Back to Journals » OncoTargets and Therapy » Volume 13
CD47 Overexpression Is Associated with Epstein–Barr Virus Infection and Poor Prognosis in Patients with Nasopharyngeal Carcinoma
Authors Wang ZH, Pei XF, Zhu ZQ, Lin Z, Mao YY, Xu XL, Luo YL, Zhang L, Peng PJ
Received 7 January 2020
Accepted for publication 4 April 2020
Published 21 April 2020 Volume 2020:13 Pages 3325—3334
DOI https://doi.org/10.2147/OTT.S245023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Zhi-Hui Wang,1– 3,* Xiao-Feng Pei,2,3,* Zhi-Quan Zhu,2,3,* Zhong Lin,2,3 Yin-Yan Mao,2,3 Xiao-Lu Xu,2,3 You-Li Luo,4 Li Zhang,1 Pei-Jian Peng2,3
1State Key Laboratory of Oncology in South China, Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, People’s Republic of China; 2Department of Thoracic Oncology, The Cancer Center of the Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, People’s Republic of China; 3Guangdong Provincial Key Laboratory of Biomedical Imaging, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, People’s Republic of China; 4Department of Interventional Oncology, The Cancer Center of the Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Zhang
State Key Laboratory of Oncology in South China, Department of Medical Oncology, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou 510060, Guangdong, People’s Republic of China
Tel +86-20-87343533
Fax +86-20-87343292
Email [email protected]
Pei-Jian Peng
Guangdong Provincial Key Laboratory of Biomedical Imaging, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong, People’s Republic of China, Department of Thoracic Oncology, The Cancer Center of the Fifth Affiliated Hospital of Sun Yat-sen University, 52 Meihua Road East, Zhuhai 519000, Guangdong, People’s Republic of China
Tel +86-756-2528015
Fax +86-756-2528022
Email [email protected]
Purpose: Little is known about the clinical significance of CD47 expression and its association with Epstein–Barr virus (EBV) infection in patients with nasopharyngeal carcinoma (NPC). The aim of this study was to clarify the prognostic value and role of CD47 in EBV-associated NPC.
Materials and Methods: Sixty-six cases of non-metastatic NPC were retrospectively reviewed. Tissues were collected for immunohistochemical staining of CD47 and the EBV-encoded oncoprotein latent membrane protein 1 (LMP1). Western blotting and quantitative real-time PCR were performed to determine the CD47 and LMP1 levels in common human NPC cell lines. Additionally, CD47 and LMP1 expression in a constructed EBV-positive human NPC cell (CNE-2-EBV+) and a stable cell line transfected with LMP1 plasmid (CNE-2-LMP1) was assessed. Next, we used Western blotting to assess the decrease in CD47 expression on CNE-2-LMP1 cells after transfecting them with small interfering RNA (siRNA)-targeting LMP1.
Results: In NPC patients, CD47 overexpression was significantly associated with disease recurrence (P=0.010), leading to poorer disease-free survival (DFS; P=0.002) and overall survival (P=0.021). Multivariate Cox proportional hazards models demonstrated that CD47 (HR=5.452, P=0.016) was an independent prognostic factor of DFS. Moreover, CD47 expression was associated with plasma EBV-DNA copy number and LMP1 tissue expression. Among the human NPC cell lines, CD47 and LMP1 expression was notably higher in the EBV-positive C666-1 cell line than in the EBV-negative cell lines. Furthermore, EBV infection upregulated CD47 expression via LMP1-mediated pathways in human NPC cells.
Conclusion: This study indicated that CD47 is related to EBV infection in NPC patients, and it is a feasible biomarker.
Keywords: CD47, Epstein–Barr virus, latent membrane protein 1, nasopharyngeal carcinoma
Introduction
Nasopharyngeal carcinoma (NPC), a common malignancy derived from the nasopharyngeal epithelium, is endemic in southern China and Southeast Asia.1 Intensity-modulated radiotherapy with chemotherapy leads to favorable long-term survival for non-metastatic NPC patients, mainly due to improvement of local disease control,2,3 making distant metastasis the prominent reason for treatment failure.3,4 To address the issue of disease reoccurrence, new prognostic biomarkers and therapeutic targets are urgently demanded.
Epstein–Barr virus (EBV) is an important risk factor for NPC.5 The close relationship between EBV infection and non-keratinizing NPCs indicates that EBV infection is a key event in endemic regions.5,6 The role of EBV in tumorigenesis is multifactorial and complex.5 Genetic alterations are the only initiation event in the premalignant epithelium,7 and the immune microenvironment may play a critical role among the subsequent events. However, the immune escape mechanism of EBV-transformed nasopharyngeal epithelial cells is still not fully understood.
Macrophages are the main innate immune cells to infiltrate into the microenvironment of tumors. However, most cancer cells highly express CD47, which plays a crucial role in the “don’t eat me” signal related to the innate immune system; it inhibits phagocytosis by binding to the signal regulatory protein alpha (SIRPα) on macrophages.8 These interactions between CD47 and SIRPα result in immune evasion by the cancer cells.9 Several studies have demonstrated that high CD47 expression predicts poor prognosis in several cancers.10–14 However, little is known about the expression and role of CD47 in NPC.
It has been shown by the research that many macrophages infiltrate into the tumor microenvironment in NPC tissue, which is positively correlated to prognosis.15 The reason why macrophages help the growth of NPC cells may be related to immune evasion mediated by CD47.9,16 Recently, the research has shown that EBV infection induces CD47 expression on EBV-transformed B cells.17 Additionally, CD47 expression was significantly upregulated on EBV-positive gastric carcinoma cells compared to EBV-negative gastric carcinoma cells.18 NPC is considered to be an EBV-associated cancer, but the relationship between EBV infection and CD47 expression is unclear.
In this study, we first evaluated CD47 expression in NPC tissues and the prognostic value of CD47 in NPC patients. Additionally, we determined the association between CD47 expression and EBV infection and then further explored the mechanism underlying how EBV infection upregulates CD47 expression. The aim of this study was to clarify the prognostic value and role of CD47 in EBV-associated NPC.
Materials and Methods
Ethics Approval
This study was approved by the Ethics Committee of the Fifth Affiliated Hospital of Sun Yat-sen University (approval no. K44-1). After providing a detailed description of the purpose of the study, written informed consent was obtained from all the patients. All cell lines, approved by the Ethics Committee Board of our institution, were nicely provided by Prof. Fang Wenfeng and Prof. Zhang Li (Sun Yat-Sen University Cancer Center, Guangzhou, China). All experiments described in this study were conducted according to the Helsinki Declaration and relevant laws and regulations.
Tissue Samples
Formalin-fixed paraffin-embedded non-metastatic NPC tissues and case histories of patients diagnosed with NPC (n=66) between January 2011 and March 2018 were retrospectively obtained from the medical records of the Department of Pathology, at the Fifth Affiliated Hospital of Sun Yat-sen University. Clinicopathological data on age, gender, clinical stage, T stage (describing the primary tumor site), N stage (describing regional lymph node involvement), M stage (describing distant metastatic spread), EBV-DNA copy, treatment procedures, and survival were collected by clinical records. The American Joint Committee on Cancer (AJCC) staging system (8th version) was used to stage the cancers. All the patients were followed up until September 30th, 2019 or until death. The follow-up data were collected from the medical records or by contacting the patients’ relatives.
Immunohistochemical Staining
The formalin-fixed paraffin-embedded tissues were cut into 4-μm sections, which were then deparaffinized with xylene followed by subjecting them to a graded alcohol series. Antigen retrieval was carried out with ethylenediaminetetraacetic acid (EDTA) antigen repair buffer (pH 9.0) in a steam bath at 95°C for 45 min. After cooling the sections naturally, they were washed with phosphate-buffered saline (PBS; pH7.4) and endogenous peroxidase activity was blocked using 3% hydrogen peroxide for 25 min. The sections were then washed in Tris-buffered saline (pH7.4) and incubated at 4°C overnight with anti-CD47 antibody (D3O7P, 63000s; 1:50; Cell Signaling Technology, Danvers, MA, USA) or anti-EBV latent membrane protein 1 (LMP1) antibody (1:50, ab78113, Abcam, Cambridge, MA, USA). Immunoreactivity was tested using an EnVision horseradish peroxidase (HRP) staining system (Dako Cytomation, Carpinteria, CA, USA) according to the manufacturer’s instructions. Negative controls were prepared by omitting the primary antibody in the above procedure. CD47 and LMP1 expression was identified based on membranous staining with/without cytoplasmic staining in tumor cells. The expression was quantified based on the membranous tumor proportion score (TPS), with high CD47 or LMP1 expression being defined as TPS ≥10%. The results were determined by two independent pathologists by using a blinded scoring approach.
NPC Cell Lines and Cell Culture
Six human NPC cell lines employed in this study (CNE-2, SUNE-1, CNE-1, HONE-1, HK-1, and C666-1) were obtained from Prof. Zhang Li (Sun Yat-Sen University Cancer Center, Guangzhou, China). These cell lines had been established and were described in a previous study.19 Among these cell lines, C666-1, which is defective in lytic EBV reactivation, was the only EBV-positive NPC cell line. Additionally, CNE-2-EBV+ (constructed EBV-positive CNE-2 cell line) and the stable cell lines CNE-2-LMP1 (CNE-2 cell line transfected with LMP1 plasmid) and CEN-2-vector (CNE-2 cell line transfected with control vector) were kindly provided by Prof. Fang Wenfeng (Sun Yat-Sen University Cancer Center, Guangzhou, China). All the NPC cell lines were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA) at 37°C in 5% CO2.
Western Blotting
Cells (5×106 cells) of all the cell lines investigated in this study were harvested and total proteins were obtained with radioimmunoprecipitation assay (RIPA) buffer containing phenylmethylsulfonyl fluoride (PMSF) (Applygen, Beijing, China). The protein concentration was quantified using a bicinchoninic acid (BCA) assay kit (Beyotime, Shanghai, China). The protein samples were immediately heated at 100°C for 5 min and then stored at −20°C until use. On gel containing 12% (wt/vol) acrylamide, the proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). After blocking the membranes using Tris-buffered saline with Tween 20 (TBST) containing 5% skim milk, a standard immunoblot analysis was performed using primary antibody and HRP-conjugated secondary antibody. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control. The following primary antibodies were employed: anti-GAPDH antibody (ab181602; Abcam, Cambridge, MA, USA), anti-CD47 antibody (bs-2386R; Bioss, Beijing, China), and anti-LMP1 antibody (ab78113; Abcam). The bands were detected using enhanced chemiluminescence with a ChemiDoc™ XRS+ System (Bio-Rad, Hercules, CA, USA) and analyzed using ImageJ 1.8 software (National Institutes of Health, Bethesda, MD, USA).
RNA Extraction and PCR
Total RNA was extracted from all the cell lines investigated in this study, and cDNA was synthesized by reverse transcription in a C1000 Thermal Cycler (Bio-Rad) with a PrimeScriptTM RT reagent kit (RR047A; Takara, Otsu, Japan). Quantitative real-time PCR analysis was performed using a CFX96™Real-Time PCR Detection System (Bio-Rad) with an SYBR Premix EX Taq II Kit (RR802A; Takara). The primers for CD47 were as follows: forward primer, 5ʹ-CGATTGGATTAACCTCCTTCGT-3ʹ and reverse primer, 5ʹ-CATTGGTATACACGCCGCAA-3ʹ. The primers for GAPDH were as follows: forward primer, 5ʹ-GATTCCACCCATGGCAAATT-3ʹ and reverse primer, 5ʹ-TCTCGCTCCTGGAAGATGGT-3ʹ.
Small Interfering RNA (siRNA) and Transfection
CNE-2-LMP1 and CNE-2-EBV+ cells were cultivated in a 6-well plate with RPMI-1640 medium supplemented with 10% fetal bovine serum without antibiotics. After a further 24 h of incubation, Lipofectamine RNAiMAX Transfection Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) was used to transfect the cells with LMP1 siRNA or negative control siRNA, which were chemically synthesized by RiboBio Co., Ltd. (Guangzhou, China). The negative control siRNA sequences were as follows: sense sequence, 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ and anti-sense sequence, 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ. The LMP1 siRNA sequences were as follows: sense sequence, 5ʹ-GGAAUUUGCACGGACAGGCTT-3ʹ and anti-sense sequence, 5ʹ-GCCUGUCCGUGCAAAUUCCTT-3ʹ, as in a previous study.19
Statistical Analysis
The associations between categorical variables were analyzed using the chi square test. Correlations between variables were assessed using Pearson’s correlation analysis. Kaplan–Meier analysis was used to estimate the survival curves, and the Log rank test was used to compare the patient groups with positive and negative CD47 expression, and to compare the groups between positive and negative LMP1 expression. Cox proportional hazards models were then used to estimate the independent prognostic values (for predicting DFS and OS) of the variables in a multivariate analysis, with hazard ratios (HRs) and 95% CIs being calculated. SPSS version 22.0 (USA) was used for the statistical analyses and P<0.05 was considered statistically significant.
Results
Clinicopathological Characteristics and CD47 and LMP1 Expression
A total of 66 patients with non-metastatic NPC were included in the study. The clinicopathological characteristics are shown in Table 1. There were 50 (75.8%) male patients and 16 (24.2%) female patients. The median age of the patients was 49 years (range: 24–66 years). According to the AJCC staging system (8th edition), there were six (9.1%) stage II patients, 34 (51.5%) stage III patients, and 26 (39.4%) stage IVA patients at diagnosis. All patients underwent chemoradiotherapy with or without adjuvant chemotherapy, achieving complete remission by treatment. A total of 26 (39.4%) patients underwent 3-dimensional conformal radiotherapy and 40 (60.6%) patients underwent intensity-modulated radiotherapy.
 |
Table 1 Associations of Clinicopathological Characteristics with CD47 and LMP1 Expression in 66 Non-Metastatic NPC Patients |
Both CD47 and LMP1 proteins were largely localized to the cell membrane and/or cytoplasm of cancer cells in NPC tissues (Figure 1). A total of 37 (56.1%) and 36 (54.5%) samples were positive for CD47 and LMP1 expression, respectively. CD47/LMP1 co-expression occurred in 29 (43.9%) samples.
Next, we analyzed the associations of baseline clinicopathological characteristics with CD47 and LMP1 expression. CD47 expression was significantly associated with N stage (χ2=6.71, P=0.035) and age (χ2=4.52, P=0.034). However, there were no associations between clinicopathological characteristics and LMP1 expression (Table 1).
Prognostic Values of CD47 and LMP1 Expression in NPC Patients
At the time of data extraction, 41 (62.1%) patients had relapsed and 25 (37.9%) patients had died. The mean follow-up time was 49.3 months. The median disease-free survival (mDFS) and median overall survival (mOS) were 33.6 and 45.0 months, respectively. CD47 expression was significantly associated with disease recurrence (χ2=6.575, P=0.010; Table 1), but LMP1 expression was not (χ2=3.434, P=0.064; Table 1). Kaplan–Meier analysis and the Log rank test showed that patients with positive CD47 expression (34.9 vs 67.7 months, P=0.002) and positive LMP1 expression (34.9 vs 62.8 months, P=0.012) had a significantly poorer disease-free survival (DFS) compared with those with negative expression (Figure 2). Likewise, associations between positive CD47 expression and mOS (52.7 vs 80.4 months, P=0.021) and between positive LMP1 expression and mOS (46.0 vs 80.4 months, P=0.010) were also observed using Kaplan–Meier analysis and the Log rank test.
Multivariate Cox proportional hazards models demonstrated that CD47 expression (HR=5.452, 95% CI: 1.375–21.615, P=0.016; Table 2) and age (HR=0.136, 95% CI: 0.047–0.400; P<0.001; Table 2) were independent prognostic factors of DFS. However, LMP1 (HR=2.698, 95% CI: 0.588–12.833; P=0.202) was not an independent predictor of DFS. This indicates that CD47, but not LMP1, may be a prognostic factor of NPC.
 |
Table 2 Univariate and Multivariate Analyses of Prognostic Factors Predicting Disease-Free Survival (DFS) in 66 Non-Metastatic NPC Patients |
CD47 Expression Associated with EBV Infection in NPC
CD47/LMP1 co-expression (CD47+LMP1+) was observed in 43.9% (29/66) of NPC patients. In addition, the correlation between CD47 and LMP1 expression in NPC tissue was remarkably significant (r=0.541, P<0.001; Table 3). Moreover, high CD47 expression was significantly associated with EBV-DNA copy number (χ2=11.86, P=0.001; Table 1). These results indicate that CD47 expression was associated with LMP1 expression and EBV infection.
 |
Table 3 Correlation Between the Expression of CD47 and LMP1 |
CD47 and LMP1 Expression in Various Human NPC Cell Lines
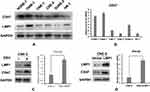
To assess CD47 and LMP1 protein expression in human NPC cell lines, Western blotting was performed in EBV-negative human NPC cell lines (SUNE-1, CNE-1, CNE-2, and HONE-1 HK-1) and an EBV-positive human NPC cell line (C666-1). As expected, CD47 and LMP1 protein expression in the EBV-positive C666-1 cell line was notably higher compared to the EBV-negative cell lines (Figure 3A and B). To confirm whether CD47 protein expression was associated with EBV infection, we further assessed CD47 and LMP1 protein expression in EBV-positive human NPC cells (CNE-2-EBV+) and the EBV-negative parental cells (CNE-2). The protein expression of CD47 and LMP1 in CNE-2-EBV+ cells was obviously higher than in CNE-2 cells (Figure 3C). Thus, the results confirmed that EBV infection upregulated CD47 expression in EBV-positive human NPC cells (CNE-2-EBV+) compared to EBV-negative human NPC cells (CNE-2). Additionally, CD47 expression was positively associated with LMP1 expression in EBV-positive NPC cell lines.
EBV Infection Enhanced CD47 Expression via LMP1-Mediated Pathways in Human NPC Cells
CNE-2-LMP1 cells (CNE-2 human NPC cell line transfected with LMP1 plasmid) were used to further explore the potential mechanisms of EBV-induced upregulation of CD47 in NPC cells. The CD47 protein and mRNA levels in CNE-2-LMP1 cells were significantly higher compared with those in the control CNE-2-vector cells (Figure 3D). In addition, we used siRNA to lower LMP1 expression in CNE-2-LMP1 and CNE-2-EBV+ cells, which decreased the CD47 expression (Figure 4A and B). These results indicated that CD47 was induced by EBV infection via LMP1-mediated pathways.
Discussion
In this study, we explored the expression and clinical significance of CD47 and LMP1 in NPC, and we demonstrated that CD47 expression, but not LMP1 expression, was an independent prognostic factor of NPC. We further observed the association between CD47 expression and EBV infection in NPC and determined that EBV infection enhanced CD47 expression via LMP1-mediated pathways in human NPC cells.
CD47 functions as a crucial “ don’t eat me” signal on tumor cells and is known to be a biomarker in several carcinomas; it is overexpressed in various cancers, and can be used to predict poor prognosis. To the best of our knowledge, CD47 has not been previously investigated as a potential NPC biomarker. We found that the positive expression rate of CD47 protein was 56.1% in NPC tissues, which is consistent with its expression rate in breast cancer,10 pulmonary sarcomatoid carcinoma,12 and head and neck squamous cell carcinoma.20 Thereafter, we explored the associations between CD47 expression and clinicopathological characteristics, along with the prognostic value of CD47 expression, in NPC patients. CD47 expression was significantly associated with N stage and age but not with T stage or clinical stage (Table 1). CD47 expression was also significantly associated with disease recurrence and poorer DFS and OS, indicating that CD47 predicts poor prognosis in NPC patients. However, the clinical significance of CD47 status in cancers appears to be inconsistent. A study of squamous cell carcinoma of the head and neck revealed that CD47 could not predict several clinicopathological characteristics but could predict OS.20 CD47 also predicted poor prognosis of other cancer types, including T-cell acute lymphoblastic lymphoma/leukemia,13 and lung cancer.14 However, recent studies revealed that CD47, which was associated with several clinicopathological characteristics, was not an independent predictor of poor DFS in breast cancer.10,11 The inconsistency across different studies may be due to variations in immunohistochemical techniques and differences between the cancers, including type, stage, and treatment history. We consider that CD47 is a feasible prognostic biomarker of NPC patients. However, the factors that upregulate CD47 expression were unclear.
We found that CD47 expression at the protein and mRNA levels in NPC cells was positively associated with EBV infection. NPC has been known as an EBV-driven malignancy, characterized pathologically by prevailing EBV infection5 and a microenvironment involving massive immune infiltration.15,21 Detection of EBV-DNA in serum by PCR can be used to diagnose primary EBV infection with favorable sensitivity and specificity.22 EBV-DNA detection has been used to screen for NPC in high-risk populations23 and for predicting prognosis in early-stage or metastatic/recurrent NPC.24,25 We observed that high CD47 expression was significantly associated with plasma EBV-DNA copy number in NPC patients. Furthermore, CD47 was expressed more highly on EBV-positive human NPC cells (C666-1) and constructed EBV-positive human NPC cells (CNE-2-EBV+) compared to EBV-negative cells. This result is similar to the results of previous studies of CD47 expression on EBV-driven transformed B cells17 and EBV-driven gastric cancer cells.18 These results, found in the present study and previous studies, implies that EBV-driven cancers undergo immune escape by upregulating CD47 expression.26,27 However, how CD47 is upregulated during EBV transformation was unclear. The EBV-encoded oncoprotein LMP1 may be essential for EBV-mediated malignant transformation. Thus, we further explored the role of LMP1 in EBV transformation.
LMP1 is a well-recognized initiator in EBV-associated NPC.7 Emerging evidence shows that LMP1 mediates immune evasion by NPC by modulating the tumor microenvironment.19,28 In accordance with a previous study,29 LMP1 expression was significantly associated with poorer DFS and OS in our study. There was a remarkably significant correlation between CD47 expression and LMP1 expression in both NPC tissue and cell lines. Furthermore, we found that upregulating or downregulating LMP1 expression significantly increased or decreased CD47 expression, respectively. Our study demonstrated that LMP1 upregulated CD47 expression in EBV-driven NPC. However, the detailed mechanism underlying the LMP1-mediated regulation of CD47 during EBV transformation needs to be further explored in a future study. Casey et al revealed that the MYC oncogene can regulate the antitumor immune response by directly binding to the promoters of the CD47 and programmed death ligand 1 (PD-L1) genes.30 Interleukin (IL)-6 has been demonstrated to upregulate CD47 expression on hepatoma cells by activating the signal transducer and activator of transcription 3 (STAT3) pathway.31 In addition, LMP1 upregulated c-myc via IL-6 induction followed by Jak3/STAT3 activation32 and it also upregulated PD-L1 via the STAT3, activator protein (AP)-1, and nuclear factor (NF)-kappa B pathways.19 Therefore, we infer that LMP1 may upregulate CD47 expression via the c-myc/IL-6/STAT3 pathway. We will seek an answer to this puzzle in our next study.
This study has several limitations. First, the study is a retrospective study with a limited number of samples, which might have resulted in bias in the conclusions. Second, CD47 expression was assessed only at the protein level in some experiments, and it is necessary to use PCR and immunofluorescence assays to analyze the CD47 expression in more detail. Third, no definitive mechanisms underlying CD47 upregulation were determined, and they need to be investigated further in future research. However, our study still provided useful clues for exploring CD47-targeted therapy and understanding immune escape during EBV transformation.
In conclusion, higher CD47 expression is associated with poor DFS and OS. In EBV-driven NPC, EBV enhances CD47 expression via LMP1-mediated pathways. To our knowledge, this is the first study to explore the expression and prognostic value of CD47 in NPC and the mechanism underlying CD47 upregulation in EBV-driven NPC. Our results imply that CD47 could be used as a new prognostic marker and a potential therapeutic target in NPC.
Acknowledgment
This study was supported by a grant from the Youth Science Fund Project of China National Natural Science Foundation (no. 81702808).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a “cantonese cancer”? Chin J Cancer. 2010;29(5):517–526.
2. Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group Phase II trial 0225. J Clin Oncol. 2009;27(22):3684–3690. doi:10.1200/JCO.2008.19.9109
3. Tian YM, Liu MZ, Zeng L, et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Head Neck. 2019;41(5):1246–1252.
4. Sun XS, Liu DH, Liu SL, et al. Patterns of failure and survival trends in 3808 patients with stage II nasopharyngeal carcinoma diagnosed from 1990 to 2012: a large-scale retrospective cohort study. Cancer Res Treat. 2019;51(4):1449–1463. doi:10.4143/crt.2018.688
5. Tsao SW, Tsang CM, To KF, Lo KW. The role of Epstein-Barr virus in epithelial malignancies. J Pathol. 2015;235(2):323–333. doi:10.1002/path.4448
6. He YQ, Xue WQ, Xu FH, et al. The relationship between environmental factors and the profile of Epstein-Barr virus antibodies in the lytic and latent infection periods in healthy populations from endemic and non-endemic nasopharyngeal carcinoma areas in China. EBioMedicine. 2018;30:184–191. doi:10.1016/j.ebiom.2018.02.019
7. Yoshizaki T, Kondo S, Wakisaka N, et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett. 2013;337(1):1–7. doi:10.1016/j.canlet.2013.05.018
8. Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109(17):6662–6667. doi:10.1073/pnas.1121623109
9. Vonderheide RH. CD47 blockade as another immune checkpoint therapy for cancer. Nat Med. 2015;21(10):1122–1123. doi:10.1038/nm.3965
10. Yuan J, He H, Chen C, Wu J, Rao J, Yan H. Combined high expression of CD47 and CD68 is a novel prognostic factor for breast cancer patients. Cancer Cell Int. 2019;19(1):238. doi:10.1186/s12935-019-0957-0
11. Yuan J, Shi X, Chen C, et al. High expression of CD47 in triple negative breast cancer is associated with epithelial-mesenchymal transition and poor prognosis. Oncol Lett. 2019;18(3):3249–3255. doi:10.3892/ol.2019.10618
12. Yang Z, Xu J, Li R, Gao Y, He J. PD-L1 and CD47 co-expression in pulmonary sarcomatoid carcinoma: a predictor of poor prognosis and potential targets of future combined immunotherapy. J Cancer Res Clin Oncol. 2019;145(12):3055–3065. doi:10.1007/s00432-019-03023-w
13. Yang K, Xu J, Liu Q, Li J, Xi Y. Expression and significance of CD47, PD1 and PDL1 in T-cell acute lymphoblastic lymphoma/leukemia. Pathol Res Pract. 2019;215(2):265–271. doi:10.1016/j.prp.2018.10.021
14. Barrera L, Montes-Servin E, Hernandez-Martinez JM, et al. CD47 overexpression is associated with decreased neutrophil apoptosis/phagocytosis and poor prognosis in non-small-cell lung cancer patients. Br J Cancer. 2017;117(3):385–397. doi:10.1038/bjc.2017.173
15. Peng J, Ding T, Zheng LM, Shao JY. Influence of tumor-associated macrophages on progression and prognosis of nasopharyngeal carcinoma. Ai Zheng. 2006;25(11):1340–1345.
16. Takimoto CH, Chao MP, Gibbs C, et al. The macrophage ‘do not eat me’ signal, CD47, is a clinically validated cancer immunotherapy target. Ann Oncol. 2019;30(3):486–489. doi:10.1093/annonc/mdz006
17. Park GB, Bang SR, Lee HK, et al. Ligation of CD47 induces G1 arrest in EBV-transformed B cells through ROS generation, p38 MAPK/JNK activation, and Tap73 upregulation. J Immunother. 2014;37(6):309–320. doi:10.1097/CJI.0000000000000042
18. Abe H, Saito R, Ichimura T, et al. CD47 expression in Epstein-Barr virus-associated gastric carcinoma: coexistence with tumor immunity lowering the ratio of CD8(+)/Foxp3(+) T cells. Virchows Arch. 2018;472(4):643–651. doi:10.1007/s00428-018-2332-2
19. Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5(23):12189–12202. doi:10.18632/oncotarget.2608
20. Sakakura K, Takahashi H, Kaira K, et al. Relationship between tumor-associated macrophage subsets and CD47 expression in squamous cell carcinoma of the head and neck in the tumor microenvironment. Lab Invest. 2016;96(9):994–1003. doi:10.1038/labinvest.2016.70
21. Lin X, Gudgeon NH, Hui EP, et al. CD4 and CD8 T cell responses to tumour-associated Epstein-Barr virus antigens in nasopharyngeal carcinoma patients. Cancer Immunol Immunother. 2008;57(7):963–975. doi:10.1007/s00262-007-0427-8
22. Chan KH, Ng MH, Seto WH, Peiris JS. Epstein-Barr virus (EBV) DNA in sera of patients with primary EBV infection. J Clin Microbiol. 2001;39(11):4152–4154. doi:10.1128/JCM.39.11.4152-4154;2001
23. Zheng XH, Lu LX, Li XZ, Jia WH. Quantification of Epstein-Barr virus DNA load in nasopharyngeal brushing samples in the diagnosis of nasopharyngeal carcinoma in southern China. Cancer Sci. 2015;106(9):1196–1201. doi:10.1111/cas.12718
24. An X, Wang FH, Ding PR, et al. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011;117(16):3750–3757. doi:10.1002/cncr.25932
25. Chan AT, Lo YM, Zee B, et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94(21):1614–1619. doi:10.1093/jnci/94.21.1614
26. Chikamatsu K, Tada H, Takahashi H, et al. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral Oncol. 2019;89:34–39. doi:10.1016/j.oraloncology.2018.12.002
27. Wu L, Yu GT, Deng WW, et al. Anti-CD47 treatment enhances anti-tumor T-cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma. Oncoimmunology. 2018;7(4):e1397248. doi:10.1080/2162402X.2017.1397248
28. Yoshizaki T, Kondo S, Endo K, et al. Modulation of the tumor microenvironment by Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Cancer Sci. 2018;109(2):272–278. doi:10.1111/cas.13473
29. Chen YP, Zhang WN, Chen L, et al. Effect of latent membrane protein 1 expression on overall survival in Epstein-Barr virus-associated cancers: a literature-based meta-analysis. Oncotarget. 2015;6(30):29311–29323. doi:10.18632/oncotarget.4906
30. Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227–231. doi:10.1126/science.aac9935
31. Chen J, Zheng DX, Yu XJ, et al. Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology. 2019;8(11):e1652540. doi:10.1080/2162402X.2019.1652540
32. Tudor CS, Dawson CW, Eckhardt J, et al. c-Myc and EBV-LMP1: two opposing regulators of the HLA class I antigen presentation machinery in epithelial cells. Br J Cancer. 2012;106(12):1980–1988. doi:10.1038/bjc.2012.197
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.