Back to Journals » International Journal of Women's Health » Volume 15
Case Report. Diagnostic Challenges: Liver Metastases from Mammary Gland Origin or Cholangiocarcinoma?
Authors Tocu G , Popa IT, Ivan I, Anghel L , Nechita LA, Musat CL, Rebegea LF, Tutunaru D
Received 28 February 2023
Accepted for publication 11 July 2023
Published 27 July 2023 Volume 2023:15 Pages 1205—1211
DOI https://doi.org/10.2147/IJWH.S408055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
George Tocu,1,* Ioana Teodora Popa,2 Iuliana Ivan,3 Lucretia Anghel,4 Luiza A Nechita,4 Carmina Liana Musat,5 Laura Florentina Rebegea,4,* Dana Tutunaru1,*
1Pharmaceutical Sciences Department, “Dunărea de Jos” University, Galati, Romania; 2Clinical Surgical Department, Fundeni Clinical Institute, Bucharest, Romania; 3Oncology Department of “Sf. Apostol Andrei” Emergency County Hospital, Galati, Romania; 4Clinical Medical Department, “Dunărea de Jos” University, Galati, Romania; 5Morphological and Functional Sciences Department, “Dunarea de Jos” University, Galati, Romania
*These authors contributed equally to this work
Correspondence: George Tocu, Department of Pharmaceutical Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, 35 Alexandru Ioan Cuza Street, Galați, 800008, Romania, Tel +40773819438, Email [email protected]
Abstract: Liver metastases are secondary malignant tumor formations due to the dissemination of primary malignant tumors, which are often the first clinical manifestation of mammary cancer. We present the case of a 52-year-old female patient from an urban area who came to the walk-in service at the “Sf. Ap. Andrei” Emergency County Hospital, Galati, for laboratory investigations. These revealed a significant increase in CA 15– 3 tumor markers and a slight increase in CA 125 and CA 19– 9 markers; however, clinical examination did not reveal tumors in the breast and there were no axillary adenopathies. CT exam revealed a large tumor formation in the hepatic right lobe and, close to it, a smaller one. The mammograph showed millimetric lesions at the level of the left mammary gland and bilateral axillary adenopathies. Subsequently, the patient underwent two liver biopsies in two different hospitals, which produced different histological and immunohistochemical results. PET-CT drew attention to a lung tumor and disclosed a different origin of metastases. In the end, correlating all investigations, the final diagnosis was cholangiocarcinoma with liver metastases and lung tumor with lung and bone metastases.
Keywords: tumor, breast, biopsy, markers, immunohistochemistry
Introduction
Liver metastases are secondary malignant tumor formations resulting from the dissemination of primary malignant tumors, being most often the first clinical manifestation of breast, lung, pancreatic, gastric, and colorectal neoplasm. Some authors have revealed that liver metastases of breast origin occur in 15% of cases in newly diagnosed patients and in almost 50% of cases in patients with stage IV breast cancer.1 There are many paraclinical investigations to detect the origin of metastases, the most commonly used being immunohistochemistry and positron emission tomography and computed tomography (PET-CT). In some circumstances, taking into account the histopathological features of the tumors, imaging diagnosis may become more helpful.2 In addition to these elective exams, laboratory analyses of tumor markers and inflammatory biomarkers3 can provide important tumor information not only for diagnosis but also for monitoring treatment, screening, or prognosis.4 This study was performed to report a difficult diagnosis in a woman with two primary cancers and multiple metastases.
Case Report
We report the case of a 52-year-old woman from an urban area who presented herself in October 2018 to the walk-in service at the “Sf. Apostol Andrei” Emergency County Clinical Hospital, Galati, for some laboratory investigations. Investigation of tumor markers was particularly recommended following a previous abdominal ultrasound examination, in which hepatic tumor formations were highlighted.
Laboratory investigations revealed increased tumor markers CA 15–3 = 3942 U/mL, CA 19–9 = 40.2 U/mL and CA 125 = 29.2 U/mL, with CEA and AFP markers in the normal range. The patient was admitted to the Internal Medicine Department. The breast ultrasound examination revealed bilateral fibrocystic mastopathy; in the left breast, a small non-homogeneous formation with a relatively net contour of 4–5 mm; and bilateral free axillas without adenopathy. The bilateral mammography (Figure 1) highlighted breasts with a mixed density appearance; in the inferior-inner dial of the left breast were two nodular images with a diameter of <12 mm, homogeneously weakly radiopaque; micronodular opacities bilateral axillary.
 |
Figure 1 Bilateral mammography with nodular images in the left breast. |
A thorax-abdomen-pelvis CT scan with contrast agent (Figure 2) revealed the following: hepatomegaly (in the lower edge of the right lobe under the iliac crest); a central macronodular mass delimited with a diameter of 117/110/112 mm in the right hepatic lobe, with minimal polycyclic contours, native hypodensial, iodophilic, with “washing” in late time; adjacent, several nodular images 21 mm in diameter with a similar structure; anterior, the described mass was in direct contact with the portal vein and the right branch of the portal vein, which it moved slightly anterior, without invading them; stasis in the left port vein, diameter 11 mm; posterior contact directly on an enlarged portion with the inferior vena cava; and vascular disorders on a geometric area located peripherally in the VIth segment.
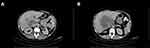 |
Figure 2 CT scan of thorax-abdomen-pelvis: (A) Hepatic tumor at the portal bifurcation; (B) Liver tumor at maximum diameter. |
Bone scan revealed an increased capture of scapulo-humeral articulations. At chest X-ray, the bilateral infrahilar interstitial appears accentuated. The surgical examination did not reveal any tumor formations on the left or right breast and breast biopsy was not indicated. Laboratory investigations revealed the following changes: hemoglobin = 150 g/L, ESR = 25 mm/h; fibrinogen = 5.272 g/L; ALT = 1.0167 µmol/L; AST = 0.75 µmol/L; GGT = 1.7333 µmol/L; ALP = 6.7167 µmol/L; CA 15–3 = 5725 U/mL; CA 19–9 = 45.8 U/mL; and CA 125 = 33.9 U/mL. The patient was referred to the Fundeni Clinical Institute for further investigation, including liver biopsy puncture. In November 2018, the patient underwent a histopathological exam. The liver biopsy highlighted a fragment represented by hepatic parenchyma with conserved lobular architecture and an extensive tumor proliferation area of epithelial type with poorly differentiated carcinoma layers with ovalar cube cells and areas of tumor necrosis. Immunohistochemistry highlighted P63 – nuclear positive in tumor cells, diffuse with marked to moderate density in more than 80% of tumor cells; mammaglobin – negative; GCDFP15 – negative; Ck7 – negative; Ck20 – negative; ER – negative; TTF1 – negative. Histological aspect and immunohistochemical profile supported the diagnosis of moderately to poorly differentiated non-keratinized epidermoid carcinoma with tumor necrosis areas; in a clinical and imagistic context, the origin of tumor proliferation can be in the mammary gland as metaplastic carcinoma. In January 2019, the patient attended the Oncology Department for re-evaluation, and at discharge was directed to “Sf. Maria” Hospital in Bucharest for a new histopathological exam. The liver biopsy highlighted a fragment of fibrous tissue with poorly differentiated carcinoma of solid architecture, focal formation of lumen and necrotic detritus. Immunohistochemistry highlighted the following: P63 – zonally positive in tumor cells; Ck7 – zonally positive in tumor cells; Ck20 – negative; CA19.9 – focal positive; GCDFP15 – negative; glypican-3 – negative; ER – negative; PR – negative; CDX2 – negative. The histopathological and immunohistochemical aspect supported the diagnosis of a poorly differentiated squamous differentiation carcinoma; the histological aspects of the tumor correlated with the imaging data supporting the diagnosis of a tumor with biliary origin of adenosquamous carcinoma type. In March 2019, the patient returned to the Oncology Department, presenting severe thoraco-lumbar pain, functional impotence in the left upper member, vomiting, inappetence, and anxiety. At mammography, the left breast showed two pale radio-opaque nodules with a perfectly regular contour, 4–6 mm in diameter, BIRADS II. A bone scan (Figure 3) revealed multiple metastases to: left humeral head, right humerus diaphysis, VIIth left and VIth right ribs, toraco-lumbosacral spine, left iliac crest, right pubis, and bilateral femur.
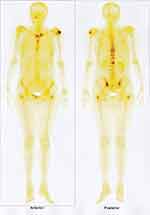 |
Figure 3 Bone scan: multiple bone metastases. |
Abdominal ultrasound showed: a fatty liver; in the right hepatic lobe region, a macronodular mass of 132/118 mm, hyperecogenic; in the proximity of the described formation, several hyperechogenic ovalar images with diameter below 24 mm; spleen of 119 mm; normal kidney; and normal urinary bladder. The patient received a first dose of chemotherapy with GemOx (Gemcitabine 1700 mg + Oxaliplatin 170 mg) and treatment with bisphosphonates, opioids, antiemetics and hydro-electrolytical rebalancing. In April 2019, the patient presented herself to the hospital to begin radiotherapy and for repeated chemotherapy with GemOx. A new CT highlighted the tumoral formation from the level of the liver with a diameter of 135/150 mm. In July 2019, the patient underwent a mammography that showed pale opaque micronodular images of 2–4 mm in the left breast and an ovalar radio-opaque image with a radiotransparent center of 15/8 mm in the left axilla. Laboratory analyses showed the following changes: WBC = 14.18 x 109/L; hemoglobin = 106 g/L; ESR = 110 mm/h; ALT = 1.75 µmol/L; AST = 1.2333 µmol/L; GGT = 10.3 µmol/L; ALP = 35.8667 µmol/L; chloride = 95.5 mmol/L; sodium = 130 mmol/L; CA 15–3 = 6350 U/mL; CA 125 = 343.6 U/mL; and CA 19–9 = 142.8 U/mL. In September 2019, the patient had a PET-CT examination, which revealed: pulmonary, the apical-posterior segment of the upper left lobe showed a solid nodule with infiltrative contours, metabolically active with SUV = 1.77 and dimensions of 13/9mm; multiple bilateral pulmonary micronodular images located centrally and subpleurally, most with dimensions up to 5mm without increased metabolic activity – secondary determinations; the posterobasal segment of the lower left lobe showed an ovoid nodule with dimensions of 12/11 mm, with increased minimal metabolic activity, SUV = 0.8; metabolically active subaortic adenopathy with SUV = 6.59 and dimensions of 27/17 mm; normal distribution of activity at bilateral breast level; tumor-transformed liver (Figure 4), with multiple metabolically active lesions confluent with portal wash-out and SUV = 11.25; and multiple metabolically active lesions (SUV max. = 8.19), with osteocondensating aspect, visible at the bilateral humeral level, bilateral clavicle, bilateral scapula, sternum, anterior rib arch III right, in all segments of the spine, pelvic bones and bilateral femur. In conclusion, metabolically active secondary determinations of bilateral lung, mediastinal ganglion, liver and bone, with a probable starting point of the left lung, was evident. The reporting of this study conforms to CARE guidelines.5
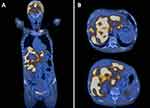 |
Figure 4 PET-CT: (A) overall image; (B) liver tumor. |
Discussion
During repeated admissions, many laboratory investigations were conducted. The inflammatory biomarkers that make up the biological picture attest to the inflammation present in all cancers.6,7 As the disease progressed, metabolic modifications 8 and hydroelectrolytic changes occurred,9 and cholestasis syndrome worsened, indicating hepatic dysfunction.10 Fejzić et al revealed that the CA 15–3 tumor marker was elevated in more than 90% of patients with advanced disease and metastasis to the lungs, bones or liver, and is the tumor-specific marker in diagnosis and especially monitoring of breast cancer patients.11 In this case, the increased values of CA 15–3 indicate an advanced stage of the disease with multiple metastases; however, BIRADS II and the absence of palpable nodules constitute an uncertainty in establishing the origin of the metastases. Also, the presence of markers CA 125 and CA 19–9, which are continuously increasing, enlarged the area of uncertainty. The histopathological examination described the liver tumor from the cellular perspective; however, supplementing with a cytogenetic examination of the tumor piece would allow a deeper description of the tumor type.12,13 On the other hand, the nodules in the breast were well defined, which makes it difficult to appreciate their type, so other investigation options should be explored.14 At both immunohistochemical examinations, P63-positive was detected in tumor cells, which is a highly sensitive and specific marker for metaplastic carcinoma.15 P63-positive together with CA 15–3 could support the origin of breast metastases from a metaplastic carcinoma. However, the first biopsy did not support with certainty evidence of metaplastic carcinoma, which is very rare and aggressive.16,17 As a result, must be taken into account and the biliary origin of adenoscuamos carcinoma, specified in the second immunohistochemical examination, where the expression of CA 19–9 marker was recorded, this marker being more specific for identifying a cholangiocarcinoma. Also, the second immunohistochemical examination highlighted Ck7-positive/Ck20-negative, the markers being expressed in the breast metaplastic carcinoma, cholangiocarcinoma and lung cancer,18 which could not link the diagnosis to one of these origins. The bone scan was an extremely useful investigation, which specified the extension of bone metastases to the upper members (the spine, ribs, pubis, and iliac crest) and the lower members. Dispersed pain and functional impotence are explained in this framework.19 The PET-CT exam provided additional information, highlighting a lung tumor, which was not highlighted in the other imaging investigations. Spine metastases are most commonly caused by lung or breast tumors.20 Thus, the marker CA 15–3, which is grown in mucin-secreting formations, becomes significant for the lung tumor.
This study had limitations because while we were conducting it our hospital had not yet acquired a PET-CT and, in the absence of an immunohistochemistry laboratory, the patient had to go to another center that offered a wider range of paraclinical explorations. Thus, precious time was lost both in the final diagnosis and in the correct treatment of the patient.
Conclusion
The particularity of the case is the lack of correlation between the CA 15–3 marker, with extremely high value, and the clinical lesions in the mammary gland (fibrocystic mastosis and benign nodules), which has attracted many question about the origin of hepatic metastases from the level of the breast. As for the immunohistochemistry, it was expressed from two different points of view and could not accurately specify the origin of the metastases. PET-CT has drawn attention to a lung tumor and, correlating previous data, the final diagnosis was cholangiocarcinoma with liver metastases and lung tumor with lung and bone metastases. The CA 15–3 marker should be interpreted in this context in correlation with a mucin-secreting lung tumor.
Abbreviations
PET-CT, positron emission tomography (PET) and computed tomography (CT); SUV, standardized uptake value; BIRADS, breast imaging-reporting and data system; CA 15-3, cancer antigen 15-3; CA 19-9, cancer antigen 19-9; CA 125, cancer antigen 125; CEA, carcinoembryonic antigen; AFP, alpha-fetoprotein; WBC, white blood cells; ESR, erythrocyte sedimentation rate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase; ALP, alkaline phosphatase; P63, tumor protein 63; CK7, cytokeratin 7; CK20, cytokeratin 20; TTF1, thyroid transcription factor 1; GCDFP15, gross cystic disease fluid protein 15; ER, estrogen receptor; PR, progesterone receptor; CDX2, human caudal type homeobox 2.
Data Sharing Statement
Access to the information herein will be granted on reasonable request.
Ethics Approval
Our ethics committee does not require approval for case reports.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Târcoveanu E, Vasilescu A, Zugun-Eloae F, et al. Metastazele hepatice ale cancerului de sân. Jurnalul de chirurgie. 2008;4(3):201–216.
2. Sava I, Sava A, Şapte E, et al. Intraventricular metastatic clear cell renal carcinoma. Rom J Morphol Embryol. 2013;54(2):447–450.
3. Barzoi R, Rezus E, Badescu C, et al. The impact of proinflammatory cytokynes of rheumatoid polyarthritis on the generalized loss of bone mass. Revista de Chimie. 2018;69(9):2541–2545. doi:10.37358/RC.18.9.6572
4. Sharma S. Tumor markers in clinical practice: general principles and guidelines. Indian J Med Paediatr Oncol. 2009;30(1):1–8. doi:10.4103/0971-5851.56328
5. Gagnier JJ, Kienle G, Altman DG, et al.; CARE Group. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache. 53;2013:1541–1547. doi:10.1111/head.12246
6. Mozos I, Jianu D, Gug C, et al. Links between high-sensitivity C-reactive protein and pulse wave analysis in middle-aged patients with hypertension and high normal blood pressure. Dis Markers. 2019;2019:2568069. doi:10.1155/2019/2568069
7. Stefanache T, Jitaru D, Dragos ML, et al. Tumor immune response in the presence of a cytotoxic peptide. Rev Chim. 2018;69(5):1179–1186. doi:10.37358/RC.18.5.6284
8. Gug C, Mihaescu A, Mozos I. Two mutations in the thiazide-sensitive NaCl co-transporter gene in a Romanian Gitelman syndrome patient: case report. Ther Clin Risk Manag. 2018;14:149–155. doi:10.2147/TCRM.S150483
9. Jurcă AD, Jurcă MC, Bembea M, et al. Clinical and genetic diversity of congenital hyperammonemia. Rom J Morphol Embryol. 2018;59(3):945–948.
10. Pop L, Popa I, Popa Z, et al. Trisomy 21, cholelithiasis and positive sweat test at infant – diagnostic difficulty. J Pediatr. 2009;12:25–26.
11. Fejzić H, Mujagić S, Azabagić S, et al. Tumor marker CA 15-3 in breast cancer patients. Acta Med Acad. 2015;44(1):39–46. doi:10.5644/ama2006-124.125
12. Caba L, Rusu C, Plăiaşu V, et al. Ring autosomes: some unexpected findings. Balkan J Med Genet. 2012;15(2):35–46. doi:10.2478/bjmg-2013-0005
13. Diaconescu BM, Jitaru D, Dragos ML, et al. The effect of some natural cytotoxic peptides on tumor cells. Rev Chim. 2018;69(3):597–601. doi:10.37358/RC.18.3.6157
14. Gug C, Anghel A, Tamas L, Seclman E, Willems P. Neurofibromatosis type 1 molecular testing and clinical presentation of two cases. Analele Stiintifice ale Universitatii Alexandru Ioan Cuza Iasi. 2010;11(2–3):33–38.
15. Koker MM, Kleer CG. p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am J Surg Pathol. 2004;28(11):1506–1512. doi:10.1097/01.pas.0000138183.97366.fd
16. Takala S, Heikkilä P, Nevanlinna H, et al. Metaplastic carcinoma of the breast: prognosis and response to systemic treatment in metastatic disease. Breast J. 2019;25(3):418–424. doi:10.1111/tbj.13234
17. Gortman A, Aherne NJ, Westhuyzen J, et al. Metaplastic carcinoma of the breast: clinicopathological features and treatment outcomes with long-term follow up. Mol Clin Oncol. 2021;15(3):178. doi:10.3892/mco.2021.2340
18. Selves J, Long-Mira E, Mathieu MC, et al. Immunohistochemistry for diagnosis of metastatic carcinomas of unknown primary site. Cancers. 2018;10(4):108. doi:10.3390/cancers10040108
19. Grigoriță L, Gug C, Vaida MA, et al. An unusual case of unilateral supernumerary extensor carpi radialis muscle. Folia Morphol. 2019;78(4):888–892. doi:10.5603/FM.a2019.0037
20. Ziu E, Viswanathan VK, Mesfin FB. Spinal Metastasis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
