Back to Journals » Cancer Management and Research » Volume 13
Caudate Lobe Hepatocellular Carcinoma Treated with Sequential Transarterial Chemoembolization and Iodine 125 Seeds Implantation: A Single-Center Retrospective Study
Authors Yan L, Chen L , Qian K , Kan X, Zhang H, Liang B , Zheng C
Received 4 March 2021
Accepted for publication 20 April 2021
Published 13 May 2021 Volume 2021:13 Pages 3901—3912
DOI https://doi.org/10.2147/CMAR.S309310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Liangliang Yan,1,2,* Lei Chen,1,2,* Kun Qian,1,2 Xuefeng Kan,1,2 Hongsen Zhang,1,2 Bin Liang,1,2 Chuansheng Zheng1,2
1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Hubei Key Laboratory of Molecular Imaging, Wuhan, 430022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chuansheng Zheng
Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
Email [email protected]
Purpose: Resection of the hepatocellular carcinoma (HCC) in the caudate lobe (CL) is challenging even for accomplished surgeons. This retrospective study evaluated the safety and efficacy of transarterial chemoembolization (TACE) and iodine 125 seeds implantation (ISI) for unresectable or “ablation unsuitable” HCC-CL detected at the initial presentation in clinical practice.
Patients and Methods: A total of 20 HCC-CL patients undergoing sequential TACE and ISI from January 2014 to October 2018 were enrolled in this study. The overall survival (OS), progression-free survival (PFS), tumor response rate, and complication rates were analyzed and compared to non-caudate lobe (NCL) HCC patients. Multivariate analyses for potential clinical and radiological factors were performed using the Cox proportional hazard model.
Results: The technical success rate was 100%, as all the patients received 28 ISI treatments. The median OS was 35 months. The 1-, 3-, and 5-year OS rates were 100%, 63.2%, and 11.1%, respectively. The median PFS was 16 months. The objective response rate was 60.0%. The puncture tract bleeding (2/20) and pneumothorax (1/20) were the most common complications in operation, but no operation-related deaths occurred. One year after the surgery, biliary tract injury occurred in 1 patient, necessitating percutaneous biliary intervention. No statistical difference was observed between the CL and NCL groups. Multivariable analysis revealed that Barcelona Clinic Liver Cancer stage B and tumor size > 3 cm were two significant factors associated with OS.
Conclusion: Sequential TACE and ISI were associated with the survival benefits in HCC-CL and should be considered as a reliable therapy for surgeons and interventional radiologists.
Keywords: transarterial chemoembolization, iodine 125 seeds implantation, hepatocellular carcinoma, caudate lobe, survival
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy, with incidence and mortality rates ranking sixth and fourth in the world, respectively.1 An annual prevalence growth trend has been observed in some Asian countries and the USA.2,3 Cases of HCC originating in the caudate lobe (HCC-CL) are rare, with an incidence rate between 1.9% and 12.4%.4–7 The implementation of surveillance programs for high-risk populations and advances in diagnostic imaging technology has increased the diagnosis rate of HCC-CL.8,9
The surgical resection and percutaneous radiofrequency ablation (RFA) can be used as a curative treatment for HCC-CL.10 However, the resection of the HCC-CL is challenging for accomplished surgeons owing to the tumor’s deep location that is adjacent to the inferior vena cava and hepatic vein and has a narrow surgical margin.4,5,11,12 Thus, radiofrequency therapy cannot be administered safely under certain circumstances, such as thermal injury of adjacent structure, heat sink effect (near major vessels), and limited tumor necrosis range.13–15
Currently, transarterial chemoembolization (TACE) therapy that effectuates tumor necrosis through the occlusion of blood flow and the slow release of chemotherapeutic drugs into tumors is the primary palliative treatment option for unresectable HCC.16,17 Some studies have reported that ultraselective chemoembolization via the caudate artery for solitary HCC-CL is possible in most patients, the consequence is prolonged longer overall survival (OS) and progression-free survival (PFS).18,19 However, after repeated TACE therapy, TACE failure/refractoriness often occurs, resulting in decreased OS. Thus, effective adjuvant therapy is essential to prevent or delay recurrence. Iodine 125 seeds implantation (ISI) is an internal radiation source that has been applied widely for the treatment of intrahepatic and extrahepatic tumors.20–22 Several studies have confirmed that TACE combined with ISI for HCC with portal vein tumor thrombus (PVTT) and beneath the diaphragm (segment VII and VIII) is safe with significant clinical efficacy in some Asia-Pacific regions.23–26
However, no studies have focused on the sequential use of TACE and ISI for the treatment of HCC-CL. Thus, this retrospective study was conducted to evaluate the efficacy and safety of this novel combined therapy for the treatment of HCC-CL at initial presentation.
Patients and Methods
Study Design and Patient Selection
This retrospective study was performed according to the guidelines of the Helsinki Declaration and approved by the ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. The need for informed consent was waived by the Institutional Review Board. The study involved only a minimal risk to the patients. Moreover, the waiver did not adversely affect the rights and welfare of the patients. All data of patients were used confidentiality and anonymously.
The radiological database of our hospital was searched, and a total of 121 consecutive patients diagnosed with HCC receiving TACE with ISI therapy between January 2014 and October 2018 were collected. Next, we analyzed the clinical features and long-term prognosis of 20 patients whose target lesions were in the CL that could be measured at initial chemoembolization, and 57 cases of non-caudal lobe (NCL) HCC were also included in the analysis to compare the difference in the efficacy between CL and NCL HCC.
The patient enrolment and categorization flowchart is illustrated in Figure 1. The inclusion criteria were as follows: (1) Patients diagnosed with primary HCC by biopsy or imaging based on the European Association for the Study of the Liver (EASL) guidelines;10 (2) Liver function status at Child–Pugh class A or B; (3) East Coast Oncology Group (ECOG) performance status value 0 or 1; (4) No severe coagulopathy (platelets ≥50,000/mL); (5) Available medical records. Patients who fulfilled the following criteria were excluded from this study: (1) Incomplete clinical data; (2) Tumor thrombus in the portal vein, inferior vena cava, or hepatic vein; (3) Extrahepatic metastasis at preprocedural imaging study; (4) Undergone liver resection or RFA.
 |
Figure 1 Patient enrolment and categorization flow chart. Flow chart shows the screening procedure for patients with HCC-LC who were included in this study. |
TACE Procedure
TACE was performed according to the standard protocol of our Institute and that reported previously.27 All operators had at least eight years of experience in performing TACE procedures using digital subtraction angiography (DSA) (Artis zee floor; Siemens, Germany). Herein, TACE was performed using transfemoral arterial access route with a micro-puncture system by placing a 5-F vascular introducer (Cook, Bloomington, IN, USA) and celiac or superior mesenteric arteriography was conducted to assess the arterial anatomy, tumor supplying vessel, and patency of the portal vein. A 2.6-Fr microcatheter (Terumo, Japan) was inserted into the tumor donor arteries as superselectively as possible to identify the staining and arteries feeding the target lesions. First, an emulsion of 10–20 mL lipiodol (Lipiodol Ultrafluido, Guerbet, France) mixed with 20–40 mg doxorubicin hydrochloride (Hisun Pharmaceutical Co. Ltd, Zhejiang, China) was injected into the tumor feeding branch of the hepatic artery. Then, the gelatin sponge particles (300–500 µm, Cook) mixed with contrast material were administered into the tumor-feeding arteries until stasis of the arterial flow was achieved.
ISI Procedure
The ISI procedure was performed in accordance with the standard treatment regimen described in our previous study.28 The patients underwent computed tomography (CT) scans under the circumstances of the disappearance of the syndrome after embolization and the recovery of liver function before implantation. Residual tumors were evaluated by a senior radiologist, and the decision about administering 125I seed implantation was determined based on the doctor’s experience and patient’s preference. 125I seeds (China Institute of Atomic Energy, Beijing, China) with length 4.5 mm and diameter 0.8 mm, and encapsulated in NiTinol capsules were implanted within an average interval of 5.3 (2–12) days after TACE. Then, the CT images were transmitted to the treatment-planning system (TPS). The TPS software simulates the cancer tissue puncture and the placement of 125I seeds, uses 3D-printing technology to create a three-dimensional (3D) conformation template of the surface of the cancer tissue, and directs the puncture needle into the tissue based on the template. The numbers and positions of 125I seeds were determined by TPS according to the minimum peripheral dose prescribed to each tumor (90–165 Gy), such that the X-rays and c-rays could cover the planning target volume, including the tumor and 0.5–1 cm of the adjacent non-tumor tissues. Needle (18-gauge; XinKe Pharmaceutical Ltd, Shanghai, China) was placed under the guidance of a CT scan, and the seeds were implanted into the tumor via the needle at intervals of 1–1.5 cm.
Definition and Evaluation of Data
The procedural success was defined as the completion of both TACE and ISI in one treatment session. Successful selective TACE was defined as the catheterization of all tumor-feeding arteries and puncture, and the placement of the seeds on the preset path was defined a successful ISI. Modified Response Evaluation Criteria in Solid Tumors (mRECIST) was used to evaluate the efficacy of the technique, carried out at 1–1.5-month interval by two radiologists,29 after the initial TACE procedure. Complete response (CR) refers to the absence of enhancement in all target lesions; partial response (PR) is classified as at least a 30% decrease in the sum of the diameters of viable tumors; progressive disease (PD) is an increase of at least 20% in the sum of the diameters of target lesions; stable disease (SD) refers to any case that does not qualify as either PR or PD. Disease control rate (DCR) was used to represent the proportion of patients who reached CR+PR+SD. For each patient, the OS and PFS were calculated from the date of the initial TACE to the date of death or the last follow-up and to the date of tumor progression (intrahepatic recurrence or new intrahepatic or extrahepatic lesions developed) or the last follow-up, respectively. Adverse events and complications after the therapy were identified and described according to both the Common Terminology Criteria for Adverse Events (CTCAE-v4.0) and the Society of Interventional Radiology Classification system for Complications by Outcomes.30,31 The major complications were defined as events leading to death and disability.
Follow-Up
The follow-up contents included laboratory tests, contrast-enhanced computed tomography (CT) or magnetic resonance (MR) imaging examinations performed 4–6 weeks after the treatment. When a residual or recurrent tumor was detected, decisions about additional treatment were made according to the recurrence pattern, underlying liver function, and overall clinical condition of the patient. Imaging (contrast-enhanced CT or MR) and laboratory examinations were conducted every 2–3 months, and follow-up continued until the patient died or the endpoint of this study’s follow-up in October 2020.
Statistical Analyses
All statistical analyses were performed using SPSS software (version 24.0; IBM, Armonk, NY, USA). Continuous variables were summarized as mean ± standard deviation (SD). Pearson’s χ2 test, correction χ2 test, Fisher’s exact test, independent-samples t-test, and Mann–Whitney U-test were used for the comparison of the baseline characteristics and the occurrence rates of adverse events between the two groups. The OS and PFS curves were obtained using the Kaplan–Meier method, and the differences between the two groups were compared by Log rank test. Multivariate analysis was performed using the Cox regression model for significant variables (P < 0.1) in the univariate analysis, and the risk factors that affected the OS were determined. All statistical tests were two-sided, and P < 0.05 indicated statistical significance.
Results
Study Population and Patient Characteristics
From January 2014 to October 2018, a total of 121 consecutive patients diagnosed as HCC undergoing TACE combined ISI (TACE followed by ISI) therapy at our hospital were collected for this study; 44 patients were excluded because they did not meet the study requirements. Finally, 77 patients were included in this analysis: 20 patients consisted of target lesions in CL, while 57 patients showed the lesions in the NCL. Before these patients underwent initial TACE, a treatment strategy was recommended by the multidisciplinary tumor board. The patients with early-stage HCC were recommended to receive surgical resection, liver transplantation, or RFA. Some patients were not candidates for RFA treatment due to a suboptimal location of the tumor, while some declined the surgery because they had severe portal hypertension or difficulty in ensuring effective surgical margins. The surgeon informed the patients and/or their family members of the possible survival benefits of TACE and ISI treatment, the possible complications, and alternative treatment. The patients and/or their family members expressed their understanding, agreed to the operation, and signed the informed consent for the TACE and ISI operation. The baseline characteristics of the two groups of patients are summarized in Table 1. The clinical features and treatment of the HCC-CL group are summarized in Supplementary Table 1. A total of 34 tumors were detected in 20 patients, and the mean tumor diameter was 4.2 (2.1–7.8 range) cm. The subsegmental locations of the 20 CL tumors were the spiegel lobe (n = 12), the paracaval portion (n = 6), and the caudate process (n = 2). Moreover, 7 patients had one tumor-feeding artery, while 13 patients had multiple tumor-feeding arteries.
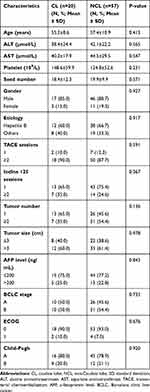 |
Table 1 Baseline Characteristics of Patients Enrolled in This Study |
A superselective chemoembolization was achieved via a 2.6-Fr microcatheter in 16/20 (80%) patients. Nonselective chemoembolization was performed in 4/20 (20%) patients because of failed catheterization of the tumor-feeding vessels that were not clearly indicated on DSA and the technical difficulty due to the small tumor-feeding vessels caliber or the acute angulation. All the 20 patients received 28 ISI treatments, and an average of 18.4 ± 12.3 particles was implanted in each patient; no particle displacement or shedding occurred. ISI had a technical success rate of 100%. Repeated TACE or ISI could be used on demand when the multidisciplinary tumor board considered that TACE or ISI was promising for the control of intrahepatic lesions, such as localized progression or metastasis in the liver. Before the final follow-up, 18/20 (90%) patients had experienced varying degrees of disease progression: 13 had intrahepatic metastasis, 8 had local progression, and 5 had extrahepatic metastasis, including 3 pulmonary metastasis and 2 lymph node metastases. Subsequently, 18/20 (90%) patients received repeated TACE treatments, and 7/20 (35%) patients received multiple TACE treatments. Finally, 15/20 (75%) patients died due to extensive metastasis or liver failure during the observation period.
Treatment Response and Complications
The treatment response in the CL group at the first follow-up CT or MR was CR in 6 patients (30%) and PR in 6 patients (30%). The objective response rate (ORR) was 60%. The NCL group comprised 71.9% (41/57) patients, and no statistically significant difference (P = 0.322) was detected between the two groups (Table 2). Bleeding of puncture tract (2/20) and pneumothorax (1/20) were the most common complications during the operation. One year after surgery, biliary tract injury occurred in 1 patient, requiring percutaneous biliary intervention. However, no statistical difference was noted between the CL and the NCL groups. The common complications were as follows: 16 (80.0%) patients had a fever, 13 (65.0%) patients had abdominal pain, and 11 (55.0%) patients presented vomiting (Supplementary Table 2), but no statistically significant difference was observed between the two groups. These symptoms lasted 2–7 days and were relieved by symptomatic treatment before discharge; no treatment-related deaths occurred in this study.
 |
Table 2 Tumor Responses After Initial Combination in the CL and NCL Groups Results Reported as N (%) |
OS and PFS Analysis
Survival curves of patients are shown in Figure 2. The median OS was 35 (95% CI: 27.6–42.4) months in the CL group and 39 (95% CI: 30.9–47.1) months in the NCL group (P = 0.256) (Figure 2A). The 1-, 3-, and 5-year survival rates were 100% vs 100% (P > 0.999), 63.2% vs 81.6% (P = 0.196), and 11.1% vs 14.6% (P = 0.712), respectively. The median PFS was 16 (95% CI: 8.7–23.3) months in the CL group and 20 (95% CI: 14.9–25.1) months in the NCL group (P = 0.455) (Figure 2B). The 1- and 3-year cumulative recurrence rate was 30% vs 33.3% (P = 0.784) and 100% vs 96% (P > 0.999), respectively.
 |
Figure 2 Kaplan–Meier curve of all patients; (A) Kaplan–Meier curve of overall survival (OS); (B) Kaplan–Meier curve of progression-free survival (PFS). |
Prognostic Factors Affecting OS and PFS
The risk factors for PFS are analyzed in Table 3. Although univariate analysis showed that gender, tumor number, tumor size, and BCLC stage were associated with PFS, multivariate analysis identified BCLC stage B (HR = 3.495, 95% CI: 1.185–6.728, P<0.001) as a significant factor associated with PFS. The risk factors for OS are analyzed in Table 4. Univariate analysis showed that tumor size, α-fetoprotein level, and BCLC stage were associated with OS, and multivariate analysis showed that tumor size >3 cm (HR = 2.102, 95% CI: 1.058–4.178, P = 0.034) and BCLC stage B (HR = 3.318, 95% CI: 1.673–6.581, P = 0.001) indicated a poor prognosis.
 |
Table 3 Univariable and Multivariable Analysis for Progression-Free Survival |
 |
Table 4 Univariable and Multivariable Analysis for Overall Survival |
Subgroup Analysis
Kaplan–Meier curve of OS and PFS of patients in the subgroups is shown in Figure 3.
The median OS and PFS were 43 (95% CI: 37.9–48.1) months and 22 (95% CI: 18.7–25.3) months, 49 (95% CI: 38.1–59.9) months and 24 (95% CI: 21.9–26.1) months for patients with maximum diameter of the tumor < 3 cm in the CL and NCL groups (P = 0.665, P = 0.502, respectively) (A-B). The median OS and PFS were 29 (95% CI: 24.5–33.5) months and 12 (95% CI: 8.6–15.4) months, 31 (95% CI: 27.3–34.7) months and 11 (95% CI: 8.7–13.3) months for patients with maximum diameter of the tumor > 3 cm in the CL and NCL groups (P = 0.217, P = 0.807, respectively) (C-D). The median OS and PFS were 41 (95% CI: 35.2–46.8) months and 24 (95% CI: 18.3–29.7) months, 69 months (95% CI: 41.9–96.1) months and 25 (95% CI: 22.7–27.3) months for patients with BCLC stage A in the CL and NCL groups (P = 0.225, P = 0.471, respectively) (E-F). The median OS and PFS were 27 (95% CI: 22.3–31.7) months and 11 (95% CI: 8–14) months, 31 (95% CI: 26.5–31.7) months and 11 (95% CI: 8.7–13.3) months for patients with BCLC stage B in the CL and NCL groups (P = 0.375 and 0.238, respectively) (G-H).
Discussion
Although surgery and percutaneous ablation treatment provide survival benefits to some patients with HCC in the CL,4,32 they are challenging and have a high local recurrence rate owing to the deep location of the tumors, involvement of major adjacent vessels, and limited therapeutic margin.6,11,13 The current study proved that the novel treatment approach, TACE plus ISI, is safe and tolerable for some selected patients who gained significant benefits of survival and disease control.
To the best of our knowledge, Tanaka et al reported that the resection of HCC-CL had worse 5-year OS rates (25.9% vs 54.1%, P=0.01) and higher intrahepatic recurrence (40% vs 17.6%; P < 0.05) compared to the resection of HCC originating in other locations.33 In addition, these patients showed significant intraoperative blood loss, prolonged surgical time, and postoperative complications. Nishigaki et al34 have confirmed that RFA is a promising alternative to hepatic resection for the treatment of HCC-CL, but the 4-year cumulative local recurrence rate after RFA in the caudate and non-caudate groups was 22.3% and 4.5%, respectively (P < 0.001), and a revised method to reduce local recurrence should be pursued. Multipolar RFA and sequential TACE have been reported by Hirooka et al32 and Hyun et al,35 respectively, which improves the efficacy of RFA in the treatment of HCC-CL. Liu et al6 indicated that tumor size > 2 cm increases the risk of local tumor progression (LTP), and intrahepatic tumor number is associated with distant recurrence (DR) after ablation. On the other hand, some studies reported that no significant difference was observed in the OS or disease-free survival rates between the two groups.36,37 Herein, we proposed a novel treatment approach, TACE plus ISI, wherein 125I seeds have a long half-life and can irradiate the dividing tumor cells at close range (< 1 c m). The irradiation is not affected by “heat sink effect” and reduces the formation of new collateral blood supply to the HCC-CL caused by initial or repeated TACEs, such as the right inferior phrenic artery,38 thereby achieving effective local control of the tumors.
In the current group of patients, we found that TACE combined with ISI was well tolerated and showed a 100% procedural success rate. No major complications occurred. Also, no statistically significant difference was detected in the technical success rate, complication rate, and objective response rate between the CL and the NCL groups. Therefore, our results may serve as a safe and effective alternative treatment option as a treatment plan for unresectable or unsuitable ablation HCC-CL.
Several studies have reported various clinical results of surgical resection, including 3- and 5-year OS of 47–83% and 21–76%, respectively.5,36,39 The reported OS rates with RFA or chemoembolization for HCC-CL varied as follows: 59–86.6% at 3 years, and 44–67.5% at 5 years,19,40–43 which were similar to those after surgical resection. However, the tumor size > 2 cm and intrahepatic tumor number are associated with poor prognosis after ablation.6 In this study, the 3- and 5-year OS rates were 63.2% and 11.1%, respectively, which may be similar to those after surgical resection if the difference in the tumor size was considered for comparison; also, no difference was detected in the curative effect between caudate and non-caudal liver cancer. However, the cumulative recurrence rate was higher in this study than reported previously. This phenomenon could be attributed to the heavy tumor burden or the inclusion of patients with severe cirrhosis and a high rate of distant metastasis in the liver. Although our results are not inferior to that of radiofrequency treatment, and the diameter of tumors that can be treated is wider than that for radiofrequency, additional retrospective studies or prospective randomized controlled trials (RCT) comparing caudate and non-caudal HCC are essential to verify this hypothesis.
Nevertheless, the present study has some limitations. First, this was a retrospective study, lending some inevitable selection biases. Second, due to the limited number of included patients, the heterogeneity was large. Thus, the current findings should be expanded to a multicenter study for statistical and substantial medical evidence. Third, not all patients were diagnosed with HCC on a pathological basis, and the diagnostic efficiency might be insufficient only based on imaging findings combined with tumor markers. Further pathological results may be required.
Conclusion
In conclusion, the current study indicated that TACE combined with ISI treatment might be associated with comparable survival benefits in unresectable or “ablation unsuitable” HCC in the CL and should be considered as a reliable therapy for surgeons and interventional radiologists.
Abbreviations
HCC, hepatocellular carcinoma; CL, caudate lobe; PVTT, portal vein tumor thrombus; RFA, radiofrequency ablation; BCLC, Barcelona clinic liver cancer; TACE, transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group; mRECIST, modified response evaluation criteria in solid tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; CTCAE, common terminology criteria for adverse events; CT, computed tomography; MR, magnetic resonance; AFP, alpha-fetoprotein; SD, standard deviation; CI, confidence intervals; HR, hazard ratio; TPS, treatment-planning system; EASL, European Association for the study of the liver; DSA, digital subtraction angiography; HBV, hepatitis B virus; LTP, local tumor progression; DR, distant recurrence.
Ethics Approval and Informed Consent
This was a retrospective study and performed according to the guidelines of the Helsinki Declaration. This study was approved by the ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (UHCT-IEC-SOP-016-03-01). The need for informed consent was waived by this institution.
Acknowledgments
This work was supported by grants from the National Nature Science Foundation of China (81873919 and 81701800).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Nature Science Foundation of China (81873919 and 81701800).
Disclosure
All authors declare that they have no conflicts of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/s0140-6736(19)30427-1
4. Takayama T, Midorikawa Y, Higaki T, et al. Algorithm for resecting hepatocellular carcinoma in the caudate lobe. Ann Surg. 2019. doi:10.1097/SLA.0000000000003384
5. Shimada S, Kamiyama T, Yokoo H, et al. Prognoses and clinicopathological characteristics for hepatocellular carcinoma originating from the caudate lobe after surgery. World J Surg. 2019;43(4):1085–1093. doi:10.1007/s00268-018-4869-2
6. Liu B, Long J, Wang W, et al. Predictive factors of treatment outcomes after percutaneous ablation of hepatocellular carcinoma in the caudate lobe: a retrospective study. BMC Cancer. 2019;19(1):699. doi:10.1186/s12885-019-5881-0
7. Kim HC, Miyayama S, Chung JW. Selective chemoembolization of caudate lobe hepatocellular carcinoma: anatomy and procedural techniques. Radiographics. 2019;39(1):289–302. doi:10.1148/rg.2019180110
8. Son JH, Choi SH, Kim SY, et al. Accuracy of contrast-enhanced ultrasound liver imaging reporting and data system: a systematic review and meta-analysis. Hepatol Int. 2020;14(6):1104–1113. doi:10.1007/s12072-020-10102-5
9. Kierans AS, Kang SK, Rosenkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: a meta-analysis. Radiology. 2016;278(1):82–94. doi:10.1148/radiol.2015150177
10. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
11. Ahanatha Pillai S, Sathyanesan J, Perumal S, et al. Isolated caudate lobe resection: technical challenges. Ann Gastroenterol. 2013;26(2):150–155.
12. Chaib E, Ribeiro MA
13. Nault JC, Sutter O, Nahon P, Ganne-Carrie N, Seror O. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68(4):783–797. doi:10.1016/j.jhep.2017.10.004
14. Lee S, Kang TW, Cha DI, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol. 2018;69(1):70–78. doi:10.1016/j.jhep.2018.02.026
15. Chen J, Peng K, Hu D, et al. Tumor location influences oncologic outcomes of hepatocellular carcinoma patients undergoing radiofrequency ablation. Cancers. 2018;10(10). doi:10.3390/cancers10100378
16. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
17. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52(2):762–773. doi:10.1002/hep.23725
18. Choi WS, Kim HC, Hur S, et al. Role of C-arm CT in identifying caudate arteries supplying hepatocellular carcinoma. J Vasc Interv Radiol. 2014;25(9):1380–1388. doi:10.1016/j.jvir.2014.02.028
19. Kim HC, Chung JW, Jae HJ, et al. Caudate lobe hepatocellular carcinoma treated with selective chemoembolization. Radiology. 2010;257(1):278–287. doi:10.1148/radiol.10100105
20. Lu J, Huang W, Wang Z, et al. The safety and efficacy of interstitial (125)I seed implantation brachytherapy for metastatic epidural spinal cord compression. J Cancer Res Ther. 2018;14(7):1549–1555. doi:10.4103/jcrt.JCRT_938_17
21. He C, Liu Y, Li Y, et al. Efficacy and safety of computed tomography-guided (125)I brachytherapy for lymph node metastatic from hepatocellular carcinoma. J Cancer Res Ther. 2018;14(4):754–759. doi:10.4103/jcrt.JCRT_245_17
22. Denecke T, Stelter L, Schnapauff D, et al. CT-guided interstitial brachytherapy of hepatocellular carcinoma before liver transplantation: an equivalent alternative to transarterial chemoembolization? Eur Radiol. 2015;25(9):2608–2616. doi:10.1007/s00330-015-3660-0
23. Yuan D, Gao Z, Zhao J, Zhang H, Wang J. (125)I seed implantation for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Brachytherapy. 2019;18(4):521–529. doi:10.1016/j.brachy.2019.01.014
24. Li J, Zhang L, Xie Q, et al. 125I seeds implantation for treating residual hepatocellular carcinoma located beneath the diaphragm after transcatheter arterial chemoembolization. Brachytherapy. 2019;18(3):420–425. doi:10.1016/j.brachy.2018.12.008
25. Zhu ZX, Wang XX, Yuan KF, Huang JW, Zeng Y. Transarterial chemoembolization plus iodine-125 implantation for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 2018;20(9):795–802. doi:10.1016/j.hpb.2018.03.015
26. Zhang ZH, Zhang W, Gu JY, et al. Treatment of hepatocellular carcinoma with tumor thrombus with the use of iodine-125 seed strand implantation and transarterial chemoembolization: a propensity-score analysis. J Vasc Interv Radiol. 2018;29(8):1085–1093. doi:10.1016/j.jvir.2018.02.013
27. Kan X, Liang B, Zhou G, et al. Transarterial chemoembolization combined with apatinib for advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol. 2020;10:970. doi:10.3389/fonc.2020.00970
28. Chen L, Kan X, Sun T, et al. Transarterial chemoembolization combined with iodine 125 seeds versus transarterial chemoembolization combined with radiofrequency ablation in the treatment of early- and intermediate-stage hepatocellular carcinoma. BMC Gastroenterol. 2020;20(1):205. doi:10.1186/s12876-020-01355-3
29. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
30. Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14(9 Pt 2):S199–S202. doi:10.1097/01.rvi.0000094584.83406.3e
31. National Cancer Institute.Common Terminology Criteria for Adverse Events (CTCAE) v4.0.CTCAE_4.03_2010–06–14_QuickReference_5x7.pdf. 2010. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/.
32. Hirooka M, Ochi H, Hiraoka A, et al. Multipolar versus monopolar radiofrequency ablation for hepatocellular carcinoma in the caudate lobe: results of a propensity score analysis. Hepatol Res. 2017;47(7):658–667. doi:10.1111/hepr.12791
33. Tanaka S, Shimada M, Shirabe K, et al. Surgical outcome of patients with hepatocellular carcinoma originating in the caudate lobe. Am J Surg. 2005;190(3):451–455. doi:10.1016/j.amjsurg.2004.12.005
34. Nishigaki Y, Tomita E, Hayashi H, et al. Efficacy and safety of radiofrequency ablation for hepatocellular carcinoma in the caudate lobe of the liver. Hepatol Res. 2013;43(5):467–474. doi:10.1111/j.1872-034X.2012.01095.x
35. Hyun D, Cho SK, Shin SW, Rhim H, Koh KC, Paik SW. Treatment of small hepatocellular carcinoma (</=2 cm) in the caudate lobe with sequential transcatheter arterial chemoembolization and radiofrequency ablation. Cardiovasc Intervent Radiol. 2016;39(7):1015–1022. doi:10.1007/s00270-016-1314-5
36. Sakamoto Y, Nara S, Hata S, et al. Prognosis of patients undergoing hepatectomy for solitary hepatocellular carcinoma originating in the caudate lobe. Surgery. 2011;150(5):959–967. doi:10.1016/j.surg.2011.03.005
37. Yamamoto T, Kubo S, Shuto T, et al. Surgical strategy for hepatocellular carcinoma originating in the caudate lobe. Surgery. 2004;135(6):595–603. doi:10.1016/j.surg.2003.10.015
38. Miyayama S, Yamashiro M, Sugimori N, Ikeda R, Ishida T, Sakuragawa N. Blood supply to the caudate lobe of the liver from the right inferior phrenic artery: observation by cone-beam computed tomography during arteriography. Abdom Radiol. 2020;45(9):2851–2861. doi:10.1007/s00261-020-02489-4
39. Sakoda M, Ueno S, Kubo F, et al. Surgery for hepatocellular carcinoma located in the caudate lobe. World J Surg. 2009;33(9):1922–1926. doi:10.1007/s00268-009-0110-7
40. Schullian P, Laimer G, Putzer D, Effenberger M, Bale R. Stereotactic radiofrequency ablation of primary liver tumors in the caudate lobe. HPB (Oxford). 2020;22(3):470–478. doi:10.1016/j.hpb.2019.09.008
41. Li P, Kong L, Wang Y, Lv X, Wang J, Gao H. Comparative analysis of radiofrequency ablation and resection for colorectal liver metastases in caudate lobe: a retrospective study. Acta Chir Belg. 2020;120(5):321–328. doi:10.1080/00015458.2019.1631614
42. Jiang K, Zhang W, Su M, et al. Laparoscopic radiofrequency ablation of solitary small hepatocellular carcinoma in the caudate lobe. Eur J Surg Oncol. 2013;39(11):1236–1242. doi:10.1016/j.ejso.2013.08.002
43. Fujimori M, Takaki H, Nakatsuka A, et al. Combination therapy of chemoembolization and radiofrequency ablation for the treatment of hepatocellular carcinoma in the caudate lobe. J Vasc Interv Radiol. 2012;23(12):1622–1628. doi:10.1016/j.jvir.2012.09.005
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

