Back to Journals » Cancer Management and Research » Volume 14
Case Report: First-Line Immune Checkpoint Inhibitor Plus Chemotherapy for Oral Metastasis in a Patient with Ultra High-Risk Gestational Choriocarcinoma
Authors Chen Y, Ye H, Tang J, Weng Y, Zhang J, Liu J
Received 17 February 2022
Accepted for publication 31 May 2022
Published 3 June 2022 Volume 2022:14 Pages 1867—1875
DOI https://doi.org/10.2147/CMAR.S351165
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Yu Chen,1,* Haiyan Ye,2,* Jiming Tang,3 Yihan Weng,4 Jie Zhang,5 Jianhua Liu4
1Department of Pathology, Guangdong Provincial People’s Hospital, Second Clinical Medical College of Southern Medical University, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, People’s Republic of China; 2Department of Gynecology, Guangdong Provincial People’s Hospital, Second Clinical Medical College of Southern Medical University, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, People’s Republic of China; 3Department of Thoracic Surgery, Guangdong Provincial People’s Hospital, Second Clinical Medical College of Southern Medical University, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, People’s Republic of China; 4Department of Oncology, Guangdong Provincial People’s Hospital, Second Clinical Medical College of Southern Medical University, Guangdong Academy of Medical Sciences, Guangzhou, Guangdong, People’s Republic of China; 5Department of Radiology, Zhuhai City People’s Hospital, Zhuhai Hospital of Jinan University, Zhuhai, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianhua Liu, Department of Oncology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, 123 Huifu Road West, Guangzhou, 510180, People’s Republic of China, Tel + 86 20 8188 4713, Fax + 86 20 8188 3300, Email [email protected] Jie Zhang, Department of Radiology, Zhuhai City People’s Hospital/Zhuhai Hospital of Jinan University, 79 Kangning Road, Xiangzhou District, Zhuhai, 519000, People’s Republic of China, Tel +86 756 2222 569, Fax +86 756 2218 950, Email [email protected]
Abstract: Choriocarcinoma (CC) tends to metastasize early into various organs and may exhibit peculiar clinical behaviors specific to metastases. Although chemotherapy has revolutionized the survival of most patients, the mortality rate remains high in cases at ultra high-risk, which may be associated with multiple organs involvement and intolerable toxicity resulting from combination chemotherapy. Here, we illustrate a 46-year-old woman patient with oral and lung lesions whose clinical and morphological heterogeneity misled the preliminary diagnosis. According to the initial pathological report of oral squamous cell carcinomas with lung metastasis and a combined positive score = 100, she received first-line immunotherapy plus two-drug chemotherapy, which obtained a surprisingly favourable outcome. Then, CC was identified by a high level of beta human chorionic gonadotropin (β-HCG) in serum and biopsies. DNA polymorphic analysis revealed its gestational origin, and a more aggressive standard regimen was subsequently implemented. However, the patient suffered repeated vomiting and myelosuppression, and the duration of treatment was significantly prolonged. Ultimately, she succumbed to death. The clinical course of this report helps to improve the understanding of this disease. We consider immune checkpoint inhibitors as potential first-line alternatives for ultra-high-risk CC patients, which provide a therapeutic reference for clinicians.
Keywords: choriocarcinoma, oral metastasis, ultra high-risk, immunotherapy
Plain Language Summary
Choriocarcinoma (CC) tends to metastasize early into various organs. Although chemotherapy has improved the survival of most patients, the mortality rate remains high in cases at ultra high-risk. In this report, we illustrated a special case of metastatic CC in the oral cavity and lungs without detectable gynaecological lesions. She was well treated with first-line chemotherapy combined with immunotherapy due to an initial misdiagnosis. Immune checkpoint inhibitors are clearly superior to conventional regimens in terms of efficacy and toxicity and may play a better role in early treatment. Based on these clues, first-line immunotherapy combined with chemotherapy can be an attractive alternative for ultra high-risk CC patients.
Introduction
Choriocarcinoma (CC), a rare subtype of gestational trophoblastic neoplasia (GTN), tends to metastasize early to distant organs via haematogenous spread and may exhibit peculiar clinical behaviours specific to metastases.1,2 However, spontaneous gingival haemorrhage (SGH) at first presentation is extremely rare and easily misdiagnosed,3 especially in the absence of detectable gynaecological lesions. It is well known that CC can be divided into gestational (GCC) and nongestational CC (NGC) based on different pathogenetic origins.4–6 The diversity of clinical characteristics and morphological heterogeneity of tumour cells pose challenges for accurate diagnosis and treatment.7,8 Although chemotherapy has radically revolutionized the survival of most CC patients, the risk of early death for cases at ultra high-risk remains high.9 The more extensive the spread of this disease, the worse the effect. Also, intolerable toxicity resulting from combination chemotherapy may facilitate organ failure, highlighting the urgent need for the development of optimal treatment strategies.
Currently, the tumor microenvironment has received remarkable attention in cancer research.10 Increasing literatures11,12 reported NOD-like receptor protein 7 (NLRP7), a member of the NLR, involved in the innate immune response and regulating the expression of programmed cell death-ligand 1 (PD-L1). As the master gene that contributes to trophoblast tumorigenesis, its abnormality facilitates the foundation of immunosuppressive microenvironment. It has been reported that PD-L1 is highly expressed in CC,13 and that programmed cell death-1 (PD-1)/PD-L1 blockade can yield clinical benefit for metastatic patients. However, the ideal treatment time for ICIs remains controversial. In this report, we describe an extremely rare case of SGH secondary to CC in a female patient, and her clinical course helped to improve the understanding of this disease and provide a therapeutic reference for clinicians.
Case Report
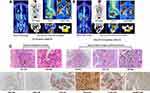
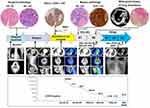
A 46-year-old woman was admitted to a local hospital complaining of SGH. She denied significant nausea, vomiting, abdominal pain or weight loss. Her past medical history was remarkable for orofacial cleft repair. She had regular menstrual cycles, three normal pregnancies and vaginal deliveries, and no history of abortion. On clinical examination, a 2.0-cm diameter exophytic mass on her left upper gum was found, which was diagnosed as squamous cell carcinoma by needle biopsy. Chest X-rays showed an isolated high-density mass in the right lung. The patient underwent extensive gingival resection. Ten days later, her left cheek was visibly distended and she had a dry cough. Full-body computed tomography (CT) revealed that another 6.0-cm diameter mass developed in the palate and almost compressed the airway, and there were multiple solid nodules throughout the bilateral lungs, the largest of which was located in the right lower pulmonary lobe. Then, the patient urgently presented to our department, and her rapidly deteriorating condition was better characterized by 18-fluorine-fluorodeoxyglucose positron-emission tomography/CT (18F-FDG PET/CT), which showed markedly enlarged oral and lung lesions with hypermetabolism, and no other specific abnormalities were found (Figure 1A). Pathological examination of the palatal mass revealed a tumour similar to a previous gingival mass in morphological and immunohistochemical (IHC) features, with massive tissue necrosis, atypical neoplastic cells with abundant cytoplasm and karyopyknosis, as well as positive IHC staining for P40 and P63. Thus, the preliminary diagnosis was poorly differentiated squamous carcinoma with lung metastasis. Notably, strong membranous labelling for PD-L1 with combined positive score (CPS) = 100 was observed using PD-L1 IHC 22C3 pharmDx (DAKO). Microsatellite stability status and high tumour mutation burden were identified by targeted next-generation sequencing analysis (483-gene panel, Cloudgene, China) (Supplementary Table and Figure 2A). Moreover, the expression levels of CD8, CD20, CD27, tryptase and Fc-epsilon in baseline tumor tissues were determined using mIF, and a large amount of CD8+ T-cell and CD20+/ CD27+ B-cell infiltration were observed (Figure 2B). The patient received docetaxel and cisplatin (DC, both 75 mg/kg every 3 wk) chemotherapy combined with immunotherapy (tirelizumab, an anti-PD-1 antibody developed in China, 200 mg every 3 wk) and was then transferred to a community hospital for palliative care. Unexpectedly, a laboratory investigation during hospitalization showed a high serum beta-human chorionic gonadotropin (β-HCG) level of 225,000 mIU/mL, and she was highly suspicious of CC, although no abnormality was detected by subsequent gynaecological examinations. All pathological sections were carefully double-checked by experienced pathologists. Although the microscopic appearance was very variable, the biphasic growth patterns of cytotrophoblasts and syncytiotrophoblasts were eventually identified. Immunostains showed that the tumour cells were strongly positive for cytokeratin (CK), HCG, Ki-67 and the master gene NLRP7 (Figure 1C), confirming the diagnosis of CC. Based on the World Health Organization (WHO) criteria, the patient was classified into FIGO IV with a prognostic score ≥14, representing ultra high-risk.
According to the patient’s preference, the DC+tirelizumab regimen was continued, and she had a surprisingly favourable outcome. Repeated PET-CT displayed a significant reduction in these lesions and a remarkable decrease in the standardized uptake value (SUV) (Figure 1B). CT-guided multipoint biopsy of lung lesions revealed no viable cancer cells, and the serum β-HCG level sharply fell to 374.71 mIU/mL. To distinguish the genetic origin, DNA polymorphic analysis was performed, and genetic profiles of 21 highly polymorphic short tandem repeats (STRs) from tumours, patients and spouses were compared. At 7/21 valid loci examined, the tumour sample was found to contain both the maternal and paternal alleles (nonhydatidiform triploid), demonstrating its gestational origin (Figure 3A–D). Subsequent treatment was switched by our multidisciplinary team to a more aggressive regimen of 5-fluorouracil (24 mg/kg/d on Days 1–5, 3 wk apart), actinomycin D (4 μg/kg/d on Days 1–5, 3 wk apart), vincristine (2 mg on Day 1, 3 wk apart) and etoposide (250 mg on Days 1–5, 3 wk apart) (FEVA), which is one of the standard first-line therapies in China. However, degree-II myelosuppression and vomiting occurred repeatedly after FEVA chemotherapy, and the decline in serum β-HCG slowed considerably. Despite the dose reduction in the 3rd cycle of chemotherapy, the patient’s status worsened with sepsis and multiorgan failure and ultimately succumbed to death (Figure 4).
Discussion
GCC generally arises from cytotrophoblast and syncytiotrophoblast cells, which develop into the placenta. The phenotype of early trophoblast cells is similar to that of tumour cells, such as fast growth and dissemination, whose persistence leads to tumorigenesis.14 Several factors, such as heredity, race, viral infection and folate deficiency, may contribute to malignant transformation. Notably, GCC is characterized by a high propensity for metastasis, often before it is clinically apparent. Given the unique “burn out” hypothesis of CC,15 it was possible that the primary tumour regressed early in the disease course, leaving only distant metastases, which easily resulted in confusion with other cancers. Here, we presented such an entity.
It is well known that oral malignancies are substantially primary, and only 1–3% of them are metastatic, originating mainly from lungs (30.6%), breasts (22.2%), liver (15.5%) and other organs.16 The low incidence of GCC with oral metastasis and atypical clinical features limit our understanding of this disease. To date, a total of 26 cases of oral metastases from CC were retrieved from major literature sources, including the Web of Science and PubMed. After excluding NGC,6–8,17–21 and 12 GCC prior to 1960 which had been summarized by Japanese experts,22 our search yielded 5 patients (Table 1).2–5,23 Among them, the mean onset age was approximately 28.6 years, and 3 patients presented oral mass as the first sign, accompanied by a history of abnormal pregnancy or uterine lesions. The concurrent multiple metastases beyond the oral cavity usually lead to poor prognosis.
 |
Table 1 Cases of Oral Metastasis by Choriocarcinoma with Survival Data in Reviewed Literatures |
Although the haematogenous route is considered to be the preferred mode for CC metastasis,24 the definite pathophysiological mechanism remains controversial. For patients with oral metastasis, oral inflammation was thought to play an important role in tumour progression. The abundant capillary network and activated cytokines during the inflammatory process may favour the metastasis of tumour cells.25 In this case, the patient was born with an orofacial cleft, a polygenic hereditary disease, and we speculated that orofacial lesions may be a predisposing factor for oral metastasis. More studies are needed.
Early diagnosis and treatments of metastatic oral lesions are important and could improve patient survival. In this case, microscopically, we observed that cytotrophoblasts displayed sheet-like proliferation, and syncytiotrophoblasts were few and inconspicuous and were easily mistaken for multinucleated tumour giant cells. Some syncytiotrophoblasts with red-stained cytoplasm and karyopyknosis can be erroneously regarded as keratinocytes or apoptotic cells. Consequently, timely IHC detection of β-HCG is essential. In addition, highly specific STR analysis was performed to further confirm its genetic origin, and the results showed that all tumour specimens were triploid, which conformed to the nuclear heteromorphism of tumour cells, suggesting that this disease originated from an unknown spontaneous abortion.
So far, chemotherapy remains the mainstay treatment. In European and American countries, the combination of etoposide, methotrexate and dactinomycin, followed by cyclophosphamide and vincristine (EMA/CO), is regarded as a standard first-line regimen for high-risk patients.26 In contrast, the 5-FU-based multidrug schedule is more popular in China.27 Notably, for cases at ultra high-risk, it is recommended to begin with gentle induction chemotherapy prior to standard multi-drug combinations, which may result in organ failure and even death.28 In recent years, the success of PD-1/PD-L1 blockade, a high-efficiency and minimally toxic ICI, in the treatment of multiple cancer types has heralded the beginning of a novel era in tumour therapy.29 ICIs can induce durable clinical responses that often transform into an overall survival benefit for malignancies. Because of the presence of paternal genes in GCC, it is generally regarded as a nonself, which might stimulate the host immune reaction. Early in the 1970s, Brewer et al30 proposed that CC was correlated with some immunologic aspects. In recent years, accumulating data11,12 have indicated the ubiquitous overexpression of NLRP7 and PD-L1 in GTN, including CC. NLRP7 contributed to protumoral activities in an inflammasome-independent manner, and its interaction with PD-L1 may play a vital role in immune tolerance and tumor development. Besides, we observed the infiltration of T cells (CD8+ upregulation) and memory B cells (CD20+ and CD27+ upregulation), which were identified as key orchestrators of antitumor immunity and modulators of the cancer stroma, implying favorable response to immunotherapy. In 2017, experts from Charing Cross Hospital in the United Kingdom reported for the first time that drug-resistant CC patients who received pembrolizumab achieved a complete response.31 Next, Huang et al29 and Paspalj et al32 showed the dramatic efficacy of pembrolizumab in cases with recurrent CC. Despite the favorable outcome and safety of ICIs for CC, they are currently implemented only in salvage treatment.
In this ultra-high-risk case, first-line ICIs combined with two-drug chemotherapy can be an attractive alternative. First, upregulation of PD-L1, high tumor mutation burden and infiltration of lymphocytes predicted better efficacy of immunotherapy. Secondly, logarithmic decrease of β-HCG occurred during the first two cycles, accompanied by a remarkable reduction of tumor foci. By contrast, subsequent standard chemotherapy led to repeated side effects, prolonged therapy period and deterioration of physical condition. Therefore, ICIs had a clear superiority over the conventional regimen in terms of efficacy and tolerance. Moreover, increasing clinical practice showed that ICIs played a better role when used earlier in treatment.10 Due to the rarity of CC and limited data available, large-scale clinical trials are difficult to conduct, and multicentre participation is needed.
Conclusions
The diagnosis and treatment of oral metastases as a result of CC is challenging for clinicians, and we highlight the importance of serum β-HCG testing in women of reproductive age at admission. Case reports, an indispensable part of evidence-based medicine, may be valuable in advancing physicians’ knowledge. Altogether, enhanced recognition, multidisciplinary cooperation and appropriate management helped to improve survival expectations of this rare disease.
Data Sharing Statement
The original contributions presented in the study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. Institutional approval was required to publish the case details. The study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital. This study was performed in accordance with the declaration of Helsinki.
Consent for Publication
The authors confirm that the details of any images, videos, recordings, etc. in the manuscript can be published.
Acknowledgments
The authors wish to gratefully acknowledge the patient’s spouse for allowing us to publish her clinical case. Moreover, we thank AJE’s English editing for editing our manuscript. Yu Chen and Haiyan Ye are co-first authors of this study.
Funding
This work was supported by grants from the Research Start-up Funds for the National Natural Science Foundation of China (Grant No. 8200110771, 8190110562).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Schrader E, Stephens AJ, Shroff S, et al. Widespread choriocarcinoma metastases from de-differentiated gastro-esophageal junction primary adenocarcinoma: a case report with literature review. Gynecol Oncol Rep. 2019;31:100513. doi:10.1016/j.gore.2019.100513
2. Kösem M, Cankaya H, Kaya Z. Choriocarcinoma metastatic to mandibular gingiva: case report and review of metastatic gingival tumours. J Otolaryngol. 2004;33(5):310–314. doi:10.2310/7070.2004.03094
3. Bakeen G, Hiyarat AM, Al-Ubaidy SS. Chorioepithelioma presenting as a bleeding gingival mass. Oral Surg Oral Med Oral Pathol. 1976;41(4):467–471. doi:10.1016/0030-4220(76)90274-7
4. Altintas A, Vardar MA, Aridoğan N, et al. Choriocarcinoma metastatic to the maxillary gingiva. Eur J Surg Oncol. 1995;21(5):579–580. doi:10.1016/s0748-7983(95)97712-0
5. Shafiee MN, Ismail NM, Shan LP, et al. A case report: metastatic choriocarcinoma to the gum. Sex Reprod Healthc. 2011;2(2):91–92. doi:10.1016/j.srhc.2011.02.001
6. Khanfir A, Kaffel T, Gouiaa N, et al. Primary choriocarcinoma of the maxillary gingival. Rev Stomatol Chir Maxillofac. 2012;113(5):382–384. doi:10.1016/j.stomax.2011.12.001
7. Velasco I, Aguilar L, Pastrian J, et al. Gingival metastasis from a testicular choriocarcinoma: an unusual case report and review of the literature. Int J Morphol. 2013;31(1):140–143. doi:10.4067/S0717-95022013000100023
8. Scolozzi P, Marret N, Bouzourene H, et al. Mixed testicular germ cell tumor presenting as metastatic pure choriocarcinoma involving the maxillary gingiva. J Oral Pathol Med. 2006;35(9):579–581. doi:10.1111/j.1600-0714.2006.00443.x
9. Al-Husaini H, Soudy H, Darwish A, et al. Gestational trophoblastic neoplasia: treatment outcomes from a single institutional experience. Clin Transl Oncol. 2015;17(5):409–415. doi:10.1007/s12094-014-1251-1
10. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
11. Abi Nahed R, Elkhoury Mikhael M, Reynaud D, et al. Role of NLRP7 in normal and malignant trophoblast cells. Biomedicines. 2022;10(2):252. doi:10.3390/biomedicines10020252
12. Reynaud D, Abi Nahed R, Lemaitre N, et al. NLRP7 promotes choriocarcinoma growth and progression through the establishment of an immunosuppressive microenvironment. Cancers. 2021;13(12):2999. doi:10.3390/cancers13122999
13. Clair KH, Gallegos N, Bristow RE. Successful treatment of metastatic refractory gestational choriocarcinoma with pembrolizumab: a case for immune checkpoint salvage therapy in trophoblastic tumors. Gynecol Oncol Rep. 2020;34:100625. doi:10.1016/j.gore.2020.100625
14. Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376(9742):717–729. doi:10.1016/S0140-6736(10)60280-2
15. Nakazaki H, Tokuyasu H, Takemoto Y, et al. Pulmonary metastatic choriocarcinoma from a burned-out testicular tumor. Intern Med. 2016;55(11):1481–1485. doi:10.2169/internalmedicine.55.5679
16. Owosho AA, Xu B, Kadempour A, et al. Metastatic solid tumors to the jaw and oral soft tissue: a retrospective clinical analysis of 44 patients from a single institution. J Craniomaxillofac Surg. 2016;44(8):1047–1053. doi:10.1016/j.jcms.2016.05.013
17. Tsuge S, Shibata K, Yamamoto H. Case of metastasis to the gingiva of chorioepithelioma malignum. Iryo. 1962;16:196–197.
18. Roa HS, Mizrahi SJ. Choriocarcinoma of the testicle with metastasis to the gingiva (report of a case). Rev Guatem Estomatol. 1972;2(3):96–99.
19. Mori M, Amano Y, Sakamoto M, et al. A case of metastatic choriocarcinoma of the gingiva (author’s transl). Nihon Koku Geka Gakkai Zasshi. 1974;20(6):621–626.
20. Nespeca JA, Sass JK. Choriocarcinoma metastatic to maxillary gingiva. J Oral Surg. 1980;38(7):534–537.
21. Bhatia K, Vaid AK, Rawal S, et al. Pure choriocarcinoma of testis with rare gingival and skin metastases. Singapore Med J. 2007;48(3):e77–e80.
22. Nishimura Y, Yakata H, Kawasaki T, et al. Metastatic tumours of the mouth and jaws. A review of the Japanese literature. J Maxillofac Surg. 1982;10(4):253–258. doi:10.1016/s0301-0503(82)80050-7
23. Sato M, Nishio J, Yoshida H, et al. Metastatic choriocarcinoma involving the gingiva. Int J Oral Surg. 1978;7(3):192–196. doi:10.1016/s0300-9785(78)80024-6
24. Sebire NJ, Lindsay I. Current issues in the histopathology of gestational trophoblastic tumors. Fetal Pediatr Pathol. 2010;29(1):30–44. doi:10.3109/15513810903266120
25. Auguste P, Fallavollita L, Wang N, et al. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol. 2007;170(5):1781–1792. doi:10.2353/ajpath.2007.060886
26. Lurain JR, Singh DK, Schink JC. Primary treatment of metastatic high-risk gestational trophoblastic neoplasia with EMA-CO chemotherapy. J Reprod Med. 2006;51(10):767–772.
27. Zong L, Yang J, Wang X, et al. Management and prognosis of patients with liver metastases from gestational trophoblastic neoplasia: a retrospective cohort study. Cancer Manag Res. 2018;10:
28. Ngan HYS, Seckl MJ, Berkowitz RS, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynaecol Obstet. 2021;155 Suppl 1(S1):86–93. doi:10.1002/ijgo.13877
29. Huang M, Pinto A, Castillo RP, et al. Complete serologic response to pembrolizumab in a woman with chemoresistant metastatic Choriocarcinoma. J Clin Oncol. 2017;35(27):3172–3174. doi:10.1200/JCO.2017.74.4052
30. Brewer JI, Torok EE, Kahan BD, et al. Gestational trophoblastic disease: origin of choriocarcinoma, invasive mole and choriocarcinoma associated with hydatidiform mole, and some immunologic aspects. Adv Cancer Res. 1978;27:89–147. doi:10.1016/s0065-230x(08)60931-8
31. Ghorani E, Kaur B, Fisher RA, et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet. 2017;390(10110):2343–2345. doi:10.1016/S0140-6736(17)32894-5
32. Paspalj V, Polterauer S, Poetsch N, et al. Long-term survival in multiresistant metastatic choriocarcinoma after pembrolizumab treatment: a case report. Gynecol Oncol Rep. 2021;37:100817. doi:10.1016/j.gore.2021.100817
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.