Back to Journals » Infection and Drug Resistance » Volume 16
Case Report: Metagenomic Next-Generation Sequencing Confirmed a Case of Spine Infection with Brucella melitensis in Non-Endemic Area
Authors Du J, Tao Y, Yang J, Cai J, Zhou H , Zhang R , Hu Y
Received 22 August 2023
Accepted for publication 4 November 2023
Published 13 November 2023 Volume 2023:16 Pages 7219—7225
DOI https://doi.org/10.2147/IDR.S436278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Jingjing Du,1 Yiqing Tao,2 Jiaxing Yang,1 Jiachang Cai,1 Hongwei Zhou,1 Rong Zhang,1 Yanyan Hu1
1Department of Clinical Laboratory, Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou, People’s Republic of China; 2Department of Orthopedics, Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Rong Zhang; Yanyan Hu, Department of Clinical Laboratory, Second Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou, 310009, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Brucellosis is a zoonotic disease caused by Brucella spp., with the highest prevalence found in the northern cities of China. In this case report, we present an occurrence of spinal infection caused by B. melitensis in a 67-year-old man residing in a non-endemic area of southern China. The patient initially presented with chest and back pain, which was not accurately diagnosed and treated at a local hospital. Subsequently, due to worsening pain, he was admitted to our hospital. To determine the cause of the infection, we performed CT-guided aspiration biopsy and collected biopsy tissue for metagenomic next-generation sequencing (mNGS) on the second day of hospitalization. Imaging investigations revealed involvement of the thoracic vertebrae, specifically thoracic 4– 7 with the main focus on 5– 6, accompanied by stenosis of the intervertebral space. The mNGS results indicated that the spine infection was caused by B. melitensis. The patient’s history as a shepherd and a positive Rose Bengal plate test (RBPT) further supported the diagnosis of brucella spondylitis. In order to alleviate pain and restore spinal function, the patient underwent posterior internal fixation of the thoracic spine. Treatment was initiated with cefoperazone/sulbactam, followed by doxycycline. Subsequently, the patient was switched to a combination therapy of rifampicin and doxycycline for a duration of six weeks. The patient responded well to treatment, and his condition remained stable. In conclusion, brucellosis is a common disease that can be easily misdiagnosed. This case report highlights the potential value of mNGS in early and rapid diagnosis. We believe that mNGS can serve as an effective tool to improve the diagnosis of spine infections caused by this pathogen.
Keywords: spine infection, Brucella melitensis, metagenomic next-generation sequencing, Rose Bengal plate test
Introduction
Brucellosis is a common zoonotic infection in most of the developing regions of the world, severely affecting livestock productivity and human health. It caused by a small, nonmotile gram-negative coccobacillus of the genus Brucella. Among reported human brucellosis cases, the pathogenic strain B. melitensis is the most frequently encountered.1,2 Symptoms of human brucellosis are nonspecific and can include fever, malaise, headache, cough, fatigue, and osteoarthritis.3 The presence of these diverse symptoms, combined with the varying sensitivity of conventional laboratory tests, often complicates the diagnosis and leads to delays in treatment.4 In China, the northern provinces exhibit a high prevalence of human brucellosis, with 95.5% of cases nationally centralized in North China, with an average annual incidence rate ranging from 2.8 to 75.7 per 100,000 individuals, while the southern provinces report an incidence rate of less than 1.1 per 100,000, and the data in Zhejiang province was 0.28 per 100,000 in 2021.5 Metagenomic next-generation sequencing (mNGS), as an emerging pathogen diagnostic tool, have shown robust potential in improving clinical diagnosis of infections.6 In this case report, we describe the clinical presentation of a 67-year-old male residing in a non-endemic area, who presented with persistent chest and back pain for over three months. Subsequent mNGS confirmed the diagnosis of brucella spine infection.
Case Presentation
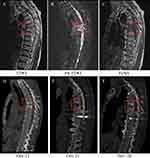
A 67-year-old male patient presented with persistent chest and back pain without an obvious cause and sought initial treatment at a local hospital. Despite receiving conservative treatment (painkillers were taken and no invasive procedures were performed) for three months, his symptoms worsened over time. Seeking further care, he visited our hospital’s outpatient clinic. The patient had a medical history of a surgical operation for a left forearm fracture resulting from a traumatic injury the previous year. He denied any history of hypertension, infectious diseases, or other medical conditions. In addition, he had no recent history of prolonged residence in foreign countries or areas with known infections (such as COVID-19, dengue fever, etc.), and his family medical history was unremarkable. His vital signs were normal, and he did not have a fever before or after admission. Physical examination revealed a right-sided convexity of the thoracic spine and tenderness in the intervertebral space of thoracic spine 5 and 6. Laboratory tests showed a slightly low white-cell count of 3.8×109/L (normal reference range: 4.0–10.0×109/L), an elevated erythrocyte sedimentation rate (ESR) of 22 mm/h (normal reference range: <15 mm/h), and an elevated C-reactive protein (CRP) level of 14.9 mg/L (normal reference range: <10.0 mg/L). Computed tomography (CT) revealed destruction of the thoracic 5–6 and cervical 6–7 vertebral bodies, an unclear intervertebral space, and a soft tissue mass surrounding the affected areas (Figure 1D). Magnetic resonance imaging (MRI) showed vertebral involvement at thoracic 4–7, predominantly at 5–6, with accompanying intervertebral space stenosis (Figure 1A–C), indicative of infectious spondylitis.
On October 16, 2022, the patient was admitted to the Second Affiliated Hospital of Zhejiang University, School of Medicine with no significant symptoms other than chest and back pain. The following day, CT-guided aspiration biopsy and mNGS were performed on the biopsy tissue to identify a potential etiology. Pathological examination of the bone marrow tissue revealed fibrosis and focal infiltration of lymphatic and plasma cells, suggesting inflammatory lesions. Both the tuberculosis infection T-cell test and bacterial culture of his tissue were negative. To relieve pain and restore spinal function, the patient underwent posterior internal fixation of the thoracic spine on October 19, 2022. Intravenous administration of cefoperazone/sulbactam sodium for injection (2.0g q12h) was initiated as an anti-infective treatment post-operation. Follow-up laboratory tests showed an elevation in CRP levels from 68.1 mg/L to 74.2 mg/L, an increase in ESR from 16 mm/h to 36 mm/h, and an interleukin-6 (IL-6) level of 19.18 pg/mL. Despite an extended duration of 10 days, both traditional blood and tissue cultures remained negative. However, 20 reads of Brucella spp. (out of a total of 247,467,502 sequence reads) were detected by mNGS in the tissue, with only one read identical to B. melitensis. No other bacteria, fungi, viruses, or parasites were detected. We further extracted the specie specific sequence fragment of B. melitensis (5’-GCTTCAAATCGGAAACAGAGGTACCGACAGCAGTATTGACGGC-3’) and found a 100% nucleotide sequence identity with B. melitensis using nucleotide blast (https://blast.ncbi.nlm.nih.gov). In light of the mNGS result, we conducted further investigation and confirmed the patient’s history as a shepherd one year prior. We then performed the Rose Bengal plate test (RBPT) for brucellosis using the RBPT Brucella antigen (Zhongchuang Biotechnology Co., LTD) obtained from the Institute of Infectious Disease of the China Centers for Disease Control and Prevention. As expected, the RBPT yielded a positive result (Figure 2). Consequently, a preliminary diagnosis of brucella spondylitis was made based on the patient’s medical history and laboratory findings.
 |
Figure 2 Rose-Bengal plate test for brucellosis. (A) Negative for saline control; (B) Positive for agglutination of the patient’s serum. |
During the course of treatment, the patient received doxycycline (0.1g bid) for five days, followed by rifampicin (0.45g qd) as an anti-Brucella infection therapy. After six days of treatment, the CRP level decreased from 68.1 mg/L to 13.5 mg/L, IL-6 decreased from 19.18 pg/mL to 13.34 pg/mL, and ESR returned to a normal range. A follow-up CT scan of the thoracic vertebrae revealed no remarkable findings (Figure 1E). The patient experienced significant relief from his back pain compared to before. He recovered well and was discharged from the hospital on October 26, 2022, with prescriptions for doxycycline (0.1g bid) and rifampicin (0.45g qd) (Figure 3). At the one-month follow-up after discharge, the patient reported the elimination of back pain, and both the hemogram and CT scan of the thoracic spine were normal, indicating the effectiveness of the initial treatment (Figure 1F). Therefore, the patient continued the oral anti-infective regimen for two weeks by adjusting the dosage of doxycycline to 0.1g qd.
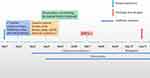 |
Figure 3 Timeline of the patient’s hospitalization and discharge. |
Bioinformatics Analysis and Pathogen Identification of mNGS
The raw sequencing data underwent bioinformatics analysis using a pipeline developed by BGI. The main procedure was as follows: short reads, duplicates, and human host sequences were removed using the Burrows-Wheeler-Alignment Tool (BWA) to obtain high-quality sequencing data.7 Next, the remaining sequences were efficiently aligned to PMDB (PMseq metagenomic Database, version 3.1.2, BGI-locally established database) consisting of 1798 whole genome sequences of viral taxa, 6350 bacterial genomes or scaffolds, 1064 fungi related to human infection, and 234 parasites associated with human diseases, which were downloaded from NCBI (ftp://ftp.ncbi.nlm.nih.gov/genomes/). The obtained results were interpreted and utilized for generating the final report. The decision to report mNGS results to the clinic was based on the following criteria: (1) Microbial species: Sequencing Depth of the Single-Most-Represented Non-Human Genus (SDSMRNG) ≥ 3; (2) Parasite: SDSMRNG ≥ 100; (3) Mycobacterium tuberculosis complex (MTCP): SDSMRNG ≥ 1.8,9
The metagenome raw sequence read generated in this study have been deposited and is available in the Sequence Read Archive (SRA) (http://www.ncbi.nlm.nih.gov/sra) under BioProject ID: PRJNA971259 with the specific BioSample number: SAMN35054986.
Discussion
Brucellosis is recognized as a significant zoonotic disease, leading to significant public health concerns and economic losses. Human infection with Brucella spp. typically occurs through direct contact with infected animals, inhalation of aerosolized particles containing the bacteria, or ingestion of contaminated raw meat or unpasteurized dairy products.2 Among the various species of Brucella, four have the potential to cause human disease: B. melitensis, B. abortus, B. suis, and B. canis. Among them, B. melitensis is the most virulent and prevalent in China.10 According to a report from the Centers for Disease Control and Preventionin China,5 a total of 117,012 cases of brucellosis were reported during 2020 to 2021, with an exponential average annual incidence from 3.4/100,000 in 2020 to 5.0/100,000 in 2021. The majority of cases were concentrated in northern China, although there has been a rapid increase in incidence in certain areas of southern China.11 Occupations such as farming and herding have been identified as high-risk groups for brucellosis, as individuals in these occupations are more likely to be exposed to the bacteria.11 In the present case, the patient contracted B. melitensis infection, probably related to contact with infected animals, and eventually progressed to brucella spondylitis.
Brucella spp. as a facultative bacterium, has the ability to evade the host’s immune response, allowing it to replicate and persist within the host’s professional phagocytic cells. Following uptake by local tissue lymphocytes, Brucella can disseminate throughout the body via the lymphoreticular system or bloodstream, leading to potential damage to various organs or body systems.2,12,13 Traditional culture methods for brucellosis typically require one to four weeks for detection.14
Clinical manifestations of brucellosis are characterized by undulant fever, malaise, arthralgias, hepatomegaly, splenomegaly, lymphadenopathy, or focal involvement of specific organs such as the epididymis, joints, cardiovascular system, and central nervous system.13,15 Among these, osteoarticular involvement represents the most common focal complication of brucellosis, with the spine being a frequently affected site.16,17 Brucella spondylitis, a serious complication of brucellosis, occurs in adults with an incidence rate ranging from 2% to 60%.13,16–18 Initially, spinal infection occurs at the endplate due to its rich blood supply, but it can progress to involve the entire vertebral body or adjacent vertebral bodies.19 The lumbar spine is the most commonly affected region, followed by the thoracic and cervical spine.17,20 Clinical presentation typically includes back pain, sometimes accompanied by fever, fatigue, weakness, anorexia, and sweating.21 However, the nonspecific and subtle nature of the initial symptoms often make the diagnosis of brucella spondylitis challenging, leading to delays in treatment and potential long-term adverse effects on the patient’s overall well-being. In this study, the patient presented with a single symptom of chest and back pain, which was initially not accurately diagnosed and treated at the local hospital. Over the subsequent three months, the pain persisted and gradually worsened.
The diagnosis of brucella spondylitis poses clinical challenges and requires a comprehensive approach combining medical history, clinical manifestations, and various clinical examinations. Clinical manifestations consistent with the disease and an epidemiological history can provide important clues for diagnosing brucella spondylitis. Radiological techniques, including X-rays, CT scans, and MRI, can also contribute to the diagnosis. However, in the early and acute stages of infection, there may be no obvious bone destruction or morphological changes, making X-rays or CT scans less sensitive for diagnosis. In contrast, MRI is considered the primary imaging modality for evaluating patients with spinal brucellosis due to its high sensitivity in detecting early changes in the vertebral body, intervertebral discs, and surrounding soft tissues.18,19,22–24 Laboratory tests are crucial for timely diagnosis of spinal brucellosis. In combination with imaging results, general laboratory tests often reveal elevated levels of ESR and CRP, which serve as indicators for diagnosing spinal infection and assessing the response to anti-brucellosis therapy.17,21,24–26 Isolation of Brucella spp. from blood, bone marrow, or other tissues is considered the gold standard for diagnosis. However, the positive rate of bacterial culture is influenced by various factors and decreases as the infection progresses, which may result in missed or incomplete diagnoses.2,14,18,24 In the absence of etiologic confirmation, serological tests for antibodies to Brucella species play a crucial role in definitive diagnosis. RBPT is a simple and rapid agglutination assay frequently used as a screening tool for human brucellosis, and its positive results still need to be confirmed by other tests.27 However, indirect diagnosis by serological tests, and direct rapid diagnosis by molecular PCR-based methods are not routinely performed in clinical laboratories in Zhejiang province (southern China).
mNGS is a recently developed technique used for diagnosing infectious diseases. It combines high-throughput sequencing with bioinformatic analysis to comprehensively detect the genetic sequences of all microorganisms in clinical samples, allowing comparison with a pathogen reference database to determine the pathogen in the tested sample.28 mNGS is increasingly used for diagnosing atypical and rare infectious diseases and has shown significantly higher sensitivity than traditional microbial culture for identifying pathogens in spinal infections, thereby improving pathogen detection rates.29,30
In the present case, the imaging findings showed infectious lesions of the thoracic spine including destruction of the 5–6 vertebral bodies, intervertebral space stenosis, and a soft-tissue mass surrounding the affected area. Traditional microbial cultures were negative. However, mNGS analysis of the biopsy tissue indicated the presence of B. melitensis. To further confirm the diagnosis, RBPT was performed, and the results were in line with the mNGS findings. Despite some limitations in this study, as we did not conduct further serological tests and molecular PCR-based methods, the final diagnosis of spine infection with B. melitensis was established through a comprehensive analysis, which included a detailed medical history and a combination of laboratory and radiographic examinations. Furthermore, it is worth noting that the patient exhibited a positive response to the treatment.
Given the high pathogenicity of B. melitensis, early and prompt antimicrobial treatment is required. The most commonly antibiotic treatment regimen for brucellosis is doxycycline with either rifampicin or streptomycin. The duration of treatment typically spans six weeks, although it may be adjusted based on the patient’s overall health and any underlying conditions. Additionally, for patients experiencing severe local pain and spinal deformity, early surgical intervention may be necessary to alleviate pain and provide spinal stability.31,32 In this particular case, posterior fusion and internal fixation of the spine proved to be effective and safe for the patient.
Conclusion
In summary, brucellosis is characterized by diverse and atypical manifestations, making its diagnosis particularly challenging, especially in non-endemic areas. In this case, mNGS exhibited remarkable efficacy in identifying the specific pathogen responsible for the spinal infection. We believe that mNGS would be helpful in guiding clinical treatment and enhancing patient outcomes. However, the high cost associated with mNGS might restrict its widespread use.
Data Sharing Statement
The metagenome raw sequence read generated in this study have been deposited and is available in the Sequence Read Archive (SRA) (http://www.ncbi.nlm.nih.gov/sra) under BioProject ID: PRJNA971259 with the specific BioSample number: SAMN35054986. The other original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Statement
Permission for using the information in the medical records of the patients was granted by the ethical committee of The Second Affiliated Hospital of Zhejiang University, School of Medicine (Number: 2023-0280).
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of case details and images.
Disclosure
The authors declare that they have no competing interests.
References
1. Lai S, Chen Q, Li Z. Human brucellosis: an ongoing global health challenge. China CDC Wkly. 2021;3(6):120–123. doi:10.46234/ccdcw2021.031
2. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352(22):2325–2336. doi:10.1056/NEJMra050570
3. Zheng R, Xie S, Lu X, et al. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. Biomed Res Int. 2018;2018:5712920. doi:10.1155/2018/5712920
4. Al Dahouk S, Nockler K. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev Anti Infect Ther. 2011;9(7):833–845. doi:10.1586/eri.11.55
5. Yang H, Chen Q, Li Y, Mu D, Zhang Y, Yin W. Epidemic characteristics, high-risk areas and space-time clusters of human brucellosis - China, 2020–2021. China CDC Wkly. 2023;5(1):17–22. doi:10.46234/ccdcw2023.004
6. Fang X, Cai Y, Chen X, et al. The role of metagenomic next-generation sequencing in the pathogen detection of invasive osteoarticular infection. Int J Infect Dis. 2022;122:996–1001. doi:10.1016/j.ijid.2022.07.061
7. Li H, Durbin R. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics. 2010;26(5):589–595.
8. Chen X, Ding S, Lei C, et al. Blood and bronchoalveolar lavage fluid metagenomic next-generation sequencing in pneumonia. Can J Infect Dis Med Microbiol. 2020;2020:6839103. doi:10.1155/2020/6839103
9. Wang H, Lu Z, Bao Y, et al. Clinical diagnostic application of metagenomic next-generation sequencing in children with severe nonresponding pneumonia. PLoS One. 2020;15:6.
10. Whatmore AM, Koylass MS, Muchowski J, Edwards-Smallbone J, Gopaul KK, Perrett LL. Extended multilocus sequence analysis to describe the global population structure of the genus Brucella: phylogeography and relationship to biovars. Front Microbiol. 2016;7:2049. doi:10.3389/fmicb.2016.02049
11. Tao Z, Chen Q, Chen Y, et al. Epidemiological characteristics of human brucellosis - China, 2016–2019. China CDC Wkly. 2021;3(6):114–119. doi:10.46234/ccdcw2021.030
12. de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol. 2015;185(6):1505–1517. doi:10.1016/j.ajpath.2015.03.003
13. Zhang Z, Zhang X, Chen X, et al. Clinical features of human brucellosis and risk factors for focal complications: a retrospective analysis in a tertiary-care hospital in Beijing, China. Int J Gen Med. 2022;15:7373–7382. doi:10.2147/IJGM.S380328
14. Yagupsky P. Detection of Brucellae in blood cultures. J Clin Microbiol. 1999;37(11):3437–3442. doi:10.1128/JCM.37.11.3437-3442.1999
15. Adetunji SA, Ramirez G, Foster MJ, Arenas-Gamboa AM. A systematic review and meta-analysis of the prevalence of osteoarticular brucellosis. PLoS Negl Trop Dis. 2019;13:1.
16. Esmaeilnejad-Ganji SM, Esmaeilnejad-Ganji SMR. Osteoarticular manifestations of human brucellosis: a review. World J Orthop. 2019;10(2):54–62. doi:10.5312/wjo.v10.i2.54
17. Lim KB, Kwak YG, Kim DY, Kim YS, Kim JA. Back pain secondary to Brucella spondylitis in the lumbar region. Ann Rehabil Med. 2012;36(2):282–286. doi:10.5535/arm.2012.36.2.282
18. Rizkalla JM, Alhreish K, Syed IY. Spinal brucellosis: a case report and review of the literature. J Orthop Case Rep. 2021;11(3):1–5. doi:10.13107/jocr.2021.v11.i03.2060
19. Tu L, Liu X, Gu W, et al. Imaging-assisted diagnosis and characteristics of suspected spinal brucellosis: a retrospective study of 72 cases. Med Sci Monit. 2018;24:2647–2654. doi:10.12659/MSM.909288
20. Ali Adam A, Sheikh hassan M, Adam Osman A. Spinal brucellosis causing spondylodiscitis. Ann Med Surg. 2022;82:104782. doi:10.1016/j.amsu.2022.104782
21. Solera J, Lozano E, Martinez-Alfaro E, Espinosa A, Castillejos ML, Abad L. Brucellar spondylitis: review of 35 cases and literature survey. Clin Infect Dis. 1999;29(6):1440–1449. doi:10.1086/313524
22. Bozgeyik Z, Ozdemir H, Demirdag K, Ozden M, Sonmezgoz F, Ozgocmen S. Clinical and MRI findings of brucellar spondylodiscitis. Eur J Radiol. 2008;67(1):153–158. doi:10.1016/j.ejrad.2007.07.002
23. Roushan MRH, Ebrahimpour S, Afshar ZM, Babazadeh A. Cervical spine spondylitis with an epidural abscess in a patient with brucellosis: a case report. J Crit Care Med. 2019;5(3):103–106. doi:10.2478/jccm-2019-0013
24. Yang B, Hu H, Chen J, He X, Li H. The evaluation of the clinical, laboratory, and radiological findings of 16 cases of brucellar spondylitis. Biomed Res Int. 2016;2016:8903635. doi:10.1155/2016/8903635
25. Buzgan T, Karahocagil MK, Irmak H, et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis. 2010;14(6):e469–478. doi:10.1016/j.ijid.2009.06.031
26. Ciftdogan DY, Aslan S. Osteoarticular involvement of brucellosis in pediatric patients: clinical and laboratory characteristics. Turk J Pediatr. 2020;62(2):199–207. doi:10.24953/turkjped.2020.02.005
27. Diaz R, Casanova A, Ariza J, Moriyon I. The rose bengal test in human brucellosis: a neglected test for the diagnosis of a neglected disease. PLoS Negl Trop Dis. 2011;5(4):e950. doi:10.1371/journal.pntd.0000950
28. Fang X, Cai Y, Shi T, et al. Detecting the presence of bacteria in low-volume preoperative aspirated synovial fluid by metagenomic next-generation sequencing. Int J Infect Dis. 2020;99:108–116. doi:10.1016/j.ijid.2020.07.039
29. Guo C, Zhang G, Hu X, et al. Diagnostic efficiency of metagenomic next-generation sequencing on spinal infection and prognosis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47(7):865–871. doi:10.11817/j.issn.1672-7347.2022.220163
30. Zhang Y, Chen J, Yi X, et al. Evaluation of the metagenomic next-generation sequencing performance in pathogenic detection in patients with spinal infection. Front Cell Infect Microbiol. 2022;12:967584. doi:10.3389/fcimb.2022.967584
31. Chen Y, Yao S, He WQ, Zhao X. The application of surgical treatment in spinal brucellosis. Asian J Surg. 2021;44(5):790–791. doi:10.1016/j.asjsur.2021.03.007
32. Katonis P, Tzermiadianos M, Gikas A, Papagelopoulos P, Hadjipavlou A. Surgical treatment of spinal brucellosis. Clin Orthop Relat Res. 2006;444:66–72. doi:10.1097/01.blo.0000203455.59393.9a
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.