artificial dura mater. On day 47, the abscess was excised, the artificial dura mater was removed, and a subcutaneous drainage tube was inserted. Meropenem and CAZ-AVI were continued after the operation. Meropenem was discontinued 15 days later (day 62) since the patient’s body temperature returned to normal. The subcutaneous drainage tube was removed, and CAZ-AVI was discontinued on day 65 as the CSF glucose level returned to normal, and the patient maintained a normal body temperature. The CSF analyses are presented in Table 2. The patient underwent cranioplasty and ventriculoperitoneal shunt implantation and was transferred to a rehabilitation facility for further treatment.
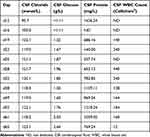 |
Table 2 CSF Analyses on Various Dates |
Post-infusion drug concentrations were measured 3, 5, and 7 h after delivering the 15th dose of CAZ-AVI in the CSF and after 3 and 5 h in the serum for therapeutic drug monitoring (TDM) on day 25. All samples were centrifuged at 2000 ×g for 10 min, and the supernatant was stored at –80°C until assayed. Moreover, we quantified the serum and CSF concentrations using liquid chromatography with tandem mass spectrometry assay as previously described.13 This analysis was performed to assess the therapy adequacy and ensure sufficient CAZ-AVI levels were maintained in the CNS after administering 1.25 g IV as a 2-h infusion every 8 h. The respective serum concentrations 3 and 5 h after administration were 57.0 and 25.8 μg/mL for CAZ and 11.3 and 4.5 μg/mL for AVI. The respective CSF concentrations 3, 5, and 7 h after administration were 15.6, 7.1, and 3.5 μg/mL for CAZ and 4.0, 2.1, and 1.2 μg/mL for AVI. CAZ and AVI penetration through the CSF/blood barrier was approximately 27.3 ± 0.4% and 40.5 ± 7.7%, respectively. The CAZ and AVI serum and CSF concentrations are presented in Table 3. A case synopsis timeline is shown in Figure 1.
 |
Table 3 Therapeutic Drug Monitoring of CAZ-AVI Concentrations |
 |
Figure 1 Timeline depicting case progression, antibiotic treatment, and TDM initiation (dates in a yyyy/mm/dd format). Abbreviation: TDM, therapeutic drug monitoring. |
Discussion
Here, we reported a case of a 4-year-old girl who developed post-neurosurgical meningitis and ESBL-producing MDR E. coli infection and was effectively treated with CAZ-AVI and meropenem.
Approximately one-third of meningitis cases are caused by gram-negative bacilli. The risk factors include head trauma, neurosurgery, the presence of a CSF shunt or other neurosurgical devices, and a CSF leak. In our case, NGS CSF analysis identified the CTX-M enzyme. Treatment with carbapenems produced the best outcomes in survival and bacteriologic clearance.14 Our patient was administered sufficient doses of meropenem, following the guideline for CNS infection.15 However, the patient remained feverish after 10 days of treatment, and the CSF culture was still positive. Therefore, a multidisciplinary consultation was organized. Considering the high drug dose, long course, and drug toxicity when treating CNS infections, aminoglycosides and quinolones were not considered in this case due to safety considerations in a pediatric patient. After multidisciplinary consultation, CAZ-AVI 1.25 g IV treatment every 8 h was added to meropenem. CSF culture became negative 3 days later. However, the fever persisted despite treatment. Enhanced magnetic resonance imaging revealed a possible abscess around the artificial dura mater; thus, exploratory craniotomy was performed to remove it. The CNS infection was successfully treated, and the patient was discharged 65 days after hospitalization.
CAZ-AVI is a combination of cephalosporin and a β-lactamase inhibitor. It has been approved in China for complicated intra-abdominal infections, hospital-acquired pneumonia/ventilator-associated bacterial pneumonia, and other infections caused by gram-negative bacteria (K. pneumoniae, Enterobacter cloacae, E. coli, Proteus mirabilis, and P. aeruginosa) with limited treatment options. Many meningitis and ventriculitis cases were successfully treated with CAZ-AVI. Yasmin et al7 reported TDM of CAZ-AVI, showing that at 64 min after a 2-h infusion, CAZ and AVI concentrations were 19.0 and 4.2 µg/mL in the CSF and 61.3 and 13.1 µg/mL in the plasma, respectively.
According to the package insert, the standard CAZ-AVI dose was 1 g IV every 8 h. Considering the severity of the infection and convenience, a higher dose of 1.25 g was used. The CAZ 7 h after administration was 3.5 µg/mL in the CSF. The pharmacokinetic (PK)/pharmacodynamic (PD) target of CAZ is associated with bacterial killing and is estimated to be approximately 50% fT > MIC.14 The guidelines for hospital-acquired CNS infections recommend a CSF level 10 times higher than the in vitro MIC.16 Conservatively assuming a CAZ t1/2 of 2 h for our patient, its concentrations in the CSF were likely >20 times higher than that in the MIC for the entire dosing interval.14 Similarly, the PK/PD index for AVI was estimated to achieve concentrations ≥1.0 g/L during approximately 50% of the dosing interval (% time above a threshold).17,18 AVI concentration in the CSF of our patient 1 h before the next dose was 1.2 µg/mL, which seemed adequate. Meningeal inflammation is known to improve the penetration of antibiotics into the CSF. The CSF samples in our case were collected after the 15th dose (on day 5 of therapy) when inflammation was reduced.19,20 Therefore, it is reasonable to conclude that our CSF PK findings are a conservative estimate of CAZ-AVI penetration into the CSF.
CAZ-AVI is a novel agent that exhibits greater activity against ESBL-producing organisms than meropenem, and its penetration rate is promising. The levels attained and measured in our patient using this dosing regimen appear to be adequate. In a recent and only published paper for using CAZ-AVI for CNS infections in pediatrics,12 the boy was diagnosed with ventriculoperitoneal shunt infection due to MDR P. aeruginosa. The patient was treated with multiple antipseudomonal agents where the isolate was reported as susceptible, including IV meropenem, amikacin, ciprofloxacin, intraventricular (IVT) gentamicin, and IV colistin for the causative infection. Despite antibiotic therapy, CSF analyses and cultures remained persistently positive. The isolate was reported as MDR P. aeruginosa, and he was switched to CAZ-AVI and IVT colistin. CSF cultures became sterile 3 days after IV CAZ-AVI combined with IVT colistin, and subsequent CSF cultures had no growth. The treatment was very successful, though CAZ-AVI concentration in CSF was not measured.
Colistin is another agent with in vitro activity against ESBL-producing Enterobacterales. Although it has the in vitro activity, using colistin in ESBL-producing Enterobacterales treatment is still reserved because of limited evidence. Recently, one study revealed loading dose of colistin was less effective than carbapenems for treating extended-spectrum beta-lactamase-producing Enterobacterales.21 Additionally, polymyxins have a large molecular weight and are difficult to cross the blood-brain barrier when administered intravenously, making it challenging to achieve CSF concentrations.
IVT or intrathecal (ITH) administration of antibiotics (gentamicin or colistin) is considered to be another choice for CNS infection caused by multi/extensive drug-resistant (MDR/XDR) gram-negative bacilli. These procedures may increase the risk of bacterial CNS infection with a new microorganism. Toxicity is also an issue associated with IVT or ITH treatment of colistin or gentamicin. The most significant side effect reported is chemical ventriculitis, meningitis, and epilepsy.22 Chemical meningitis presents with fever, low glucose concentration, and elevated white blood cell count in the CSF, which is very similar to CNS infection. The reappearance of signs of meningitis provokes a serious dilemma for the clinician. Therefore, we will consider IVT or ITH colistin to be an alternative option when there is no better intravenous dosing regimen. Gram-negative bacillary meningitis is frequently fatal, with reported mortality rates of 40%–80% in adults and children.23 Treatment of MDR/XDR Gram-negative bacillus-associated CNS infections is more difficult due to increasing bacterial resistance and limited CNS penetration of antimicrobials. CAZ-AVI has exhibited a potential activity against MDR/XDR Gram-negative bacillus. However, ceftazidime/avibactam for CNS infections is an off-label use. Our findings and those of a previous study7 demonstrated that CAZ-AVI achieved an adequate CSF concentration throughout the drug interval. Therefore, it is crucial for clinicians and healthcare professionals worldwide to recognize that CAZ-AVI can be a good alternative.
Timely surgical intervention is vital in addition to antibiotics. Based on the infectious Diseases Society of America guidelines,15 the most effective treatment for CNS infections should include the removal of the infected shunt or extracerebral ventricular drain, reasonable extracerebral drainage, and appropriate antimicrobial therapy. CSF drainage and prompt abscess removal were important in our case and constituted the basis for successful antibiotic treatment.
Our study had some limitations. First, samples were collected during a relatively reduced meningeal inflammation (after administration of the 15th dose). Meningeal inflammation improves the penetration of β-lactam antibiotics into the CSF. Therefore, the CSF CAZ-AVI concentrations are likely understated. Second, the PK/PD index for AVI in CNS infection was poorly established. Third, the AVI PK/PD index was referred to animal models with thigh or lung infections.18 Therefore, further studies are needed to verify these measurements.
Conclusion
This report described measurements of CAZ-AVI concentrations in the serum and CSF of a patient with meningitis and abscess due to ESBL-producing E. coli infection. A novel CAZ-AVI TDM method was successfully applied, demonstrating that CAZ-AVI achieved CSF levels higher than the MIC throughout the dosing interval. Therefore, further clinical studies are required to assess the complete profile of CAZ-AVI in the CSF. Our case provides evidence for using this agent to treat CNS infections caused by ESBL-producing E. coli.
Abbreviations
AST, antimicrobial susceptibility testing; CAZ-AVI, ceftazidime-avibactam; CNS, central nervous system; CSF, cerebrospinal fluid; ESBL, extended-spectrum β-lactamase; MDR/XDR, multi/extensive drug-resistant; MIC, minimum inhibitory concentration; NGS, next-generation sequencing; TDM, therapeutic drug monitoring; PK, pharmacokinetic; PD, pharmacodynamic; IVT, intraventricular; ITH, intrathecal.
Consent for Publication
Written informed consent was obtained from the next-of-kin of the patient for the publication of the case details. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Air Force Medical University, Shaanxi, China. EC number: TDLL-202302-02
Acknowledgments
We are grateful for the useful comments and suggestions from the anonymous referees.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No extramural funding was received for this study.
Disclosure
Mrs Jing Ren and Mr Yunfeng Xiao report a patent (application number:202210925945.9) pending to Air Force Medical University of Chinese People ‘s Liberation Army. The authors report no other conflicts of interest in this work.
References
1. Kim BN, Peleg AY, Lodise TP, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9(4):245–255. doi:10.1016/S1473-3099(09)70055-6
2. Li Z, Wu X, Yu J, et al. Empirical combination antibiotic therapy improves the outcome of nosocomial meningitis or ventriculitis in neuro-critical care unit Patients. Surg Infect (Larchmt). 2016;17(4):465–472. doi:10.1089/sur.2015.060
3. Reichert MC, Medeiros EA, Ferraz FA. Hospital-acquired meningitis in patients undergoing craniotomy: incidence, evolution, and risk factors. Am J Infect Control. 2002;30(3):158–164. doi:10.1067/mic.2002.119925
4. Holyk A, Belden V, Lee JJ, et al. Ceftazidime/avibactam use for carbapenem-resistant Klebsiella pneumoniae meningitis: a case report. J Antimicrob Chemother. 2018;73:254–256.
5. Pektezel MY, Isikay I, Gocmen R, et al. Carbapenem-resistant Klebsiella pneumoniae meningitis and abscess treated with ceftazidime-avibactam. Enferm Infecc Microbiol Clin. 2022;40:332–333.
6. Zhou Q, Wang H, Zhan TX, et al. Successful treatment of ventriculitis caused by MDR/XDR Gram-negative Bacillus using ceftazidime/avibactam: case series and literature review. Infect Drug Resist. 2021;14:1691–1701. doi:10.2147/IDR.S306222
7. Yasmin M, Hanrahan J, Marshall S, et al. Using therapeutic drug monitoring to treat KPC-producing Klebsiella pneumoniae central nervous system infection with ceftazidime/avibactam. Open Forum Infect Dis. 2020;7(9):ofaa349. doi:10.1093/ofid/ofaa349
8. Gatti M, Virgili G, Cojutti PG, et al. Real-time optimization of pharmacodynamic target attainment at infection site during treatment of post-neurosurgical ventriculitis caused by carbapenem-resistant Gram negatives with ceftazidime-avibactam-based regimens: a report of two cases. Microorganisms. 2022;10(1):154. doi:10.3390/microorganisms10010154
9. Gofman N, To K, Whitman M, et al. Successful treatment of ventriculitis caused by Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae with i.v. ceftazidime-avibactam and intrathecal amikacin. Am J Health Syst Pharm. 2018;75(13):953–957. doi:10.2146/ajhp170632
10. Daccò V, Claut L, Piconi S, et al. Successful ceftazidime-avibactam treatment of post-surgery Burkholderia multivorans genomovar II bacteremia and brain abscesses in a young lung transplanted woman with cystic fibrosis. Transpl Infect Dis. 2019;21:e13082.
11. Xipell M, Bodro M, Marco F, et al. Clinical experience with ceftazidime/avibactam in patients with severe infections, including meningitis and lung abscesses, caused by extensively drug-resistant Pseudomonas aeruginosa. Int J Antimicrob Agents. 2017;49:266–268.
12. Almangour TA, Alsubaie S, Ghonem L, et al. Ceftazidime-avibactam for the treatment of multidrug-resistant Pseudomonas aeruginosa central nervous system infection in pediatric patient: a case report. Pediatr Infect Dis J. 2022;41:436–438.
13. Wang QH, Zheng Y, Liu LL, et al. Simultaneous determination of ceftazidime and avibactam in human plasma and cerebrospinal fluid by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). Analatical Letters. 2023;56(5):816–831. doi:10.1080/00032719.2022.2105859
14. Kaye KS, Bhowmick T, Metallidis S, et al. Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA. 2018;319(8):788–799. doi:10.1001/jama.2018.0438
15. Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-associated Ventriculitis and Meningitis. Clin Infect Dis. 2017;64(6):e34–e65. doi:10.1093/cid/ciw861
16. Veillette JJ, Truong J, Forland SC. Pharmacokinetics of Ceftazidime-Avibactam in Two Patients With KPC-Producing Klebsiella pneumoniae Bacteremia and Renal Impairment. Pharmacotherapy. 2016;36(11):e172–e177. doi:10.1002/phar.1840
17. Crass RL, Pai MP. Pharmacokinetics and pharmacodynamics of β-lactamase inhibitors. Pharmacotherapy. 2019;39(2):182–195. doi:10.1002/phar.2210
18. Berkhout J, Melchers MJ, van Mil AC, et al. Pharmacodynamics of ceftazidime and avibactam in neutropenic mice with thigh or lung infection. Antimicrob Agents Chemother. 2016;60(1):368–375. doi:10.1128/AAC.01269-15
19. Lutsar I, McCracken, Jr. GH, Friedland IR. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis. 1998;27(5):1117–1127. doi:10.1086/515003
20. Nau R, Sörgel F, Prange HW. Pharmacokinetic optimisation of the treatment of bacterial central nervous system infections. Clin Pharmacokinet. 1998;35(3):223–246. doi:10.2165/00003088-199835030-00005
21. Katip W, Yoodee J, Uitrakul S, et al. Efficacy of loading dose colistin versus carbapenems for treatment of extended spectrum beta lactamase producing Enterobacteriaceae. Sci Rep;2021 11 1 . 18. doi:10.1038/s41598-020-78098-4
22. Karaiskos I, Galani L, Baziaka F, et al. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents. 2013;41 6 :499–508.
23. Tanglm CST. Klebsiella pneumoniae meningitis: prognostic factors. Scand J Infect Dis. 1994;26 1 :95–102..
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
