Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Cd34 Immunostaining Adds Specificity To Microvascular invasion Analysis in Hepatocellular Carcinoma
Authors Gama H, Albuquerque R, Campos Wanderley D, Pascoal Xavier MA, Osório FMF , Couto CA, Ferrari TCA, Lima AS, Diniz PHC , Vidigal PVT
Received 16 September 2022
Accepted for publication 10 January 2023
Published 22 January 2023 Volume 2023:10 Pages 91—98
DOI https://doi.org/10.2147/JHC.S389836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Henrique Gama,1 Ronniel Albuquerque,1 David Campos Wanderley,1 Marcelo Antônio Pascoal Xavier,1 Fernanda Maria Farage Osório,2 Cláudia Alves Couto,3 Teresa Cristina de Abreu Ferrari,3 Agnaldo S Lima,4 Paulo Henrique Costa Diniz,3 Paula Vieira Teixeira Vidigal1
1Department of Anatomic Pathology, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; 2Alpha Institute of Gastroenterology, Hospital das Clínicas Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; 3Department of Internal Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; 4Department of Surgery, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil
Correspondence: Paulo Henrique Costa Diniz, Department of Internal Medicine, School of Medicine, Universidade Federal de Minas Gerais, Prof Alfredo Balena Av. 190, Belo Horizonte, Minas Gerais, Brazil, 30130100, Email [email protected]
Introduction: Hepatocellular carcinoma is the most common primary neoplasia of the liver. Microvascular invasion predicts outcome and defines tumor staging. However, its diagnosis is still a challenge. The present study aims to evaluate inter and intraobserver agreement in identifying the presence of microvascular invasion using conventional and immunohistochemistry histology.
Methods: Three pathologists performed the analysis of 76 hepatocellular carcinoma explants to characterize the presence of microvascular invasion using the hematoxylin/eosin stain and immunohistochemistry for CD34. The evaluations were made individually, in two distinct moments. Results were analyzed by the Kappa’s coefficient and ROC curves.
Results: Our study demonstrated similar agreement for microvascular invasion between hematoxylin/eosin and CD34 methods. However, the intraobserver agreement values for both methods were higher than the interobserver ones. The accuracy of CD34 in relation to hematoxylin/eosin by ROC curves in intraobserver analysis tends to a high specificity, ranging from 82.1 to almost 100%, with sensitivity of 46.9% to 81.1%. In interobserver analysis, CD34 also has a high specificity (84.3% to 85.5%) while its sensitivity is a little shorter (81.2% to 84.3%).
Conclusion: Intraobserver higher agreement allows us to suppose that pathologists employed own criteria to evaluate vascular invasion, reinforcing the need of standardization. ROC Curves analysis showed that the CD34 method is more specific than sensitive. Therefore, immunohistochemistry for CD34 should not be used routinely, but it could be useful to help confirming invasion previously seen by conventional histology.
Keywords: hepatocellular carcinoma, vascular invasion, immunohistochemistry, interobserver, intraobserver
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignant neoplasm of the liver, and liver cirrhosis is its major risk factor.1,2 It is a highly vascularized tumor with high propensity to vascular invasion. The frequency of vascular invasion often increases with tumor growth, which alters both the disease’s staging and the patient’s prognosis3–5 and is one of the most important predictors of HCC recurrence after treatment.5–9 Therefore, accurate pathologic staging is of great importance to ensure appropriate clinical management, prognostic assessment and even recurrence prediction after liver transplantation for HCC.3,10–12
Several studies conducted to date show that identification of microscopic vascular invasion (MVI) is a great challenge for pathologists, since the criteria vary among professionals. Comparative studies on interobserver agreement, using only conventional optical microscopy – routine staining with hematoxylin-eosin (HE) – showed statistically significant degrees of disagreement, which reinforces the need for more specific criteria for the diagnosis of MVI.13–15 The use of immunohistochemistry (IHC) with blood and lymph vascular markers, such as CD34, could play a role in predicting MVI in hepatocellular carcinoma. Similar techniques have been carried out in studies of lymphovascular invasion in colorectal adenocarcinoma, with some limitations.17 IHC could also enhance agreement among examiners, resulting in less statistical variability.
The present study aims to evaluate inter and intraobserver agreement in identifying the presence of MVI, by means of both conventional optical microscopy in HE and immunohistochemistry using blood vascular marker CD34.
Methods
Seventy-six liver explants from liver transplantation performed from January 2002 to December 2017 at the Clinics Hospital, Federal University of Minas Gerais, Belo Horizonte, Brazil, were studied. Explants were retrospectively reviewed after the study approval by the local Ethics Committee (CAAE 0643.0.203.000–11, Comitê de Ética em Pesquisa, Universidade Federal de Minas Gerais – CEP-UFMG). Written informed consent was obtained from the patients or their relatives prior to study commencement, in accordance with the Declaration of Istanbul. The study was performed in compliance with the Helsinki Declaration.
The most representative slide of each case was selected and analyzed using the conventional HE staining and immunohistochemical technic. The CD34 antibody was used to mark blood vascular endothelium by using a commercial kit (Novolink Polymer, Leica Biosystems, IL), according to the manufacturer’s instructions, in a 1/100 dilution, one hour incubation.
Unidentified slides of all cases were reviewed by three pathologists with total experience time ranging from three to 18 years, and also with variable experience in liver pathology. The evaluation by the pathologists was performed independently in two distinct moments, with an interval of two months between them. Before each analysis all the slides were mixed together. Thus, all cases were evaluated twice by the same pathologist, allowing the analysis of inter- and intraobserver agreement.
Inter- and intraobserver reproducibility was analyzed using the Kappa statistic (Minitab LLC, version 17, State College, PA, USA, and MedCalc Software’s VAT, version 18.9.1 Ostend, Belgium). Interobserver agreement was considered when at least two of the three pathologists had the same evaluation concerning the presence of vascular invasion. The interpretation of the Kappa index was expressed according to the following threshold values: 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, almost perfect. The χ2 test or the two‐tailed Fisher’s exact test was used to compare the results. IHC performance was analyzed using ROC curves. The P‐value of <0.05 was considered statistically significant.
Results
During the study period, 76 liver explants from hepatic transplantation for hepatocellular carcinoma treatment were selected for this investigation. All cases of HCC in the present study were obtained from cirrhotic livers.
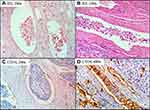
The major point of our study was to evaluate inter and intraobserver agreement in identifying the presence of MVI, by means of both conventional optical microscopy in HE and IHC using blood vascular marker CD34. The frequency of positive vascular invasion in HE by each observer in two different occasions was A (42% and 39%), B (49% and 39%) and C (42% and 50%). With CD34 staining the frequency of positive vascular invasion was A (21% and 26%), B (49% and 43%) and C (62% and 63%). Figure 1 shows examples of HCC vascular invasion in vessels stained by HE and IHC methods.
Intra and Interobserver Concordance of Microvascular Invasion
Figure 2 shows the intraobserver agreement related to HE and CD34 staining, obtained by the three observers, regarding the presence of vascular invasion at two different occasions. The intraobserver agreement in relation to examiner A occurred in 92.0% of the cases using the HE method and in 87.0% of the cases using the CD34 antibody. For examiner B, agreement was found in 78.0% of cases by HE, in 82.0% of cases by CD34. As observed, examiner C agreed with himself in 84.0% of cases by HE, 99.0% of cases by CD34.
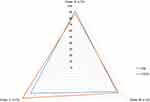 |
Figure 2 Agreement of vascular invasion in HE and CD34 according to each observer in two different occasions. Abbreviations: ExamA, examiner A; ExamB, examiner B; ExamC, examiner C. |
Interobserver agreement when at least two examiners had the same interpretation for the presence of vascular invasion at two different moments reveal that 45.0% of the cases were positive for vascular invasion by HE staining at the first moment, dropping to 39.0% at the second moment. These percentages were 46.0% of agreement when using the evaluation by CD34 in the first moment, with a slight reduction to 41.0% in the second moment.
The results of the Kappa coefficient (K) regarding intra and interobserver agreement concerning the identification of vascular invasion in HE and CD34 IHC staining are presented in Tables 1 and 2, respectively. Intraobserved agreement was superior to that of the interobserver. The intraobserver agreement by HE was almost perfect (K = 0.837) for examiner A, moderate (K = 0.546) for examiner B and substantial (K = 0.682) for examiner C (p < 0.0001). The CD34 evaluation revealed substantial agreement (0.636 and 0.629) for examiners A and B and almost perfect agreement (0.972) for examiner C (p < 0.0001). The interobserver agreement by HE was moderate in the first attempt (K = 0.485) and fair (0.320) in the second (p < 0.0001). The CD34 evaluation revealed fair agreement (0.234 and 0.218) in both attempts (p < 0.001).
 |
Table 1 Intraobserver Agreement for 3 Examiners at 2 Different Moments, Regarding Vascular Invasion in HCC |
 |
Table 2 Interobserver Agreement for 3 Examiners at 2 Different Moments, Regarding Vascular Invasion |
Evaluation of the CD34 Staining Accuracy to Determinate Microvascular Invasion
We used the ROC curve to verify the accuracy of the CD34 IHC staining to diagnose MVI, considering HE staining as the standard method, as it is routinely used in pathology laboratories. We constructed 6 curves comparing the intraobserver evaluation at 2 different moments, as shown in Figure 3A.
All ROC curves presented in Figure 3 have an area under the curve (AUC) greater than 0.700; and when considering the lower limit of the 95% CI for these AUCs, only in one graph (A1) this value was below 0.700. Regarding the examiners A and B, the comparison of CD34 IHC evaluation and the HE method presented a high specificity in the 4 graphs, ranging from 82.1% to almost 100% at the break point of the curve, while the sensitivity ranged from 46.9% to 81.1%. For examiner C, the comparison between the CD34 IHC evaluation and HE staining showed a specificity ranging from 65.9% to 71.1% and a higher sensitivity, ranging from 97.4% to almost 100%.
In order to further verify the accuracy of CD34 IHC, considering HE as the standard method, we constructed ROC curves for interobserver agreement of vascular invasion at two different moments, as seen in Figure 3B. The two ROC curves of Figure 3B are similar. The AUCs have high values, being higher than 0.800; and when considering the lower limit of the 95% CI for these AUCs, such values were higher than 0.734. We observed that when compared to the HE method, the CD34 IHC assessment presented a relatively high specificity in both graphs, ranging from 84.3% to almost 85.5% at the point of curve break, while the sensitivity was somewhat lower, but also remains high, ranging from 81.2% to 84.3%.
Discussion
Assessing the presence of MVI in HCC can potentially lead to either under staging or over staging of the tumor which, in turn, may have a significant impact on prognostic assessment and therapeutic decision-making. In this study, we demonstrated that IHC using markers of blood vessels did not perform better than standard HE staining in the identification of microvascular invasion.
Most studies dealing with vascular invasion in malignant neoplasms focus on the traditional method of optical microscopy with HE staining. In the present study, we evaluated both HE staining and vascular IHC staining, with the purpose of marking the endothelium vessels to highlight tumor emboli. To our knowledge, this is the first study comparing intra and interobserver agreement regarding MVI in HCC using both methods.
Fan et. al, studying vascular invasion in HCC using HE staining, found moderate interobserver as well as intraobserver agreement (Kappa of 0.5 and 0.45, respectively). They also found no statistically significant difference among the pathologists, regardless the time of profession or experience in hepatopathology. Based on these results, the authors concluded that there is a need to better define criteria to diagnose vascular invasion; however, they did not consider IHC as a possible contributing factor for this definition.14
The difficulty of assuring the presence of tumor emboli lies in some factors, among them, the morphology of the neoplasia. HCC generally has a structure of trabecular arrays lined by sinusoidal capillaries, which can imitate true vascular channels making it difficult to identify a possible invasion.4,14,16 This reinforces the need to establish more uniform and reproducible criteria including the use of other techniques such as IHC.
In this sense, Harris et al studied the interobserver agreement of vascular invasion in colorectal cancer, allying the conventional microscopy methods with HE and also IHC using the vascular markers CD31 and D2-40, similarly to the one used in our study. Those authors found no statistically significant difference between these methods. Using HE staining, the interobserver agreement regarding small vessels was reasonable (Kappa of 0.28) and minimal for larger vessels (Kappa of 0.18); and, there was no significant improvement when the CD31 antibodies (Kappa for small and larger vessels of 0.26 and 0.42, respectively) and D2-40 (Kappa of 0.32) were applied.17
Our study demonstrated a reasonable interobserver agreement by the conventional method of HE (Kappa 0.4025, range 0.32–0.485). When we used the CD34 antibody, the concordance was inferior (Kappa 0.226, range 0.218–0.234), although still within the range of reasonable agreement according to the classification adopted in this study. Regarding intraobserver agreement, our findings were classified as substantial for the HE method, as well as with the use of the CD34 antibody.
We believe that some morphological features of the samples may have been responsible for the low agreement index. Among them, we highlight two: retraction artifacts simulating a false vascular space, which should be clarified by the immunohistochemistry, and sinusoidal capillarization, which consists of the transformation of normal (fenestrated) hepatic sinusoids into continuous blood capillaries by stimulation of the vascular endothelial growth factor, resulting in deposition of laminin and basement membrane between endothelial cells and hepatocytes, making them physically and chemically similar to a blood vessel, with CD34-positive marking.4 This could be a confusing factor, since both a peritumoral blood vessel containing a neoplastic emboli and a modified tumoral sinusoid would be labeled by CD34.
In addition, the wear of the paraffin blocks to make all the slides to be stained by IHC may have, somewhat, modified the cut surface to be evaluated, which may have prevented, in some cases, the visualization or confirmation of the vascular invasion previously seen in HE.
The accuracy of CD34 IHC staining, considering HE as the standard method, and the results of examiner A and B (examiners with more experience in liver disease) intraobserver agreement, was more specific than sensitive. On the other hand, the intraobserver agreement of C (general pathologist) showed values of sensitivity higher than those of specificity. When analyzing the interobserver agreement, CD34 IHC staining may also be considered slightly more specific than sensitive. This data set suggests that this type of IHC examination should not be used as an initial examination in the context of vascular invasion screening, but it may have some utility in cases previously analyzed by the conventional HE method to aid in the confirmation or non-confirmation invasion.
In this line, Salizzoni et al demonstrated that CD34 IHC staining has a higher specificity in relation to HE in a comparative intraobserver study between the methods in patients who had CHC recurrence. In that study, 136 samples were examined, and vascular invasion was detected in 22 cases by HE and in 16 cases by IHC using antibodies against CD34. Of these 22 detected in HE, six had recurrence and of the 16 detected by CD34, eight recurred, corresponding to a percentage of 27.8% and 50%, respectively, pointing to a greater specificity of detection of vascular invasion by the CD34 IHC analysis.18
Our data, together with those observed by other researchers14,17–20 demonstrate the difficulty in evaluating the presence of vascular invasion in neoplasms in general. Our study, specifically on HCC, demonstrated similar agreement indexes when HE was compared to IHC using the CD34 antibody, regardless of whether the comparison was made inter or intraobserver. However, the intraobserver agreement values of the two methods were superior to the interobserver, which allows us to infer that each examiner used his own vascular invasion detection criteria and remained relatively faithful to them when they assessed at two different moments. Huber AR et al showed that interobserver agreement was higher when microvascular invasion was evaluated by pathologists with specialized in liver pathology.21 Surgical pathology subspecialty sign-out may be a tool to aid in the diagnosis of vascular invasion.
The misdiagnosis of vascular invasion can lead, in practice, to a sub-staging or super-staging of the tumor, what directly impacts on the prognosis of the patients and also on their treatment. The data in the present study did not demonstrate superiority of the IHC method using markers of blood vessels in relation to HE, but their association can contribute solve cases considered difficult. As HE and IHC methods have limitations, molecular triggers of vascular invasion in hepatocellular carcinoma are been studying as noninvasive biomarkers. Krishnan et al identified transcriptional, epigenetic, and proteomic changes driven by the MYC oncogene that were linked to vascular invasion in HCC. They proposed the use of fibronectin as promising noninvasive proteomic biomarker of vascular invasion.22 However, the use of molecular technologies is outside the reality of most centers, especially those in developing countries. We think that the definition of more precise morphological criteria for vascular invasion in HE and IHC methods seems to be the best alternative to optimize microvascular detection in hepatocellular carcinoma.
Ethics Approval
CAAE 0643.0.203.000-11. Comitê de Ética em Pesquisa, Universidade Federal de Minas Gerais – CEP-UFMG.
Informed Consent
Written informed consent was obtained from the patients or their relatives prior to study commencement, accordance with the Declaration of Istanbul. The study was performed in compliance with the Helsinki Declaration. All original liver donations were conducted voluntarily, with written informed consent and in compliance with the Declaration of Istanbul.
Acknowledgments
We thank Mrs. Fernanda Césari e Silva Barros for technical assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Minas Gerais Research Foundation – FAPEMIG.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2018;109(4):542–553. doi:10.1038/ajg.2014.11
2. Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53. doi:10.2147/JHC.S61146
3. Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi:10.1002/lt.20472
4. Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma. Anat Rec. 2008;291:721–734. doi:10.1002/ar.20668
5. Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254:108–113. doi:10.1097/SLA.0b013e31821ad884
6. Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29(4):502–510. doi:10.1111/j.1478-3231.2008.01957.x
7. Colecchia A, Schiumerini R, Cucchetti A, et al. Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol. 2014;20(20):5935–5950. doi:10.3748/wjg.v20.i20.5935
8. Dudek K, Kornasiewicz O, Remiszewski P, et al. Impact of tumor characteristic on the outcome of liver transplantation in patients with hepatocellular carcinoma. Transplant Proc. 2009;41(8):3135–3137. doi:10.1016/j.transproceed.2009.08.016
9. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi:10.1053/j.gastro.2009.06.003
10. Selcuk H. Prognostic factors and staging systems in hepatocellular carcinoma. Exp Clin Transplant. 2017;15(Suppl 2):45–49. doi:10.6002/ect.TOND16.L11
11. Shah SA, Tan JC, McGilvray ID, et al. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellularcarcinoma. Transplantation. 2006;81:1633–1639. doi:10.1097/01.tp.0000226069.66819.7e
12. Amin MB. AJCC Cancer Staging Manual.
13. Rodríguez-Perálvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi:10.1245/s10434-012-2513-1
14. Fan L, Mac MT, Frishberg DP, et al. Interobserver and intraobserver variability in evaluating vascular invasion in hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1556–1561. doi:10.1111/j.1440-1746.2010.06304.x
15. Fujita N, Aishima S, Iguchi T, et al. Histologic classification of microscopic portal venous invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855.
16. Schlageter M, Terracciano LM, D’angelo S, et al. Histopathology of hepatocellular carcinoma. World J Gastroenterol. 2014;20(43):15955–15964. doi:10.3748/wjg.v20.i43.15955
17. Harris EI, Lewin DN, Wang HL, et al. Lymphovascular invasion in colorectal cancer: an interobserver variability study. Am J Surg Pathol. 2008;32:1816–1821. doi:10.1097/PAS.0b013e3181816083
18. Salizzoni M, Romagnoli R, Lupo F, et al. Microscopic vascular invasion detected by anti-CD34 immunohistochemistry as a predictor of recurrence of hepatocellular carcinoma after liver transplantation. Transplantation. 2003;76(5):844–848. doi:10.1097/01.TP.0000083555.06337.8E
19. Franc B, De La Salmonière P, Hoang C, et al. Interobserver and intraobserver reproducibility in the histopathology of follicular thyroid carcinoma. Hum Pathol. 2003;34(11):1092–1100. doi:10.1016/S0046-8177(03)00403-9
20. Rakha EA, Abbas A, Ellis IO, et al. Diagnostic concordance of reporting lymphovascular invasion in breast cancer. J Clin Pathol. 2018;71(9):802–805. doi:10.1136/jclinpath-2017-204981
21. Huber AR, Gonzalez RS, Orloff MS, Barry CT, Whitney-Miller CL. Accuracy of vascular invasion reporting in hepatocellular carcinoma before and after implementation of subspecialty surgical pathology sign-out. Indian J Pathol Microbiol. 2017;60:501–504. doi:10.4103/IJPM.IJPM_827_16
22. Krishnan MS, Rajan KD. Genomic analysis of vascular invasion in HCC reveals molecular drivers and predictive biomarkers. Hepatology. 2021;73:2342–2360. doi:10.1002/hep.31614
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.