Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Case-Finding and Treatment Effects in COPD: Secondary Analysis of an Interdisciplinary Intervention Trial
Authors Petrie K , Abramson MJ , George J
Received 14 September 2023
Accepted for publication 26 December 2023
Published 14 February 2024 Volume 2024:19 Pages 451—458
DOI https://doi.org/10.2147/COPD.S436690
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Kate Petrie,1 Michael J Abramson,2 Johnson George1,2
1Centre for Medicine Use and Safety, Monash University, Melbourne, Victoria, Australia; 2School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia
Correspondence: Johnson George, Centre for Medicine Use and Safety, Monash University, Melbourne, Victoria, Australia, Email [email protected]
Background: US Preventive Services Taskforce recommends against screening for COPD in asymptomatic adults due to limited evidence on the efficacy of treatments for this population. However, global and Australian guidelines recommend a case-finding approach where those with symptoms and/or risk factors, including smoking, are screened. This study aims to explore patient characteristics by time of COPD diagnosis and the effectiveness of early treatment in those with or without symptoms.
Methods: Secondary analysis of a randomised controlled trial that included those with a pre-existing (n=130) or new diagnosis (n=142) of COPD. Those randomised to the intervention arm received an interdisciplinary intervention of smoking cessation support, home medicines review and home-based pulmonary rehabilitation, while controls received usual care. The primary outcome was health-related quality of life (HR-QoL) measured using St George’s Respiratory Questionnaire. To estimate the impact of early treatment, we compared the effectiveness of treatment versus control at 6- and 12-months for the new versus pre-existing diagnosis groups, and those symptomatic versus asymptomatic or minimally symptomatic based on COPD Assessment Test score.
Results: Approximately half of those newly diagnosed with COPD were already symptomatic. Early treatment in those diagnosed via case-finding had a positive non-significant impact on HR-QoL. The size of the treatment effects generally favoured the pre-existing diagnosis group when compared to case-finding and favoured those symptomatic when compared to those asymptomatic.
Conclusion: Despite useful insights into the impacts of case-finding and early treatments, this study, like most others, was not sufficiently powered. Further larger studies or combining sub-groups across studies are required.
Keywords: RCT, screening, interdisciplinary, COPD
Introduction
Despite the significant morbidity and mortality associated with chronic obstructive pulmonary disease (COPD), few trials have investigated the impact of screening. The US Preventive Services Taskforce (USPSTF) updated (May 2022) their COPD screening guidance (following a systematic review) and recommended against screening in asymptomatic adults.1 This recommendation was based on the current lack of supportive evidence for intervention(s) in those who did not recognise their respiratory symptoms at a level that would prompt them to seek consultation with a doctor.1,2 The USPSTF specifically stated, “do not screen for COPD in patients with no symptoms”, but this “does not apply to populations at very high risk for COPD, such as persons with α1-antitrypsin deficiency or workers exposed to certain toxins at their work” as these high-risk populations were not specifically included in the review.1 The recommendation in support of screening did not extend to those with a history of smoking and, therefore, the USPSTF recommended that those with a history of smoking should be symptomatic prior to screening for COPD.1
An earlier diagnosis of COPD could reduce the burden of COPD through earlier access to pharmacological and non-pharmacological treatments.3 There is also an increasing focus on a “pre-COPD” population who are at-risk of COPD but may have risk factor(s) with preserved lung function.3 Therefore, despite the USPSTF recommendations, the Global initiative for Obstructive Lung Disease (GOLD) and Australian COPD-X guidelines recommend a case-finding approach (targeted screening) where those at risk of COPD, including those with a smoking history, are screened using a COPD screening device, irrespective of symptoms.4,5 The diagnosis of COPD itself may evoke some response from a patient.6,7 To the best of our knowledge, there has been no trial that has specifically aimed to investigate the impact of COPD screening (or case-finding) on outcomes including respiratory symptoms and behavioural changes such as smoking cessation.
However, a Cochrane review investigated the potential impact of biomedical risk assessment(s), including spirometry, to aid smoking cessation.8 This review found little supportive evidence for biomedical risk assessment as smoking cessation aids, with spirometry showing some positive benefit once high risk of bias studies were excluded from analyses.8 To address the impact of a diagnosis via case-finding (or screening) and early treatments more conclusively, future studies need to be conducted that specifically recruit those who are at-risk of COPD or sub-group analyses need to be conducted so that the outcomes in those diagnosed via case-finding and/or asymptomatic or minimally symptomatic could be systematically reviewed and meta analysed.
To address the lack of trials that have aimed to investigate the impact of COPD case-finding (or screening) on outcomes, we aimed to examine the: a) distribution of demographic and clinical characteristics by time of COPD diagnosis and b) effectiveness of early treatment versus usual care in those who were diagnosed via case-finding, and in those with or without symptoms.
Methods
Study Design and Population
Detailed study methods including study design and population have been published.9 In brief, Review of Airway Dysfunction and Interdisciplinary Community-Based Care of Adult Long-Term Smokers (RADICALS) was a randomised controlled trial (RCT) that evaluated the effectiveness of an interdisciplinary community-based program against usual care at improving HR-QoL.9,10 The study followed up participants at 6- and 12-months and was undertaken between 2015 and 2018 in Melbourne, Australia.9,10 Current or former smokers ≥40 years of age with at least a 10-pack-year history of smoking were identified from 40 recruited and randomised general practice clinics and invited to participate.9,10 Those with an expected survival of <12-months, unable to provide consent, less than two visits to the practice in 12-months, interstitial lung disease, comorbidities preventing participation in pulmonary rehabilitation or a contraindication to spirometry were excluded.9
Participants underwent case-finding following the Australian COPD-X plan using a handheld COPD-6 (Vitalograph Inc., Ennis, Ireland) screening device and completed the RADICALS baseline questionnaire (demographics, smoking history, outcomes).9 Those with a forced expiratory volume in 1 s (FEV1) and a forced expiratory volume in 6 s (FEV6) ratio of ≤0.75 were further tested using spirometry and completed a COPD Assessment Test (CAT) and modified Medical Research Council (mMRC) breathlessness questionnaire.9 A diagnosis of COPD was made following recommendations in the COPD-X plan.9,11 The fixed cut-off approach (post-bronchodilator FEV1/FVC <0.70) was used with clinical correlation, including CAT and mMRC questionnaire scores. Participants with a pre-existing diagnosis of COPD were also invited to participate after being identified in practice databases.
Study Arms
RADICALS was a two-arm, cluster RCT, with the intervention group receiving an interdisciplinary model of care that included collaborative support from their regular general practitioner (GP) and practice staff and a study-specific pharmacist and physiotherapist.9,10,12 The intervention group received integrated disease management, from a multidisciplinary team, that included active smoking cessation support, home medicines review (HMR) and home-based pulmonary rehabilitation (HomeBase), and the control group received usual care, referral to Quitline® and a copy of the Lung Foundation Australia publication “Better Living with COPD – A Patient Guide”.13
Outcome Measures
Outcome data were collected from participants using validated tools at baseline, and at both 6- and 12-months post-baseline. Outcomes included in this analysis were those that were likely to demonstrate emotional or behavioural change post-diagnosis and/or used to demonstrate the impact of treatments.
The primary outcome was HR-QoL as measured using the St George’s Respiratory Questionnaire (SGRQ).14 Secondary outcomes included smoking related: carbon monoxide (CO)-verified 7-day point prevalence smoking abstinence,15 heaviness of smoking index (HSI),16 readiness-to-quit ladder (adapted) (RTQ),17,18 smoking self-efficacy scale,19 visual analogue scale (VAS) for confidence and motivation to give up smoking.9 Symptom-related outcomes were as follows: COPD Assessment Test (CAT) score20 and mMRC grade.21,22 Behavioural outcomes were as follows: anxiety and depression measured using the Hospital Anxiety and Depression Scale (HADS)23 and body mass index (BMI).
Statistical Analyses
The distributions of participant characteristics were examined by time of diagnosis (and symptoms in those newly diagnosed with COPD). Symptomatic COPD was defined as those with a CAT score of ≥10 and asymptomatic/minimally symptomatic as those with a CAT score of <10.24,25 For the majority of outcomes, higher scores indicated negative/worse impacts on health eg the higher the SGRQ (scores range from 0 to 100) the worse the HR-QoL.14 Whereas for some outcomes, higher scores indicated positive/better impact on health (RTQ, VAS-confidence, and VAS-motivation).
The within-group change or difference was determined using the outcome at follow-up minus the outcome at baseline. Controlled before and after analysis used linear regression for continuous outcomes and logistic regression for binary outcomes. All regression analyses were adjusted for age at baseline, gender, highest education, income, current smoker status and standard errors accounted for clustering at the practice level based on previously published methods.9 Analysis followed an intention to treat (ITT) principle and a secondary per-protocol analysis (PPA) was performed (Supplementary Tables S2 and S4). All analyses were performed using STATA version 16.1.26
The RADICALS trial and subsequent analyses were approved by the Monash University Human Research Ethics Committee (Project ID: 4899). All participants provided informed consent at the time of enrolment in the RADICALS trial.10 The RADICALS trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12614001155684) in compliance with the Declaration of Helsinki.10
Results
Distribution of Characteristics
The RADICALS trial included 272 participants of whom 130 had a pre-existing COPD diagnosis and 142 had an incident diagnosis post case-finding. Of the 142 newly diagnosed participants, 75 (52.8%) were symptomatic (CAT score ≥10),24,25 66 (46.5%) were asymptomatic or minimally symptomatic and one (0.7%) was excluded due to a missing CAT score at baseline. Those in the pre-existing diagnosis group were older and less likely to be current smokers compared to those newly diagnosed. The baseline participant characteristics at the time of COPD diagnosis are presented in Table 1.
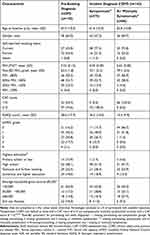 |
Table 1 Baseline Participant Characteristics by Time of COPD Diagnosis |
Effectiveness of Early Treatment in Sub-Groups
Case-Finding
There were non-significant improvements between baseline and 6-month follow-up SGRQ scores within both the control and intervention groups (Table 2). However, there were significant improvements between baseline and 12-month follow-up SGRQ scores within both groups (Table 2). For other outcomes, significant improvements were seen in HADS-anxiety, depression and CAT scores in the intervention group, and in VAS-motivation, HADS-anxiety and depression in the control group (Supplementary Table S1). A significant worsening of VAS-confidence was seen between 6-/12-months and baseline in the intervention group. Most of the within-group differences at 6-months persisted, or strengthened, at 12-months (Supplementary Table S1).
 |
Table 2 Effectiveness of Early Treatment in Those with an Incident Diagnosis: Change in SGRQ from Baseline to 6- and 12-Months |
The between-group differences (intervention versus control) in SGRQ at both 6- and 12-months follow-up favoured the intervention group, indicating a greater non-significant improvement in HR-QoL in the intervention group compared to the control group (Table 2). The between-group differences were non-significant for all other outcomes (Supplementary Table S1).
In the PPA, there was a significant between-group difference (treatment effects) in SGRQ at 12-months follow-up in favour of the intervention group, indicating a greater improvement in HR-QoL in the intervention group compared to the control group (Supplementary Table S2).
Symptomatic, Asymptomatic or Minimally Symptomatic
The improvement in SGRQ scores (between 6- and 12-months to baseline) was greater in the treatment than the control group for those with a pre-existing diagnosis and symptomatic with a new diagnosis (Supplementary Table S3). Within-group significant improvements were seen in the symptomatic incident diagnosis intervention group at 6-months (adjusted mean difference: 4.9, 95% CI: 0.5 to 9.2), symptomatic incident diagnosis control group at 12-months (6.3, 0.6 to 12.1) and symptomatic incident diagnosis intervention group at 12-months (9.2, 3.9 to 14.5) (Supplementary Table S3-ii, iii and iv). Differences in treatment effects between groups were not significant (Table 3). However, at 6-months, the point estimate favoured pre-existing compared to either incident diagnosis group, and favoured symptomatic compared to the asymptomatic incident group. At 12-months, the point estimated favoured the symptomatic incident diagnosis group compared to the pre-existing group and favoured the pre-existing and symptomatic groups when compared to the asymptomatic incident group (Table 3).
In the PPA, the differences in treatment effects between groups were not significant (Supplementary Table S4). For completeness and potential future meta-analyses purposes, the differences in other outcome measures are presented in Supplementary Table S5.
Discussion
The RADICALS study included participants who had either a pre-existing diagnosis of COPD (48%) or those newly diagnosed via case-finding (52%). Of those who were diagnosed via case-finding, 53% were already symptomatic (CAT score ≥10) and 47% were asymptomatic or minimally symptomatic (CAT score <10). Early treatment with RADICALS intervention in those with an incident diagnosis had a positive but non-significant impact on HR-QoL (as measured by SGRQ). The difference in treatment effects generally favoured the pre-existing diagnosis group when compared to either incident diagnosis groups, and favoured the symptomatic group when compared to the asymptomatic or minimally symptomatic group. These findings suggest that early treatment may have a greater positive impact in those who are symptomatic compared to those who are asymptomatic or minimally symptomatic.
The RADICALS trial was not powered to detect significant differences in outcomes in incident diagnosis versus pre-existing diagnosis sub-groups, meaning that uncertainty remains. These initial findings provide useful insights into the potential impact of screening or case-finding for COPD. COPD intervention trials often exclude either those who were newly diagnosed post screen-detection (or case-finding) and/or those with minimal or asymptomatic disease, creating a treatment effect bias towards those with more severe disease. A large European study found that, when comparing characteristics of COPD intervention trial participants to those treated with COPD in primary care, trial participants had significantly worse lung function and quality of life than those treated in primary care.27 Our results are important and could be used, along with similar subgroup analyses, as part of a meta-analysis to provide stronger evidence on the impact of screening (or case-finding) and the impact of early treatments. To the best of our knowledge, no trial has specifically considered outcomes in those screen-detected (or diagnosed via case-finding) and the USPSTF recommends against screening for COPD.1
Despite this USPSTF recommendation against asymptomatic screening, we found that approximately half of those diagnosed via case-finding were symptomatic. This suggests that there is still no strong public awareness of the symptoms of COPD, as those with respiratory symptoms were not prompted to seek consultation with a doctor and undergo COPD case-finding. A 2014 study interviewed people who were newly diagnosed with COPD and found that those with multi-morbidity had difficulty understanding the significance and long-term impact of COPD.7 These newly diagnosed patients did not place much importance on their new COPD diagnosis, with many prioritising other comorbidities such as diabetes.7 A later qualitative study found that people newly diagnosed with COPD had difficulty understanding the importance of the diagnosis and delayed quit attempts.6 Our study supports the ongoing need for COPD education and raises public awareness on risk reduction, particularly in those with a history of smoking.
Our study had several strengths. These included the pragmatic nature, broad recruitment criteria enabling comparisons between newly diagnosed via case-finding, and pre-existing diagnosis groups. The diversity of GP practices recruited in terms of size, location, socioeconomic status of patients and availability of respiratory services increased the generalisability of the findings. The intervention was designed to incorporate services that were available in primary care to ensure that the intervention could be implemented in a real-world setting.
Our study had some limitations. These included the partial uptake of the multifaceted intervention, the length of follow-up and the comparison group and the fact that this sub-group analysis was not pre-specified. There was limited uptake of full intervention, with some participants only receiving smoking cessation support. The maximum follow-up was 12 months and therefore we were unable to capture the potential longer-term impact of screening and effects of early treatment. Although we were able to make comparisons between the pre-existing diagnosis and the incident diagnosis groups, the recruitment did not include a comparison group that was not screened and therefore any potential differences in outcomes between the non-screened and screened could not be determined. The RADICALS study was not designed with this sub-group analysis in mind and therefore was not powered to measure meaningful differences in outcomes between these sub-groups. Given the significant improvements in SGRQ at 12-months, in both the intervention and control groups, usual care may be effective and/or trial participation may have positively impacted on HR-QoL.
Conclusion
Approximately half of those diagnosed with COPD via case-finding were already symptomatic. Case-finding appeared to have a positive impact on HR-QoL in the newly diagnosed group. Early treatment with RADICALS intervention in those screen-detected had a positive non-significant impact on HR-QoL. The difference in treatment effects generally favoured the pre-existing diagnosis group when compared to either incident diagnosis group and favoured the symptomatic group when compared to the asymptomatic group. Larger studies or meta-analyses of sub-group analyses are required.
Abbreviations
AUD, Australia dollars; BD, bronchodilator; BMI, Body mass index; CAT, COPD Assessment Test; CI, confidence interval(s); CO, carbon monoxide; COPD, chronic obstructive lung disease; FEV1, forced expiratory volume in 1 s; FEV6, forced expiratory volume in 6 s; FVC, forced vital capacity; GOLD, Global initiative for Chronic Obstructive Lung Disease; GP, general practitioner; HADS, Hospital Anxiety and Depression Scale; HMR, home medicines review; HR-QoL, health-related quality of life; HSI, heaviness of smoking index; ITT, intention to treat; mMRC, modified Medical Research Council dyspnoea scale; N/A, not applicable; OD, odds ratio(s); PPA, per protocol analysis; RADICALS, Review of Airway Dysfunction and Interdisciplinary Community-Based Care of Adult Long-Term Smokers; RCT, randomised controlled trial; RTQ, readiness-to-quit; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire; USPSTF, US Preventive Services Task Force; VAS, visual analogue scale.
Data Sharing Statement
If interested, reasonable requests for data can be made to the corresponding author ([email protected]) and will be considered in line with the requirements of Monash University Human Research Ethics Committee approval (Project ID: 4899).
Acknowledgments
We wish to acknowledge and thank the RADICALS: chief investigators (Nicholas Zwar, Grant Russell, Billie Bonevski, Anne Holland, Eldho Paul, Sally Wilson and Ajay Mahal), the Data Safety and Monitoring Board members, the research staff and students, clinic staff and participants, and Brigitte Borg and The Alfred Respiratory Laboratory. We also acknowledge the RADICALS trial funders and partners (Boehringer Ingelheim, Eastern Melbourne PHN, Lung Foundation Australia and National Health & Medical Research Council). KP acknowledges the support of the Australian Government’s research training program.
Disclosure
KP was supported by an Australian Government Research Training Program (RTP) Scholarship. MJA holds investigator-initiated grants for unrelated research from Pfizer, Boehringer Ingelheim, Sanofi and GSK. He has also undertaken an unrelated consultancy (paid to his employer) for Sanofi and received a speaker’s fee from GSK. JG holds investigator-initiated grants for unrelated research from Pfizer, GSK and Boehringer Ingelheim. He has received honoraria (paid to his employer) from a consultancy for GSK, AZ and for invited presentations at a continuing education event organised by Pfizer. The authors report no other conflicts of interest in this work.
References
1. Mangione CM, Barry MJ, Nicholson WK.; US Preventive Services Task Force. Screening for chronic obstructive pulmonary disease: US preventive services task force reaffirmation recommendation statement. JAMA. 2022;327(18):1806–1811. doi:10.1001/jama.2022.5692
2. Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease. JAMA. 2016;315(13):1378. doi:10.1001/jama.2016.2654
3. Han MK, Agusti A, Celli BR, et al. From GOLD 0 to Pre-COPD. Am J Respir Crit Care Med. 2021;203(4):414–423. doi:10.1164/rccm.202008-3328PP
4. Yang I, George J, McDonald C, et al. The COPD-X plan: Australian and New Zealand Guidelines for the management of chronic obstructive pulmonary disease. TSANZ [Internet]; Version 2; 2022. Available from: https://copdx.org.au/.
5. Lung Foundation of Australia. COPD case finding position paper; 2019; Available from: https://lungfoundation.com.au/resources/copd-case-finding-position-paper/.
6. Bragadottir GH, Halldorsdottir BS, Ingadottir TS, Jonsdottir H. Patients and families realising their future with chronic obstructive pulmonary disease—A qualitative study. J Clin Nurs. 2018;27(1–2):57–64. doi:10.1111/jocn.13843
7. Ansari S, Hosseinzadeh H, Dennis S, Zwar N. Patients’ perspectives on the impact of a new COPD diagnosis in the face of multimorbidity: a qualitative study. NPJ Prim Care Respir Med. 2014;24(1):14036. doi:10.1038/npjpcrm.2014.36
8. Clair C, Mueller Y, Livingstone-Banks J, et al. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2019;3(3):CD004705. doi:10.1002/14651858.CD004705.pub5
9. Liang J, Abramson MJ, Zwar N, et al. Interdisciplinary model of care (RADICALS) for early detection and management of chronic obstructive pulmonary disease (COPD) in Australian primary care: study protocol for a cluster randomised controlled trial. BMJ Open. 2017;7(9):e016985. doi:10.1136/bmjopen-2017-016985
10. Liang J, Abramson MJ, Russell G, et al. Interdisciplinary COPD intervention in primary care: a cluster randomised controlled trial. Eur Respir J. 2019;53(4):1801530. doi:10.1183/13993003.01530-2018
11. Yang IA, Brown JL, George J, et al. COPD‐X Australian and New Zealand guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 2017 update. Med J Aust. 2017;207(10):436–442. doi:10.5694/mja17.00686
12. Jessup RL. Interdisciplinary versus multidisciplinary care teams: do we understand the difference? Aust Heal Rev. 2007;31(3):330–331.
13. Schrijvers G. Disease management: a proposal for a new definition. Int J Integr Care. 2009;9(1). doi:10.5334/ijic.301
14. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi:10.1164/ajrccm/145.6.1321
15. Benowitz NL, Jacob P, Ahijevych K; SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi:10.1080/14622200210123581
16. Borland R, Yong -H-H, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12(suppl_1):S45–S50. doi:10.1093/ntr/ntq038
17. Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Heal Psychol. 1991;10(5):360. doi:10.1037/0278-6133.10.5.360
18. Niaura R, Shadel WG. Assessment to Inform Smoking Cessation Treatment. In: The Tobacco Dependence Treatment Handbook: A Guide to Best Practices. Guilford Press; 2003.
19. Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15(3):271–283. doi:10.1016/0306-4603(90)90070-E
20. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Leidy NK. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
21. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi:10.1378/chest.93.3.580
22. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
23. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
24. Jones PW, Tabberer M, Chen W-H. Creating scenarios of the impact of COPD and their relationship to COPD assessment test (CATTM) scores. BMC Pulm Med. 2011;11(1):42. doi:10.1186/1471-2466-11-42
25. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
26. StataCorp. Stata Statistical Software: Release 16.1. College Station, TX: StataCorp LLC; 2019.
27. Kruis AL, Ställberg B, Jones RCM, et al. Primary care COPD patients compared with large pharmaceutically-sponsored COPD studies: an UNLOCK validation study. PLoS One. 2014;9(3):e90145. doi:10.1371/journal.pone.0090145
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

