Back to Journals » The Application of Clinical Genetics » Volume 9
Case report of individual with cutaneous immunodeficiency and novel 1p36 duplication
Authors Hatter A, Soler D, Curtis C, Cooper K, McCormick T
Received 18 June 2015
Accepted for publication 9 September 2015
Published 13 January 2016 Volume 2016:9 Pages 1—4
DOI https://doi.org/10.2147/TACG.S90713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Martin Maurer
Alyn D Hatter,1 David C Soler,2,3 Christine Curtis,4 Kevin D Cooper,1,2,3,5 Thomas S McCormick,2,3
1University Hospitals Case Medical Center, 2Department of Dermatology, 3The Murdough Family Center for Psoriasis, Case Western Reserve University, 4Cleveland Department of Pathology and Center for Human Genetics Laboratory, 5VA Medical Center, Cleveland, OH, USA
Introduction: Crusted or Norwegian scabies is an infectious skin dermatopathology usually associated with an underlying immunodeficiency condition. It is caused when the mite Sarcoptes scabiei infects the skin, and the immune system is unable to control its spread, leading to a massive hyperinfestation with a simultaneous inflammatory and hyperkeratotic reaction. This is the first report of a novel 1p36 duplication associated with a recurrent infection of crusted scabies.
Case report: We describe a 34-year-old patient with a cutaneous immunodeficiency characterized by recurrent crusted scabies infestation, diffuse tinea, and recurrent staphylococcal cellulitis, who we suspected had an undiagnosed syndrome. The patient also suffered from mental retardation, renal failure, and premature senescence. A cytogenetic fluorescence in situ hybridization analysis revealed a 9.34 Mb duplication within the short (p) arm of chromosome 1, precisely from 1p36.11 to 1p36.21, with an adjacent 193 kb copy gain entirely within 1p36.11. In addition, chromosome 4 had a 906 kb gain in 4p16.1 and chromosome 9 had a 81 kb copy gain in 9p24.3. Over 100 genes localized within these duplicated regions. Gene expression array revealed 82 genes whose expression changed >1.5-fold compared to a healthy age-matched skin control, but among them only the lipolytic enzyme arylacetamide deacetylase-like 3 was found within the duplicated 1p36 region of chromosome 1.
Discussion: Although genetic duplications in the 1p36 region have been previously described, our report describes a novel duplicative variant within the 1p36 region. The patient did not have a past history of immunosuppression but was afflicted by a recurrent case of crusted scabies, raising the possibility that the recurrent infection was associated with the 1p36 genetic duplication.
Conclusion: To our knowledge, the specific duplicated sequence between 1p36.11 and p36.21 found in our patient has never been previously reported. We reviewed and compared the clinical, genotyping, and gene microarray results of our patient in order to characterize this novel 1p36 duplication syndrome, which might have contributed to the recurrent scabies infection in this patient.
Keywords: immunodeficiency, crusted scabies, cutaneous immunodeficiency, 1p36 duplication
Introduction
Crusted scabies is a rare, severe form of skin infection caused by the mite Sarcoptes scabiei var. homini. It is often associated with the elderly or with people who have a compromised immune system such as with HIV infection or in patients receiving immunosuppressive treatments. However, very few cases have been described of crusted scabies affecting young and relatively immune-competent patients.
Herein, we report a case of a patient with a cutaneous idiopathic immunodeficiency characterized by recurrent crusted scabies infestation as well as chronic fungal and bacterial infection. We suspected an undiagnosed syndrome. We detail her phenotype and clinical history, and report our findings from cytogenetic and cutaneous gene microarray analysis.
Case report
A 34-year-old Caucasian woman was referred to the department of dermatology for evaluation of recurrent and diffuse tinea corporis and recalcitrant crusted scabies. All studies of human subjects were approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH, USA) and the patient provided written informed consent. Earlier treatments included topical ketoconazole 2% cream, oxiconazole nitrate 1% cream, and permethrin 5% cream. The patient reported that her skin problems had been intermittent over the preceding 3 years. The medical history of this patient revealed end-stage renal disease requiring hemodialysis, obesity, non-insulin-dependent diabetes, hypertension, recurrent cellulitis, and methicillin-resistant Staphylococcus aureus bacteremia. Recently, prior to our consultation, she was diagnosed with bilateral cataracts and mild sensorineural hearing loss in one ear. Diabetes and hypertension were coincidentally diagnosed with nephrotic syndrome at age 29 during an acute illness, and she subsequently began hemodialysis. The patient had frequent admissions thereafter for dialysis catheter site cellulitis and methicillin-resistant S. aureus bacteremia secondary to catheter line infections. The patient has lived with her maternal grandmother since birth and was reportedly in good health until age 29 with no evidence of childhood infections other than the infrequent “cold”. The patient had no known siblings and the father was unknown to the family; the mother reportedly suffered from chronic pain related to an injury and died from a prescription narcotic overdose at age 29. There was no evidence of birth defects or immunodeficiency in the maternal family.
Physical examination revealed a woman appearing much older than her chronological age would suggest. The patient exhibited coarse facial features with a prominent brow ridge, midface hypoplasia, prognathia, and slight down slanting of palpebral fissures (Figure 1). The skin examination was significant for thick, hyperkeratotic, white, and flaking plaques on the palms, soles, digits, and under the distal nails (Figure 2). Moist erosions and bright erythema along many of the proximal nail folds were noted. The nail plates appeared normal except distal onycholysis overlying the plaques. There were crusted papules and erosions diffusely scattered on the face, ears, trunk, and extremities consistent with excoriations. There was no evidence of active tinea at the time of the patient visit. Earlier mineral oil scrapings had revealed multiple live mites and exam was consistent with crusted scabies. A fungal culture from the nail fold grew unidentified, non-Candida yeast. It was recommended to the referring dermatologist that the patient continues topical antifungal treatment as needed and to begin oral ivermectin 0.2 mg/kg daily on days 1, 2, 8, 9, 15, 22, and 29.1 An undiagnosed immune syndrome with cutaneous-specific immunodeficiency was suspected, so we referred the patient to medical genetics for evaluation. The laboratory results and hospital admission notes from her community physician were reviewed. Importantly, the patient was HIV negative, had a normal serum protein electrophoresis interpretation, and was negative for antinuclear antibodies. Electrolyte panels were consistent with renal failure and routine dialysis, but no renal biopsy had been performed. Although the white blood cell count would fluctuate between admissions, the patient’s lymphocyte count was consistently within normal limits. The scabies infection resolved after 3 months using routine maintenance doses of ivermectin. The tinea corporis infection resolved using the daily application of topical antifungal creams.
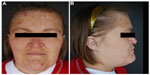  | Figure 1 Our patient demonstrates coarse facial features (A), hypoplastic midface and prognathia (B). |
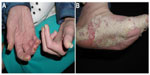  | Figure 2 Our patient had hyperkeratotic white plaques on her soles, hands, and periungual digits (A and B); manifestations of crusted scabies (B). |
Results
Cytogenetic analysis revealed a female karyotype with a duplication within the short (p) arm of chromosome 1. Duplication breakpoints were assigned to G-band regions 1p36.11 and 1p36.21 [dup(1)(p36.11p36.21)] (Figure 3A). Fluorescence in situ hybridization (FISH) was performed on metaphase cells from the sample, using two bacterial artificial chromosome probes (RP11–139H5 and –430L17) which localize to G-band regions 1p36.12 and 1p36.13, respectively (both of which lie within the duplicated segment). Each probe hybridized to two sites along the duplicated 1p arm, confirming the duplication of 1p material (Figure 3B). The relative positioning of the two probes suggests that the duplicated segment is adjacent to the original chromosomal segment in an inverted orientation. OligoArray analysis, using Signature Select OS 105K v1.1 array (Signature Genomics, Spokane, WA, USA), demonstrated an approximately 9.3 Mb copy gain within chromosome bands 1p36.11–p36.21. This gain is adjacent to an approximately 62 kb segment of a normal copy number within 1p36.11, followed by another gain of 193 kb within 1p36.11.
In addition, the array identified two smaller regions of copy gain – a 906 kb gain in chromosome 4 4p16.1 and an 81 kb copy gain in chromosome 9 9p24.3. The 4p16.1 region includes approximately seven genes and is recognized as a region of significant copy variation and segmental duplication.2 There are no FISH probes within this region.3 The small 9p24.3 region contains the 5′ portion of the DOCK8 gene and the 3′ portion of the C9orf66 gene. The copy number variation has been identified for this region3. The FISH analysis failed to confirm a duplication of this region, but a simultaneous hybridized control probe showed the normal signal number and pattern. It is therefore likely that the size of the duplication is below the technical detection limit for FISH.
Fragile X testing was negative and a plasma amino acid profile was within normal limits.
When using 1.5-fold as a threshold for measuring differences in levels of expression, we discovered 82 genes with >1.5-fold difference. Of the genes within the duplication, only one, arylacetamide deacetylase-like 3 (AADACL3, HGNC: 32037), was within the known duplicated region on chromosome 1. The AADACL3 belongs to a lipolytic enzyme family with unknown function or significance in the skin. Thus, although the duplicated regions contained more than 100 genes, these do not appear to have been upregulated at the mRNA level in unaffected tissue.
Discussion
We present our observations of a 34-year-old patient with cutaneous immunodeficiency, renal failure, and premature senescence ultimately diagnosed with an 1p36 duplication. The duplications within the 1p36 region have been previously reported in the patients with variable clinical phenotypes including, but not limited to: atrial septal defects, delayed gross motor development, mental retardation, microcephaly, craniosynostosis, minor facial anomalies, patent ductus arteriosus, transient hypogammaglobulinemia, and seizures.4,5 However, the specific duplicated sequences between 1p36.11–p36.21, 4p16.1, and 9p24.3 found in our patient have not been reported elsewhere. The comparisons of gene expression in skin samples between our patient and a control subject revealed 82 genes with at least 1.5-fold difference in levels of expression. Of these, only AADACL3 is located within our patient’s known chromosomal duplications, but the function of this gene and its significance in skin immunity is currently unknown. Phenotyping multiple patients and running microarray comparisons would increase the characterization of the 1p36 duplication genotype. However, the uniqueness of this patient’s duplicated 1p36.11–p36.21 as well as 4p16.1 and 9p24.3 regions posed a challenge and to date we have been unable to find any similar patients to study.
Finally, although skin immunodeficiency may be a result of the 1p36 duplication, caution is warranted regarding the conclusion based on only one patient observed with this genotype. The large number of genes within the duplicated regions and the limited patient availability precluded any further functional assessment in order to find a causative gene or set of genes within the duplicated regions responsible for the patient’s cutaneous immunodeficiency. A possible research direction could be undertaken by coculturing the patient’s keratinocytes with S. scabiei and quantifying by enzyme-linked immunosorbent assay the production of IL-21 and other relevant keratinocyte-derived cytokines that may be upregulated and potentially responsible for inhibiting this skin infection through recruitment of cellular immune mediators.
Conclusion
The recurrent crusted scabies infection in our patient diagnosed with a rare 1p36 duplication represents a novel phenotypic manifestation of the 1p36 duplication syndrome. To our knowledge, this is the first report of a young female patient with the 1p36 duplication associated with a recurrent crusted scabies infection of the skin. This case serves to further describe a previously unknown type of an 1p36 duplication described in the context of a recurrent skin infection normally associated with the patients suffering from immunosuppression.
Acknowledgments
This study was supported in part by the National Institutes of Health (P30AR39750), Department of Dermatology Resident and Education Fund. The funding agency had no involvement in study design, data collection, data analysis, manuscript preparation, and/or publication decisions.
Disclosure
The authors report no conflicts of interest in this work.
References
Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717–725. | |
Redon R, Ishikawa S, Fitch KR, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. | |
Zogopoulos G, Ha KC, Naqib F, et al. Germ-line DNA copy number variation frequencies in a large North American population. Hum Genet. 2007;122:345–353. | |
Chen E, Obolensky E, Rauen KA, et al. Cytogenetic and array CGH characterization of de novo 1p36 duplications and deletion in a patient with congenital cataracts, hearing loss, choanal atresia, and mental retardation. Am J Med Genet A. 2008;146A:2785–2790. | |
Brandigi E, Molinaro F, Bulotta AL, et al. Chromosome 18q-Syndrome and 1p terminal duplication in a patient with bilateral vesico-ureteral reflux: case report and literature revision. Ital J Pediatr. 2013;39:6. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

