Back to Journals » ImmunoTargets and Therapy » Volume 6
CD8+ memory T-cell inflation renders compromised CD4+ T-cell-dependent CD8+ T-cell immunity via naïve T-cell anergy
Authors Xu A, Freywald A, Xie Y, Li Z, Xiang J
Received 5 January 2017
Accepted for publication 21 February 2017
Published 15 June 2017 Volume 2017:6 Pages 39—49
DOI https://doi.org/10.2147/ITT.S131662
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Michael Shurin
Aizhang Xu,1,2 Andrew Freywald,3 Yufeng Xie,4 Zejun Li,5 Jim Xiang1,2
1Cancer Research Cluster, Saskatchewan Cancer Agency, 2Department of Oncology, 3Department of Pathology, University of Saskatchewan, Saskatoon, SK, Canada; 4Department of Oncology, First Affiliated Hospital, Soochow University, Suzhou, 5Shanghai Veterinary Research Institute, Shanghai, China
Abstract: Whether inflation of CD8+ memory T (mT) cells, which is often derived from repeated prime-boost vaccinations or chronic viral infections in the elderly, would affect late CD8+ T-cell immunity is a long-standing paradox. We have previously established an animal model with mT-cell inflation by transferring ConA-stimulated monoclonal CD8+ T cells derived from Ova-specific T-cell-receptor transgenic OTI mice into irradiation-induced lymphopenic B6 mice. In this study, we also established another two animal models with mT-cell inflation by transferring, 1) ConA-stimulated monoclonal CD8+ T cells derived from lymphocytic choriomeningitis virus glycoprotein-specific T-cell-receptor transgenic P14 mice, and 2) ConA-stimulated polyclonal CD8+ T cells derived from B6.1 mice into B6 mice with irradiation-induced lymphopenia. We vaccinated these mice with recombinant Ova-expressing Listeria monocytogenes and Ova-pulsed dendritic cells, which stimulated CD4+ T cell-independent and CD4+ T-cell-dependent CD8+ T-cell responses, respectively, and assessed Ova-specific CD8+ T-cell responses by flow cytometry. We found that Ova-specific CD8+ T-cell responses derived from the latter but not the former vaccination were significantly reduced in mice with CD8+ mT-cell inflation compared to wild-type B6 mice. We determined that naïve CD8+ T cells purified from splenocytes of mice with mT-cell inflation had defects in cell proliferation upon stimulation in vitro and in vivo and upregulated T-cell anergy-associated Itch and GRAIL molecules. Taken together, our data reveal that CD8+ mT-cell inflation renders compromised CD4+ T-cell-dependent CD8+ T-cell immunity via naïve T-cell anergy, and thus show promise for the design of efficient vaccines for elderly patients with CD8+ mT-cell inflation.
Keywords: lymphopenia, memory T-cell inflation, defective immunity, T-cell proliferation, T-cell anergy
Introduction
CD8+ cytotoxic T lymphocytes (CTLs) play an important role in protection from viral infection.1 After infection, CD8+ T cells undergo a triphasic program of rapid proliferation, contraction, and memory formation. The majority (90%–95%) of CD8+ T cells die of apoptosis in the contraction phase, and the remaining 5%–10% of CTLs become CD8+ memory T (mT) cells.2 Upon subsequent pathogen encounter, CD8+ mT cells respond swiftly by rapid proliferation and heightened effector functions.2
In cellular immunity, CD8+ T-cell responses include CD4+ T-cell-dependent and CD4+ T-cell-independent ones.3 In the former, antigen-presenting cells are required to be licensed (matured) by CD40L signaling derived from CD4+ T-helper (TH) cells prior to generating measurable CD8+ T-cell responses to noninflammatory antigens, while in the latter antigen-presenting cells mature via triggering Toll-like receptors (TLRs) by pathogen-associated molecular patterns of inflammatory antigens, leading to direct stimulation of CD8+ T-cell responses without help from CD4+ TH cells.
The original T-cell compartment theory dictated that the number of CD8+ mT cells was invariable, due to limited space,4,5 and that CD8+ mT-cell inflation derived from repeated immunizations could affect the late priming of CD8+ T-cell responses.6,7 Interestingly, Vezys et al developed a heterologous prime-boost virus-based vaccination regimen eliciting functional mT-cell inflation.8 They found that the CD8+ T-cell compartment grew in size with immunological experience, and the size of the naïve CD8+ T-cell compartment could thus be maintained at a similar level in mice with CD8+ mT-cell inflation compared to naïve mice.8 With this model, they also found that CD8+ mT-cell inflation derived from the late vesicular stomatitis virus (VSV) challenge did not affect functional CTLs primarily stimulated by lymphocytic choriomeningitis virus (LCMV), and LCMV-induced CD8+ mT-cell inflation had no impact either on the late CD4+ T cell-independent CTL immunity derived from immunization of VSV.8 However, whether CD8+ mT-cell inflation affects late CD4+ T-cell-dependent CD8+ T-cell responses was not investigated in their studies.
In chronic viral infections, such as cytomegalovirus (CMV) infection, the low-level persistent CMV provokes CD8+ T-cell responses over time, leading to CD8+ mT-cell inflation, which remain functional in protection against viral rechallenge.9 Although the maintenance of functional CD8+ mT cells is important during persistent infection, accumulating evidence suggests that infection with CMV often contributes to immunosenescence, an aging process associated with CD8+ mT-cell inflation and compromised immunity,10–12 leading to increased vulnerability to infectious pathogens, such as influenza, and progressively decreased immunity to vaccination.13 The compromised immunity has been found to be derived from altered natural killer cells and neutrophils in innate immunity and altered T-cell signaling or decreased T-cell clonal diversity in adaptive immunity.14 However, whether CD8+ mT-cell inflation contributes to compromised immunity by affecting adaptive immunity is unclear.
We previously established an animal model with functional CD8+ mT-cell inflation by transferring ConA-stimulated monoclonal CD8+ T cells derived from ovalbumin (Ova)-specific T-cell-receptor (TCR) transgenic OTI mice into irradiation-induced lymphopenic C57BL/6 (B6) mice, and found that IL15 signaling promoted transferred effector T-cell survival and memory formation in lymphopenic mice, leading to formation of an Ova-specific mT-cell inflation model.15
In this study, we generated another two animal models with CD8+ mT-cell inflation to address these questions. These included transferring, 1) ConA-stimulated monoclonal CD8+ T cells derived from LCMV glycoprotein (Gp)-specific TCR transgenic P14 mice, and 2) ConA-stimulated polyclonal CD8+ T cells derived from B6.1 mice into irradiation-induced lymphopenic B6 mice to form a Gp-specific and a CD45.1-specific mT-cell inflation model, respectively. By using the Gp- and CD45.1-specific mT-cell inflation models, we assessed whether mT-cell inflation affects Ova-pulsed dendritic cells (DCOva)-triggered CD4+ T-cell-dependent and recombinant Ova-expressing Listeria monocytogenes (rLmOva)-induced CD4+ T-cell-independent CD8+ T-cell immunity. We found that CD8+ mT-cell inflation does not affect CD4+ T-cell-independent priming of CD8+ T-cell responses derived from rLmOva infection, but does reduce DCOva-induced CD4+ T-cell-dependent priming of CD8+ T-cell responses. We found that CD8+ mT-cell inflation did not affect CD8+ mT-cell recall responses. We also found that naïve CD8+ T cells purified from splenocytes of mice with CD8+ mT-cell inflation had a defect in cell proliferation upon stimulation in vitro and in vivo, and upregulated the T-cell anergy-associated Itch and GRAIL. Therefore, our data suggest that CD8+ mT-cell inflation induces a defect in T-cell proliferation, leading to reduced CD4+ T-cell-dependent CD8+ T-cell responses via naïve T-cell anergy.
Materials and methods
Reagents, antibodies, and animals
Phycoerythrin (PE)-labeled H2Kb/Ova257–264 tetramer (PE-Ova tetramer), PE-labeled H2Kb/Gp33–41 tetramer (PE-Gp tetramer) and fluorescein isothiocyanate (FITC)-labeled anti-CD8 (KT15) antibody (FITC-CD8 Ab) were obtained from Beckman Coulter (Brea, CA, US). PE-Cy5-labeled Ab for CD8 (53-6.7) and PE-Cy5-labeled streptavidin were purchased from Thermo Fisher Scientific (Waltham, MA, US). The biotin-labeled Abs for CD44 (IM7), CD62L (MEL14) and IL7Rα (SB/199), PE-anti-CD45.1 (A20) were obtained from BioLegend (San Diego, CA, US). Anti-GRAIL (H91) and anti-Itch (H110) Abs were obtained from Santa Cruz Biotechnology (Dallas, TX, US). Cytokines IL2, IL4, and GM-CSF were purchased from PeproTech (Rocky Hill, NJ, US). Carboxyfluorescein succinimidyl ester (CFSE) was purchased from Thermo Fisher Scientific. ConA was purchased from Sigma-Aldrich (St Louis, MO, US). Cytoperm™ permeabilization buffer was obtained from BD Biosciences (San Jose, CA, US). CD3 microbeads were obtained from Thermo Fisher Scientific. MACS® anti-CD8 microbeads and anti-PE microbeads were purchased from Miltenyi Biotech (Bergisch Gladbach, Germany). Naïve CD8+ T Cell Purification kit was obtained from Stemcell Technologies (Vancouver, BC, Canada). Recombinant Ova-expressing Listeria monocytogenes (rLmOva) was obtained from DMX Inc (West Chester, PA, US). The highly metastatic Ova-expressing BL6-10Ova tumor cell line was generated in our lab.16 The Biosafety Committee of the University of Saskatchewan approved the use of the BL6-10Ova tumor cell line in this study. Female wild-type (WT) C57BL/6 (B6) mice (CD45.2), B6.1 mice (CD45.1), Ova-specific TCR transgenic OTI and LCMV Gp-specific TCR transgenic P14 mice on B6 background were purchased from Jackson Laboratory (Bar Harbor, MA, US). All mice were housed in the animal facility at the Health Sciences Building and treated according to the Animal Care Committee guidelines of the University of Saskatchewan. The Animal Care Committee of the University of Saskatchewan approved the animal experiments in this study.
Preparation of bone marrow-derived dendritic cells
Bone marrow-derived DCs were prepared as previously described.16 Briefly, bone marrow cells prepared from femora and tibiae of WT B6 mice were depleted of red-blood cells with 0.84% ammonium chloride and plated in DC culture medium (Dulbecco’s Modified Eagle’s Medium plus 10% fetal calf serum, GM-CSF [20 ng/mL] and IL4 [20 ng/mL]). On day 3, the nonadherent granulocytes, T cells, and B cells were gently removed, and fresh media were added. Two days later, the loosely adherent proliferating DC aggregates were dislodged and replated. On day 6, the nonadherent cells were mature DCs and harvested. These DCs were pulsed with Ova (0.3 mg/mL) overnight at 37°C, then washed twice with phosphate buffered saline (PBS) and termed DCOva.
Preparation of ConA-activated CD8+ T cells
Mouse splenocytes were cultured in Roswell Park Memorial Institute 1640 medium containing IL2 (20 U/mL) and ConA (1 μg/mL) for 3 days. CD8+ T cells were then purified from ConA-activated T (ConA-T) cells using MACS anti-CD8 microbeads to yield T-cell populations with 95% purity. ConA-T cells derived from B6.1 (CD45.1), P14, and OTI mice were termed CD45.1-, Gp-, and Ova-specific ConA-T cells, respectively.
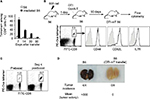
Establishment of CD8+ mT-cell inflation models
Irradiated (600 rad) B6 mice were intravenously transfused with the CD45.1, Gp, or OTI ConA-T cells (10×106 cells/mouse) 1 day after the irradiation to generate CD45.1-, Gp-, or Ova-specific mT-cell inflation models in B6 (CD45.1-mT B6, Gp-mT B6, and Ova-mT B6) mice, respectively. We found more than 5% mT cells remaining in Ova-mT B6 (Figure 1B), Gp-mT B6 and CD45.1-mT B6 (Figure 2B) mice 90 days after the T-cell transfer. In another set of experiments, irradiated (600 rad) B6 mice were intravenously transfused with OTI ConA-T cells (2×106 cells/mouse) 1 day after the irradiation to generate Ova-mT B6 mice with ~1% mT cells (Figure 1C) 90 days after the T-cell transfer for assessment of recall responses or tumor challenges. In addition, irradiated (600 rad) OTI mice were also transfused with CD45.1 ConA-T cells (10×106 cells/mouse) 1 day postirradiation to generate another CD45.1-specific mT-cell inflation model in OTI (CD45.1-mT OTI) mice with naïve Ova-specific CD8+ T cells.
Flow-cytometry analyses
To examine survival of transferred Ova-specific ConA-T cells in irradiated B6 mice, mouse peripheral blood samples were stained with PE-Ova tetramer and FITC-CD8 Ab at days 7, 14, 30, 90 after T-cell transfer. For detection of mT-cell markers, the tail-blood samples were stained with PE-Ova tetramer, FITC-CD8, and PE-Cy5-Abs for CD44, CD62L, or IL7Rα and then analyzed by flow cytometry. To assess CD8+ T-cell priming responses, DCOva (106 cells/mouse) or rLmOva (2,000 plaque-forming units/mouse) were intravenously injected into Gp-mT B6 and CD45.1-mT B6 mice (Figure 2B). Mouse peripheral blood samples were stained with PE-Ova tetramer and FITC-CD8 Ab, followed by flow-cytometry analysis at days 6 and 7 after the DCOva and rLmOva immunization. To assess recall responses, Ova-mT B6 mice (four per group) with 1% mT cells (Figure 1C) were intravenously boosted with DCOva (106 cells/mouse). In another set of experiments, 106 Ova-specific mT cells purified from Ova-mT B6 mice were intravenously transferred into Gp-mT B6 or CD45.1 mT B6 or WT B6 mice. At 1 day after the T-cell transfer, the recipient mice (four per group) were intravenously boosted with DCOva (106 cells/mouse). To assess Ova-specific CD8+ T-cell recall responses, mouse peripheral blood samples were stained with PE-Ova tetramer and FITC-CD8 Ab 4 days after the boost, followed by flow-cytometry analysis. To assess expression of intracellular molecules associated with T-cell anergy, peripheral blood samples of CD45.1-mT B6 mice were first stained with FITC-CD8 Ab and PE-CD44 Ab. After permeabilization, cells were stained with PE-Cy5-labeled anti-Itch or anti-GRAIL Abs and then analyzed by flow cytometry.
T-cell-proliferation assays
In in vitro T-cell-proliferation assays, naïve CD8+ T cells purified from Ova-specific mT-cell inflation B6 (Ova-mT B6) mice using a naïve CD8+ T-cell-purification kit were labeled with CFSE (4 μM). CFSE-labeled T cells were then incubated with CD3 microbeads at a bead:cell ratio of 1:1 in the presence of IL2 (40 U/mL) and β-mercaptoethanol (50 μM). Three days after incubation, active CD8+ T cells were analyzed for T-cell divisions by flow cytometry. In the in vivo T-cell-proliferation assay, naïve CD8+ T cells purified from CD45.1-mT OTI mice were labeled with CFSE (4 μM) and intravenously transferred (106cells/mouse) into naïve B6 recipients. DCOva (106cells/mouse) was intravenously injected to activate the CFSE-labeled OTI-CD8+ T cells in vivo. Three days later, the splenocytes were collected and analyzed by flow cytometry.
Tumor challenge
To assess the functional effect of CD8+ mT cells, Ova-mT B6 mice (four per group) with ~1% mT cells (Figure 1C) were intravenously injected with 0.5×106 Ova-expressing BL6-10Ova tumor cells. The mice were killed 3 weeks after the tumor-cell challenge, and tumor-cell lung colonization (tumor colonies) was counted in a blind fashion. Tumor-cell colonies on freshly isolated lungs appeared as discrete black-pigmented foci that were easily distinguishable from normal lung tissues and confirmed by histological examination. Tumor-cell colonies too numerous to count were assigned an arbitrary value of >300.
Statistical analysis
Data are presented as means ± standard deviation. The significance of differences was determined by Student’s t-test. A probability value of P<0.05 was considered statistically significant.
Results
Establishment of three CD8+ mT-cell inflation models in irradiation-induced lymphopenic mice
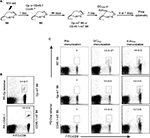
To establish a CD8+ mT-cell inflation model, we first irradiated B6 mice with 600 rad. We then transferred ConA-stimulated CD8+ T cells (10×106/mouse) derived from Ova-specific TCR transgenic OTI mice into irradiated B6 mice 1 day postirradiation15 or WT B6 mice as controls, followed by a kinetic examination of survived OTI CD8+ T cells in mice by flow cytometry. We found a much higher survival of transferred T cells 7, 14, 30 and 90 days after T-cell transfer in irradiated mice than in the control WT B6 mice (Figure 1A). After 90 days, when transferred CD8+ T cells become long-term CD8+ mT cells (5.9% in the total CD8+ T-cell population) (Figure 1B), we assessed the phenotypes of surviving OTI mT cells by flow cytometry, and found that these Ova-specific CD8+ T cells expressed mT-cell markers CD44, IL7R, and CD62L (Figure 1B), indicating that they had become CD8+ mT cells. To assess whether they were functional, Ova-mT B6 mice with ~1% of Ova-specific CD8+ mT cells 90 days after T-cell transfer (Figure 1C) were either boosted with DCOva for assessment of recall responses or challenged with Ova-expressing BL6-10Ova tumor cells for assessment of protective immunity. We found more than a tenfold (13.7%/1.23%) increase in recall responses 4 days after DCOva boost (Figure 1C), and complete (100%) protection of mice from tumor growth (Figure 1D), indicating that these CD8+ mT cells were functional. Therefore, this animal model was used as the Ova-specific mT-cell inflation model in B6 (Ova-mT B6) mice. We then similarly established another two mT-cell inflation (Gp-mT B6 and CD45.1-mT B6) models by transferring ConA-T cells derived from P14 and B6.1 mice into 600 rad-irradiated lymphopenic B6 mice at 1 day postirradiation (Figure 2A). We found that more than 5% Gp- and CD45.1-positive CD8+ mT cells remained in Gp-mT B6 and CD45.1-mT B6 mice 90 days after the T-cell transfer (Figure 2B).
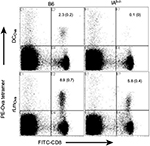
CD8+ mT-cell inflation inhibited CD4+ T-cell-dependent CD8+ T-cell priming responses
To assess whether CD8+ mT-cell inflation affected late CD4+ T-cell-dependent CD8+ T-cell priming, we first selected two stimulators – CD4+ T-cell-dependent DCOva and CD4+ T-cell-independent recombinant rLmOva – since the former triggered Ova-specific CD8+ T-cell responses only in WT but not CD4+ T-cell-deficient IAb–/– B6 mice, while the latter stimulated Ova-specific CD8+ T-cell responses in both WT and IAb–/– B6 mice (Figure S1). We then immunized Gp-mT and CD45.1-mT B6 mice with DCOva and rLmOva, respectively, and assessed Ova-specific CD8+ T-cell responses by flow cytometry after immunization. We found that Ova-specific CD8+ T-cell responses derived from rLmOva stimulation were comparable in both WT and CD8+ mT-cell inflation mice (Figure 2C). However, Ova-specific CD8+ T-cell responses derived from DCOva stimulation were significantly higher in WT B6 mice (2.8%) than in Gp-mT (0.6%) and CD45.1-mT (0.5%) B6 mice with CD8+ mT-cell inflation (Figure 2C), indicating that both monoclonal or polyclonal CD8+ mT-cell inflation inhibited CD4+ T-cell-dependent CD8+ T-cell priming responses.
CD8+ mT-cell inflation did not affect CD8+ mT-cell recall responses
To assess whether CD8+ mT-cell inflation affected late Ova-specific CD8+ mT-cell recall responses, we first transferred Ova-specific CD8+ mT cells (106/mouse) purified from Ova-mT B6 mice into Gp-mT B6, CD45.1-mT B6, and WT B6 mice. We found that there were comparable amounts of Ova-specific CD8+ mT cells (around 0.2%–0.3% in the total CD8+ T-cell population) in these mice (Figure 3). These mice were followed with DCOva boost and assessment of Ova-specific CD8+ T-cell recall responses 4 days after the boost. We found that the transferred OTI mT cells in Gp-mT and CD45.1-mT B6 mice had comparable Ova-specific T-cell increases when compared to Ova-specific T-cell increases in WT B6 mice (Figure 3), indicating that mT-cell inflation did not affect CD8+ mT-cell recall responses.
Naïve CD8+ T cells in mice with CD8+ mT-cell inflation were defective in T-cell proliferation upon stimulation
To assess potential defects in T-cell proliferation, we conducted in vitro and in vivo T-cell-proliferation assays. For in vitro T-cell-proliferation assays, we first labeled naïve CD8+ T cells with CFSE, and then stimulated them in vitro with anti-CD3 beads plus IL2 for 3 days. We found that naïve CD8+ T cells derived from CD45.1-mT-inflation B6 mice underwent fewer cell divisions (69.2%) than those derived from WT B6 mice (91.7%) (Figure 4A). For in vivo T-cell-proliferation assays, we first established a CD45.1-mT inflation model in OTI mice (CD45.1-mT OTI) by irradiating (600 rad) OTI mice followed by transfer of ConA-stimulated CD8+ T cells derived from B6.1 mice 1 day postirradiation (Figure 4B). We then purified naïve OTI CD8+ T cells from splenocytes of WT OTI or CD45.1-mT OTI mice 90 days after T-cell transfer, labeled them with CFSE, and then transferred them into WT B6 mice, followed by DCOva immunization. Three days after immunization, we analyzed mouse splenocytes by flow cytometry. We found that CD8+ T cells derived from CD45.1-mT OTI mice underwent fewer cell divisions (22%) than those derived from WT OTI mice (43.3%) (Figure 4B). Therefore, our in vitro and in vivo data indicated that naïve CD8+ T cells in mice with mT-cell inflation were defective in T-cell proliferation upon stimulation.
Naïve CD8+ T cells in mice with CD8+ mT-cell inflation upregulated T-cell anergy-associated Itch and GRAIL
We have previously shown that naïve CD8+ T cells were defective in T-cell proliferation, due to T-cell anergy.17 To assess the potential T-cell anergy responsible for defects in T-cell proliferation, we examined the expression of two T-cell anergy-associated gene-encoding molecules – Itch and GRAIL – in naïve CD44low CD8+ T cells by flow cytometry. We found that expression of Itch and GRAIL in cells from CD45.1-specific CD8+ mT-cell inflation B6 mice was significantly higher than in naïve CD8+ T cells from WT B6 mice (Figure 4C), suggesting that naïve CD8+ T cells in mice with CD8+ mT-cell inflation might be anergic.
Discussion
Whether CD8+ mT-cell inflation, which is often derived from repeated prime-boost vaccinations or chronic viral infections in the elderly, would affect late CD8+ T-cell priming and immunity is a long-standing paradox in cellular immunity. Vezys et al showed that CD8+ mT-cell inflation derived from an LCMV prime-boost virus-based vaccination regimen had no impact on the late immunity derived from immunization of VSV.8 This is consistent with some previous reports showing preexisting CD8+ mT-cell inflation did not affect late recombinant L. monocytogenes-primed CD8+ T-cell responses.18,19 Of note, both VSV and recombinant L. monocytogenes stimulate CD4+ T-cell-independent CD8+ T-cell immunity.20 However, whether CD8+ mT-cell inflation affects late CD4+ T-cell-dependent CD8+ T-cell responses was not investigated in these studies.8,18,19
In this study, we generated two CD8+ mT-cell inflation models (a monoclonal Gp-mT inflation one and a polyclonal CD45.1-mT inflation one) in irradiation-induced lymphopenic B6 mice. By using these two animal models with CD8+ mT-cell inflation, we assessed whether CD8+ mT-cell inflation affected DCOva-triggered CD4+ T-cell-dependent or rLmOva-induced CD4+ T-cell-independent Ova-specific CD8+ T-cell immunity. We found that monoclonal or polyclonal CD8+ mT-cell inflation did not affect CD4+ T-cell-independent CD8+ T-cell responses derived from recombinant rLmOva infection, consistent with the previous report,8 but did reduce DCOva-induced CD4+ T-cell-dependent CD8+ T-cell responses. The elderly, often associated with CD8+ mT-cell inflation, have increased vulnerability to infectious pathogens, such as influenza viruses,14 triggering CD4+ T-cell-dependent CD8+ T-cell immunity.21 Therefore, our finding that CD8+ mT-cell inflation rendered compromised CD4+ T-cell-dependent CD8+ T-cell immunity may partly explain the susceptibility of the elderly to influenza.
The compromised immunity in the elderly with CD8+ mT-cell inflation includes a defect in T-cell proliferation in adaptive immunity.14 However, the underlying mechanism for the defect is unclear. To assess a potential mechanism for compromised CD4+ T-cell-dependent CD8+ T-cell immunity in mice with CD8+ mT-cell inflation, we purified naïve CD8+ T cells from the mice with CD8+ mT-cell inflation and examined T-cell proliferation upon stimulation. We found that these naïve CD8+ T cells had defects in cell proliferation upon stimulation both in vitro and in vivo. Interestingly, we also found upregulation of T-cell anergy-associated Itch and GRAIL in naïve CD8+ T cells in mice with CD8+ mT-cell inflation compared to those in WT mice, indicating that naïve CD8+ T cells in mice with CD8+ mT-cell inflation are anergic. Therefore, in addition to altered natural killer cells and neutrophils and altered T-cell signaling or decreased T-cell clonal diversity,13 mT-cell inflation-induced T-cell anergy may also contribute to the compromised immunity often seen in the elderly with mT-cell inflation.
The elderly also have progressively decreased immunity to vaccination.14 For example, influenza vaccines can only induce partial immunity by protecting 59% of the elderly from influenza.22 One of the strategies to improve its efficacy in the elderly with CD8+ mT-cell inflation and compromised immunity is the use of adjuvants.23 TLR ligands have been found to induce DC maturation and licensing, leading to CD4+ TH1 and effective CTL responses and to be used as a new type of vaccine-adjuvant candidate for improvement of vaccine immunogenicity.24,25 A cationic lipid–DNA complex in Fluzone vaccine has been reported to improve protection efficacy significantly in nonhuman primates after infection with human seasonal influenza virus isolate.26 A synthetic TLR4 agonist glucopyranosyl lipid adjuvant-stable emulsion combined with split-virus vaccine has also been found to boost T-cell responses to influenza vaccination, leading to improvement in vaccine-mediated protection against influenza in the elderly.27 Our data of recombinant bacteria rLmOva expressing TLR signaling able to break naïve T-cell anergy in priming CD8+ T-cell responses under mT-cell inflation thus greatly support the use of TLR ligands as adjuvants to enhance the efficiency of vaccines for influenza viral infection in the elderly. Taken together, our data showed that CD8+ mT-cell inflation rendered compromised CD4+ T-cell-dependent CD8+ T cell immunity via naïve T-cell anergy, and thus show promise for the design of efficient vaccines for the elderly, who often show CD8+ mT-cell inflation.
Acknowledgment
This work was supported by a Bridge Grant from the College of Medicine, University of Saskatchewan, Canada.
Disclosure
The authors report no conflicts of interest in this work.
References
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.