Back to Journals » Infection and Drug Resistance » Volume 16
Case Report: Comprehensive Management of Pneumocystis Jiroveci Pneumonia (PJP) and Secondary Infections of Multiple-Drug Resistant Enterobacter cloacae complex and Pseudomonas aeruginosa in a Kidney Transplant Recipient with Sulfonamide Allergies
Authors Zhu L , Xu H, Pu Y, Fu C, Pan Q, Zhao H
Received 4 July 2023
Accepted for publication 7 September 2023
Published 13 September 2023 Volume 2023:16 Pages 6185—6193
DOI https://doi.org/10.2147/IDR.S428890
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Longyin Zhu,1 Huan Xu,2 Youmin Pu,1 Chunxiao Fu,1 Qianguang Pan,1,* Hongwen Zhao1,*
1Department of Nephrology, The First Affiliated Hospital of Army Medical University, Chongqing, People’s Republic of China; 2Department of Scientific Affairs, Vision Medicals Center for Infection Diseases, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongwen Zhao; Qianguang Pan, Department of Nephrology, The First Affiliated Hospital of Army Medical University, 30 Gaotanyanzheng Street, Shapingba District, Chongqing, 400038, People’s Republic of China, Tel/Fax +8613983360655 ; +86 18584651157, Email [email protected]; [email protected]
Abstract: We report a case of pneumocystis jiroveci pneumonia (PJP) in a 46-year-old woman, who previously underwent kidney transplant for chronic renal failure. She did not receive PJP prophylaxis treatment for the history of sulfonamide allergies. Four months after renal transplantation, the patient had cough, chest tightness, and shortness of breath. Procalcitonin (PCT) (0.06 ng/mL) and C-reactive protein (CRP) (5.33 mg/L) were normal, but the level of 1, 3-β-D-glucan test (G test, 193.89 pg/mL) were elevated. Metagenomics next-generation sequencing (mNGS) using bronchoalveolar lavage fluid (BALF) rapidly and accurately identified P. jiroveci. Through sulfonamide desensitization and sulfamethoxazole-trimethoprim (TMP-SMX) combined with caspofungin (CAS) treatment, PJP was controlled. However, the patients’ conditions were worsen for the hospital-acquired secondary pulmonary infection. A second BALF mNGS identified Enterobacter cloacae complex and Pseudomonas aeruginosa carrying carbapenem drug resistance genes, which were confirmed by subsequent culture and antimicrobial susceptibility test within 3 days. Finally, symptoms, such as chest tightness, cough, and shortness of breath, were improved and she was discharged after combined treatment with meropenem (MEM), polymyxin B (PMB), CAS, and TMP-SMX. In this case, mNGS, culture, and drug susceptibility testing were combined to monitor pathogenic microbial and adjust medication. At present, there are no case reports of mNGS use and sulfonamide desensitization in a kidney transplant recipient with sulfonamide allergies.
Keywords: metagenomics next-generation sequencing, mNGS, Pneumocystis jiroveci pneumonia, PJP, sulfonamide allergies, SA, kidney transplant, KT, multiple drug-resistant Enterobacter cloacae complex, MDR-ECC, multiple drug-resistant Pseudomonas aeruginosa, MDR-PA
Introduction
Renal transplantation is the best treatment option for patients with end-stage renal disease, but the risk of infection is significantly higher due to the long-term use of immunosuppressive drugs after transplantation. Pneumocystis jiroveci pneumonia (PJP), an acute or subacute pneumonia caused by Pneumocystis jiroveci infection, is the most common opportunistic fungal infection in renal transplant recipients. The mortality rate of PJP post renal transplantation could reach 90–100% without taking therapeutic measures.1,2 Although PJP prophylaxis is now routinely performed in patients after renal transplantation, the incidence rate of PJP is still as high as 0.4%–2.2%.3 However, if renal transplant recipients do not receive PJP prophylaxis, the incidence rate of PJP will be as high as 0.6% −14%, and the mortality rate will reach 50%.4 PJP frequently occurs within 3–6 months after kidney transplantation, when the patient’s immune function is severely compromised.5 Immunosuppression regimen, such as tacrolimus, mycophenolate and glucocorticoids, are risk factors for PJP infection. Acute rejection, cytomegalovirus infection, bacterial pneumonia, pulmonary tuberculosis, and hepatitis C virus infection are also considered risk factors for the development of PJP.6–8 We are reporting a case of a post kidney transplant patient, who first infected by P. jiroveci without PJP prophylaxis due to sulfonamide allergy, and then developed into multiple drug-resistant bacterial infection during hospitalization.
Case Presentation
On August 8, 2022 (day 1), a 46-year-old female was admitted to our hospital with a 2-day history of cough, chest tightness, and shortness of breath. She had been seen at the local hospital, where chest CT showed flocculent faint shadow in both lungs and she was prescribed with moxifloxacin (MXF), but the symptoms were not relieved. Previously, she had a history of chronic renal failure and had undergone allogeneic renal transplantation in April 2022. After renal transplantation, she orally took methylprednisolone, mycophenolate mofetil, and tacrolimus for a long time to combat rejection. She had a history of sulfonamides allergy, which manifested as localized skin urticarial.
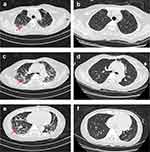
On admission, blood gas analysis suggested an oxygenation index of 361 (normal 400–500) (Figure 1), blood routine tests showed white blood cell count was 7.25×109 /L (normal 3.5–9.5) and neutrophil percentage 88.70% (normal 40–75), biochemical tests showed procalcitonin (PCT) (0.06 ng/mL, normal <0.05), C-reactive protein (CRP) (5.33 mg/L, normal 0–7.44), and GM test (0.11, normal <0.5) were within reference intervals and an increase in level of G test (193.89 pg/mL, normal <60). Conventional microbiological tests were negative, including Cytomegalovirus (CMV) PCR, Epstein–Barr virus (EBV) PCR, T-SPOT, respiratory virus antigen, sputum anti-acid staining, and sputum fungal and bacterial cultures. Creatinine was elevated (119 umol/L, normal 45–84). T-lymphocyte subsets results showed the decreased level of lymphocyte count (364.05 /μL, normal 800–4000), CD4+ T-cell count (125.58 /μL, normal 410–1590), and CD8+ T-cell count (126.78 /μL, normal 190–1140). Chest CT showed thickened vascular bronchial bundles and multiple cord-like faint shadow in both lungs (Figure 2a–c). To maintain adequate oxygenation, the nasal cannula was administered with low-flow oxygen (2 L/min). Piperacillin tazobactam (TZP, 4.5 g iv q8h) and CAS (70 mg iv qd first dose, 50 mg iv qd maintenance) were given. Oral immunosuppressants were stopped, and methylprednisolone (40 mg iv qd) was given to replace anti-rejection.
 |
Figure 2 Chest computed tomography scans of PJP. (a–c) stripe shadows (red arrow) in bilateral lungs. |
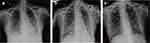
On day 3, the fiberoptic bronchoscopy showed a smooth surface of bronchial mucosa in each lobe segment of both lungs, no erosion and bleeding. Bronchoalveolar lavage fluid (BALF) was harvested for metagenomics next-generation sequencing (mNGS). Results were returned on day 4, which revealed P. jiroveci (261569 sequence reads) (Figure 3a). For the sake of sulfonamide allergies, sulfonamide desensitization therapy (Table 1) was given on day 5, followed by TMP-SMX (1.44 g po q6h) against PJP, and the rest of the anti-infection regimen remained unchanged. On the following day (day 6), oxygenation index was 130 and the patient’s shortness of breath aggravated with obvious signs of respiratory distress and hypoxemia (SaO2 <90%) requiring high flow nasal cannula at 6 L/min. Then, the patient was adjusted to non-invasive ventilator-assisted ventilation. Methylprednisolone (40 mg iv q12h) was adjusted for the suppression of pulmonary inflammatory exudation. On day 9, bedside chest X-ray illustrated bilateral lung texture increased and blurred, and scattered patchy shadows in the left middle and lower lobe plus right lower lobe (Figure 4a). However, respiratory distress worsened again and the oxygenation index dropped on day 13. She was intubated and connected to an invasive ventilator to assist breathing. On day 16, oral TMP-SMX reached 12 days but patient showed no improvement of respiratory function. Bedside chest X-ray illustrated bilateral lung exudate shadow without change (Figure 4b), so TMP-SMX was changed into intravenous therapy (1.92 g iv q8h). On day 18, oxygenation index was 213. A repeat bedside chest radiograph showed a lighter exudative shadow than before (Figure 4c). For economic reasons, TMP-SMX was changed into oral therapy (1.44 g po q6h). On Day 22, oxygenation index was 313. The ventilator was withdrawn with adequate assessment, and the high-flow transnasal humidified oxygen therapy was continued, and methylprednisolone (40 mg iv qd) was adjusted.
 |
Table 1 Sulfonamide Desensitization of the Patient on Day 5 |
On day 26, body temperature climbed into 38.5°C and she still presented with cough with sputum and felled distress of breath after activity. On day 28, multiple patchy and stripy high-density shadows were seen in both lungs by chest CT (Figure 5a, c and e). Meanwhile, the peak temperature did not decrease, and PCT was higher than before. Thus, on day 29, a second fiberoptic bronchoscopy showed foamy secretions in both bronchi, especially in the right bronchus. BALF was collected for mNGS and culture. On day 30, BALF mNGS results reported Enterobacter cloacae complex (157651) (Figure 6a), Klebsiella michiganensis (47538), Pseudomonas aeruginosa (65650) (Figure 6b), Enterococcus faecium (11112), Enterococcus faecalis (3531), Citrobacter floridis (3179), P. jiroveci (27) (Figure 3b), where E. cloacae complex and P. aeruginosa carried carbapenem drug resistance genes, including blaIMP (4), blaKPC (187), blaNDM (141), blaSHV (168), blaTEM (289), and blaVIM (30). Considering the secondary infection of multi-drug resistant bacteria, the anti-infection regimen was adjusted to meropenem (MEM, 1 g iv q8h), polymyxin B [PMB, nebulized (0.25 miu q12h) plus intravenous (first doses: 1.25 miu, maintenance dose: 0.75 miu q12h)], CAS (50 mg iv qd), linezolid (LNZ, 0.6 g po q12h), and TMP-SMX (0.96 g po qd).
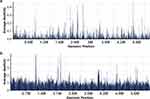 |
Figure 6 On day 30, the coverage of detected Enterobacter cloacae complex (a) and Pseudomonas aeruginosa (b) reads by mNGS using BALF was 21.36% and 58.93%, respectively. |
On day 33, BALF bacterial culture results showed E. cloacae (Table 2), only sensitive to amikacin (AMK) and colistin, and P. aeruginosa (Table 3), only sensitive to colistin and aztreonam (AZT). Sputum bacterial culture results reported Klebsiella oxytoca. On day 41, sputum culture showed K. oxytoca and P. aeruginosa, and LNZ was discontinued.
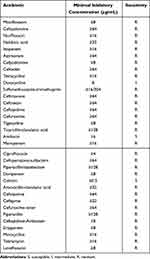 |
Table 2 Antibiotic Susceptibility Test Results of Enterobacter cloacae Isolated from BALF |
 |
Table 3 Antibiotic Susceptibility Test Results of Pseudomonas aeruginosa Isolated from BALF |
On day 50, a repeat chest CT showed that multiple patchy shadows and stripe shadows in both lungs were obviously absorbed (Figure 5b, d and f). On day 52, the patient’s cough and shortness of breath were relieved. The patient was discharged after discontinuation of MEM, PMB (nebulized + intravenous), and CAS. The patient was followed up for 2 months without any further discomfort such as cough, shortness of breath, and fever.
Discussion
In this case, PJP developed 4 months after renal transplantation. The first-line immunosuppressive regimen, including methylprednisolone, mycophenolate mofetil, and tacrolimus, was routinely used after transplantation. Due to patient’s refusal to take desensitization treatment and our hospital not have access to alternatives to TMP-SMX, the patient did not receive PJP prophylaxis post renal transplantation. Clinically, for patients with high-risk factors of PJP and clinical features and imaging manifestations consistent with PJP, the microbiological tests should be urgently completed to confirm PJP. But, the clinical manifestations of PJP lack of specificity, often starts with dry cough, and then progresses into fever, dyspnea, chest tightness and shortness of breath. More importantly, PJP progresses rapidly, which will eventually lead to death of patients due to respiratory failure if treatment is not timely.
In vitro culture of P. jirovecii has not been achieved so far due to the lack of a stable and reliable system.9 The diagnosis of P. jirovecii mainly relies on periodic acid-silver methenamine to find envelopes or trophozoites, but with relatively low detection rate. mNGS is a new technique that can detect nucleic acid of pathogens, with high sensitivity, high accuracy, and short turnaround time.10 Several studies have reported that mNGS is superior to traditional methods for the diagnosis of PJP.11,12 In this case, P. jirovecii was found by BALF mNGS at the beginning of the disease. Later, BALF mNGS was re-conducted and the sequence number of P. jirovecii was significantly reduced, but multiple other pathogens were found, which provided a strong basis for adjusting the anti-infection regimen and evaluating the anti-infective efficacy.
For the nucleic acid of microbial contaminants and colonizers can also be detected by mNGS, we should combine the clinical manifestations of the patients and the pathogenicity of the microorganisms to consider the mNGS results. The immune function of renal transplant recipients is relatively low. In the process of anti-PJP treatment, neutropenia caused by TMP-SMX, the usage of mechanical ventilation, invasive procedures such as fiberoptic bronchoscopy, and malnutrition were all predisposing factors for secondary bacterial infection. In our study, mNGS assay detected multiple-drug resistant E. cloacae complex and P. aeruginosa, which guiding the anti-infection treatment.
The first-line drug for the treatment of PJP is TMP-SMX.13 However, the incidence of sulfonamide allergy is as high as 34/1000.14 The common allergic reactions are skin erythema, urticaria, and maculopapular rash. Given that TMP-SMX is the most effective drug for the treatment of PJP, it is best to take desensitization treatment before sulfonamide treatment for those who are allergic to sulfonamide. If patients have had severe allergic reactions such as Stevens-Johnson syndrome and toxic epidermolysis bullosa in the past, desensitization is no longer recommended and second-line treatment for PJP is recommended.15 Solensky16 summarized 11 sulfonamide desensitization therapies that differed in starting dose, maintenance dose, interval between different doses, and duration of overall desensitization. As there are no studies comparing the advantages and disadvantages of various desensitization therapies, there is still no uniform and standardized desensitization process so far.
In this case, the patient had only localized skin urticaria after previous oral administration of sulfonamide, and there were no serious allergic reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis relaxation, so this patient was given desensitization therapy under close medical supervision and equipped with drugs and equipment to resuscitate systemic allergic reactions. The desensitization regimen was based on the Australian therapeutic guidelines,17 and the desensitization of sulfonamide proceeded smoothly without any allergic reactions during continuous administration (Table 2).
Oral TMP-SMX is routinely required for PJP prophylaxis after renal transplantation and other immunodeficient individuals, but sulfonamide allergy is not uncommon in the population18. In this case, the patient successfully achieved sulfonamide desensitization and was eventually discharged with improvement through close monitoring and comprehensive treatment, thus improving the patient’s long-term healing.
Conclusion
This article is the first report of a successful case of TMP-SMX treatment of PJP after sulfonamide desensitization therapy in a renal transplant recipient with sulfonamide allergies. In the process of anti-PJP treatment, the patient experienced the secondary infections of multiple-drug resistant E. cloacae complex and P. aeruginosa. In addition, this case demonstrates the important role of mNGS in the etiological monitoring of pulmonary infection in renal transplant recipients.
Ethical Approval
Ethical approval was not needed by local ethical committee, as this is a case report. Patient provided written informed consent to publish details of this case. A copy of the consent form is available for review by the Editor of this journal.
Consent to Publish
The authors hereby confirm that the kidney donation was conducted voluntarily, with written informed consent, and it was in adherence with the Declaration of Istanbul. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication. Patient gave consent to publication.
Funding
This work was supported by grants from the Clinical Technological Innovation Training Project of Army Medical University (CX2019LC104).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zou J, Wang T, Qiu T, et al. Single-center retrospective analysis of Pneumocystis jirovecii pneumonia in patients after deceased donor renal transplantation. Transpl Immunol. 2022;72:101593. doi:10.1016/j.trim.2022.101593
2. Kim JE, Han A, Lee H, Ha J, Kim YS, Han SS. Impact of Pneumocystis jirovecii pneumonia on kidney transplant outcome. BMC Nephrol. 2019;20(1):212. doi:10.1186/s12882-019-1407-x
3. Iriart X, Bouar ML, Kamar N, Berry A. Pneumocystis pneumonia in solid-organ transplant recipients. J Fungi. 2015;1(3):293–331. doi:10.3390/jof1030293
4. Yu Y, Yang H, Yu X, et al. Critical appraisal of the quality and content of clinical practice guidelines for pneumocystis jiroveci pneumonia (PJP) prophylaxis using the AGREE II instrument. J Clin Pharm Ther. 2020;45(6):1325–1333. doi:10.1111/jcpt.13213
5. Iriart X, Challan Belval T, Fillaux J, et al. Risk factors of Pneumocystis pneumonia in solid organ recipients in the era of the common use of posttransplantation prophylaxis. Am J Transplant. 2015;15(1):190–199. doi:10.1111/ajt.12947
6. Lee SH, Huh KH, Joo DJ, et al. Risk factors for Pneumocystis jirovecii pneumonia (PJP) in kidney transplantation recipients. Sci Rep. 2017;7(1):1571. doi:10.1038/s41598-017-01818-w
7. Eitner F, Hauser IA, Rettkowski O, et al. Risk factors for Pneumocystis jiroveci pneumonia (PcP) in renal transplant recipients. Nephrol Dial Transplant. 2011;26(6):2013–2017. doi:10.1093/ndt/gfq689
8. Lufft V, Kliem V, Behrend M, Pichlmayr R, Koch KM, Brunkhorst R. Incidence of Pneumocystis carinii pneumonia after renal transplantation. Impact of immunosuppression. Transplantation. 1996;62(3):421–423. doi:10.1097/00007890-199608150-00022
9. Bateman M, Oladele R, Kolls JK. Diagnosing Pneumocystis jirovecii pneumonia: a review of current methods and novel approaches. Med Mycol. 2020;58(8):1015–1028. doi:10.1093/mmy/myaa024
10. Gu W, Deng X, Lee M, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. 2021;27(1):115–124. doi:10.1038/s41591-020-1105-z
11. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi:10.1093/cid/ciy693
12. Zhang F, Chen J, Huang H, et al. Application of metagenomic next-generation sequencing in the diagnosis and treatment guidance of Pneumocystis jirovecii pneumonia in renal transplant recipients. Eur J Clin Microbiol Infect Dis. 2021;40(9):1933–1942. doi:10.1007/s10096-021-04254-x
13. Fishman JA. Pneumocystis jiroveci. Semin Respir Crit Care Med. 2020;41(1):141–157. doi:10.1055/s-0039-3399559
14. Bigby M, Jick S, Jick H, Arndt K. Drug-induced cutaneous reactions. A report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients. JAMA. 1986;256(24):3358–3363. doi:10.1001/jama.1986.03380240052027
15. Revuz J, Penso D, Roujeau JC, et al. Toxic epidermal necrolysis. Clinical findings and prognosis factors in 87 patients. Arch Dermatol Res. 1987;123:1160.
16. Solensky R. Drug desensitization. Immunol Allergy Clin North Am. 2004;24(3):425–443. doi:10.1016/j.iac.2004.03.008
17. Brookman D. Therapeutic guidelines: antibiotic. Version 12; 2003.
18. Stern A, Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2014;2014(10):CD005590. doi:10.1002/14651858.CD005590.pub3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.