Back to Journals » International Journal of General Medicine » Volume 17
Carrageenan-Containing Nasal Spray Alleviates Allergic Symptoms in Participants with Grass Pollen Allergy: A Randomized, Controlled, Crossover Clinical Trial
Authors Unger-Manhart N, Morokutti-Kurz M, Zieglmayer PU, Lemell P, Savli M, Zieglmayer R, Prieschl-Grassauer E
Received 14 November 2023
Accepted for publication 10 January 2024
Published 3 February 2024 Volume 2024:17 Pages 419—428
DOI https://doi.org/10.2147/IJGM.S447359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Nicole Unger-Manhart,1,* Martina Morokutti-Kurz,1,* Petra U Zieglmayer,2,3 Patrick Lemell,2 Markus Savli,4 René Zieglmayer,2 Eva Prieschl-Grassauer1
1Marinomed Biotech AG, Korneuburg, Austria; 2Vienna Challenge Chamber, Vienna, Austria; 3Competence Center for Allergology and Immunology, Karl Landsteiner University, Krems, Austria; 4Biostatistik & Consulting GmbH, Zurich, Switzerland
*These authors contributed equally to this work
Correspondence: Eva Prieschl-Grassauer, Marinomed Biotech AG, Hovengasse 25, Korneuburg, 2100, Austria, Tel +43 226292300, Email [email protected]
Purpose: Nonpharmacological, barrier-forming nasal sprays are used to manage symptoms of allergic rhinitis. We aim to evaluate the safety and effectiveness of Callergin (investigational product, IP), a nasal spray containing barrier-forming iota-carrageenan, in the treatment of allergic rhinitis (AR).
Methods: In this randomized, controlled, crossover trial, adults with grass pollen allergy underwent a treatment sequence with IP, VisAlpin (comparator product, CP), and no treatment in random order. Treatment blocks consisted in prophylactic administration of the assigned treatment or no treatment, followed by a 3-hr allergen exposure, and were separated by a washout period of 7 days. Primary endpoint was a mean change from baseline in “Total Nasal Symptom Score” (TNSS, sum of rhinorrhea, itching, sneezing, and congestion scores) over 3 hr, recorded every 15 min during the challenge period.
Results: A total of 42 participants underwent randomization. Exposure to grass pollen for 3 hr induced a notable TNSS increase from baseline in all participants at all times. Mean TNSS change from baseline over 3 hr was lower when participants received IP compared to no treatment, although the difference did not reach statistical significance (untreated 6.96 ± 2.30; IP 6.59 ± 1.93; difference 0.37 points [95% CI (confidence interval) − 0.17 to 0.91]; p=0.170). In a post-hoc analysis, mean TNSS at 3 hr was significantly reduced after IP treatment compared to no treatment (untreated 8.29 ± 2.64; IP 7.70 ± 2.56; difference 0.60 points [95% CI − 0.10 to 1.29] p=0.028). While all individual nasal symptoms contributed to this effect, rhinorrhea (p=0.013) and congestion (p=0.076) contributed most. Consistently, nasal secretion weight was slightly reduced with IP treatment (p=0.119). IP was safe and well-tolerated, with similar incidence of adverse events across treatment groups.
Conclusion: Prophylactic treatment with the iota-carrageenan nasal spray IP is safe, well-tolerated, and alleviates nasal allergy symptoms in adults with grass pollen-induced AR.
Trial Registration: NCT04531358.
Keywords: allergic rhinitis, nonpharmacological, drug-free, barrier, iota-carrageenan
Introduction
Allergic rhinitis (AR) is an immunoglobulin E (IgE)-mediated hypersensitivity reaction occurring after exposure to airborne allergens like pollen, mold, dust mites, or animal dander.1,2 Allergic rhinitis is one of the most common chronic conditions in high-income countries, with an estimated prevalence of between 10% and 40%, depending on the geographic region.3,4 Seasonal allergic rhinitis, also termed hay fever, is caused by seasonal peaks in the airborne load of pollens and is the most common type of allergic rhinitis. It is estimated that in Europe, up to 40% of the population suffer from pollen allergy.5 Classic symptoms include nasal itching, obstruction, sneezing, and rhinorrhea (runny nose), although some participants may also experience ocular or upper respiratory symptoms.6,7
About half of allergic participants have AR symptoms for more than 4 months/year and one-fifth for more than 9 months/year. If not treated properly, these symptoms affect people’s quality of life and are associated with substantial health-care costs (eg, exacerbations of sinusitis and asthma, nasal polyps, and hearing impairment) and other economic impacts (eg, less productivity).8
Current recommendations for the management of allergic rhinitis comprise avoidance of allergen exposure as well as pharmacotherapy. Recommended pharmacologic treatment options backed by solid scientific evidence include pharmacotherapy with intranasal saline, oral or intranasal H1-antihistamines, intranasal corticosteroids, or a combination of intranasal H1-antihistamines and intranasal corticosteroids in cases where monotherapy fails to control symptoms.9 Saline nasal sprays have an excellent safety profile but act mainly by flushing allergens out of the nasal cavity and therefore must be applied often during exposure. Symptomatic treatment with antihistamines, administered intranasally or orally, offers relief from symptoms. Oral antihistamines of the second generation have a good benefit–risk ratio in contrast to first generation, but at higher doses may cause side effects on the central nervous system and sedation. Second-generation H1 antihistamines are considered safe in infants and young children, but no antihistamines fall in the lowest FDA pregnancy risk category, denoting negative studies in animals and negative human data.10 Intranasal corticosteroids play a crucial role in alleviating inflammation and improving outcomes but may come with undesired local and systemic side effects like epistaxis or reduced bone growth in children.11 Costs associated with pharmacotherapy are considered low to moderate.9 In sum, benefits outweigh risks for both oral and intranasal antihistamines as well as intranasal corticosteroids, however drug interactions and risk of side effects need to be considered.
Barrier-forming nasal sprays represent an alternative approach to AR management: By forming an intranasal barrier, they aim to prevent physical contact between allergens and mucosal cells. A number of studies have examined barrier-enforcing measures in the context of allergic rhinitis and have found a significant reduction of AR symptoms upon topical application of cellulose powder and lipid compositions like a microemulsion (reviewed in12). Drug-free, locally acting, barrier-forming nasal sprays can be an attractive alternative treatment for mild-to-moderate allergic rhinitis and in special populations (eg, pregnant women or children), or as a complementary therapy for moderate-to-severe allergic rhinitis.
Carrageenans are linear sulphated polysaccharides extracted from red algae. Based on their high viscosity and gelling capacity as well as their excellent biocompatibility, carrageenans have gained great importance in the food industry as well as in medical research.13 Previous studies have shown that carrageenan has a broad, non-specific virus-blocking activity that is not based on chemical activity,14 but most probably on its capacity to form a viscous barrier where inhaled as well as newly synthesized and released virus particles are trapped.15 Importantly, carrageenan does not penetrate the nasal mucosa and is thus not absorbed by the human body when applied intranasally.16 Based on its barrier function, we speculate that carrageenan may block the interaction of pollen/allergens with mucosal cells, in an analogous manner as it inhibits virus-host cell interaction.
Callergin (investigational product, IP) is a nasal spray based on iota-carrageenan, a natural polymer from red seaweed. Iota-carrageenan (Carragelose®) is certified for marketing in the EU and in parts of Asia and Australia, as a component of nasal sprays, throat sprays, and lozenges. Iota-carrageenan IP nasal spray is classified as a class I substance-based medical device.
The objective of this study was to evaluate the clinical performance of IP in preventing AR symptoms in participants with grass pollen allergy when compared to a “no-treatment” control. VisAlpin Alpensalz (comparator product, CP), a marketed saline nasal spray, was added as a comparator in this study because it can be used as an accompanying treatment for stuffy nose caused by a cold or allergy.17
Methods
Study Design
This was a randomized, three‐period crossover trial to assess the safety and efficacy of IP in participants with AR caused by grass pollen and compare it to the safety and efficacy of a comparator treatment and to no treatment (NCT04531358). In each treatment period, participants were randomly assigned to receive either IP, CP, or no treatment at all, followed by exposure to the allergen under controlled conditions. The two treatments (IP and CP) were double blinded against each other, while the untreated group was inherently unblinded. After the first treatment period, participants underwent a washout period of 7 days to allow remission of allergic symptoms, after which they crossed over to one of the treatments they had not received before for the second treatment block, again followed by a 7-days washout period and cross-over to the third treatment block. The study was performed at the Vienna Challenge Chamber in Vienna, Austria. The protocol received clearance from the Austrian Competent Authority. The Ethics Committee of the City of Vienna oversaw trial conduct and documentation. Written informed consent was obtained from all participants prior enrolment in the study.
Participants
Eligible participants were aged 18–65 and had a documented history of moderate-to-severe seasonal AR to grass pollen with or without mild-to-moderate asthma. Participants were recruited by the investigator and her delegates according to the inclusion/exclusion criteria, based on an existing database of test persons with confirmed pollen allergy. After being informed on study design, procedures, and associated risks and benefits, willing and eligible participants signed informed consent forms and underwent screening examinations. At screening, participants had to score ≥6 in total nasal symptom score (TNSS) in response to approximately 1500 grass pollen grains/m3 within the first 2 hr inside the exposure chamber. Main exclusion criteria comprised ongoing diseases and treatments that may interfere with the study intervention and outcomes.
Randomization and Masking
Patients were randomly assigned to one of the three treatments per treatment period using a crossover randomization with balanced blocks. Double blinding was guaranteed by identical presentation of the nasal sprays and the use of neutral randomization numbers for the differentiation of the packs.
Interventions and Procedures
During each treatment period, participants administered one puff (140 µL) of either IP (1.2 mg/mL iota-carrageenan in 0.5% NaCl and phosphate buffer) or the CP (0.9% NaCl in water) in each nostril 5–10 min before start of the grass pollen allergen challenge or remained untreated for that period. Study drug administration was supervised by study staff and recorded in the accountability log. Study medication IP (Lot No. 8023) and CP (Lot No. 7659) were packaged and labelled by Mono chem-pharm Produkte GmbH (Vienna, Austria) on behalf of Sigmapharm Arzneimittel (Vienna, Austria). IP and CP have been re-labeled, packed, and controlled according to study specifications and in compliance with applicable GMP regulations. Participants were then exposed to a standard grass pollen allergen mixture (1500 grass pollen grains/m3, Allergopharma) for 3 hr. The grass pollen allergen challenge was carried out at screening and on day 1 of each treatment period in the Vienna Challenge Chamber (VCC), using a validated method.18,19 VCC can accommodate up to 20 participants in one session, all of whom were under constant supervision by, and could communicate with, medical staff outside the chamber. During the challenge, the chamber was charged with 100% fresh air, which was conditioned (filtered, heated, dried, cooled, and humidified) and then loaded with a qualitatively and quantitatively determined pollen amount. The challenge agent used in the chamber was a mixture of four grass pollen species (Timothy, Orchard, Perennial rye, and Sweet vernal grass) (Allergon SB, Sweden). Air temperature (24°C), humidity (40%), and allergen load (approximately 1500 grains per cubic meter) were constantly monitored and maintained. During the four grass pollen challenge sessions, participants scored their subjective symptom scores – Total Nasal Symptom Score (TNSS), Total Ocular Symptom Score (TOSS), Total Respiratory Symptom Score (TRSS) – every 15 min. As objective parameter, nasal airflow and nasal secretion weight were assessed.
Endpoints
The primary efficacy endpoint was a mean change from baseline in “total nasal symptom score” (TNSS) over 3 hr. TNSS is the sum of the nasal symptoms of congestion, rhinorrhea, itching, and sneezing. Each symptom was rated on a scale from 0 to 3, whereas “0” corresponded to “no symptoms”, “1” to “mild symptoms” (easy to tolerate), “2” to “moderate symptoms” (bothersome, but tolerable), and “3” to “severe symptoms” (hard to tolerate). Secondary endpoints included change from baseline in “total ocular symptom score” (TOSS; eye symptoms: itchy eyes, red eyes and watery eyes) and “total respiratory symptom score” (TRSS; respiratory symptoms: cough, wheeze, and dyspnea). During their 3-hr stay in the challenge chamber, participants were asked to rate their nasal, ocular, and respiratory allergy symptoms at 15-min intervals. For the assessment of nasal secretion, participants were asked to collect their secretion in pre-weighed tissues during their stay in the chamber. Used tissues were collected every 30 min and weighed by study site staff immediately after collection. Nasal airflow was measured by active anterior rhinomanometry at 30, 60, 120, and 180 min. Safety assessments included measurement of vital signs and lung function (hourly measurement of FEV1 [Forced Expiratory Volume in 1 s] by spirometry) during treatment visits, as well as electrocardiogram, physical examination, nasal examination, and blood analysis at screening and follow-up visits. All safety analyses were based on safety population defined as all participants starting challenge provocation qualification session.
Statistical Analysis
Sample size was calculated to reach a power of 90% with an alpha level p≤0.05 resulting in 36 participants needed for evaluation. Considering a dropout rate of 10–15%, 50 participants had to be screened to randomize about 42 participants to get at least 36 evaluable participants at the end of the trial. For the primary efficacy variable, the within participant comparison of IP versus no treatment was performed using a three-period analysis of variance (ANOVA) appropriate for the crossover design. Period was included in the analysis model as a fixed effect to confirm the assumption of no period effect. Participant was included in the model as a random effect. A 95% confidence interval was calculated for the difference in means between the two active treatments from a two-sided paired t-test. The Tukey procedure was applied for post-hoc comparisons to adjust for multiplicity. The hypothesis to be tested was superiority of IP in comparison to the no-treatment control. The null hypothesis is defined as
• [mean TNSS (Test)] ≥ [mean TNSS (untreated condition)]
The alternative hypothesis was defined as
• [mean TNSS (Test)] < [mean TNSS (untreated condition)]
The effects of CP were only described in an explorative manner. Therefore, superiority/non-inferiority of IP vs CP was not defined for this study.
The primary efficacy variable was summarized for participants in both the FAS (full analysis set) and PP (per protocol) population, whereas the FAS analyzed as intended to treat (ITT) was the primary analysis population.
All attempts were made to collect all data per protocol. Missing or invalid data was neither replaced nor extrapolated. Outliers were not excluded from the primary analyses. All the data recorded on the eCRF was included in data listings. R version 4.0 or above was used to generate tables, figures, and listings and statistical analyses.
Results
Between September 2020 and December 2020, a total of 47 participants with grass pollen allergies were screened after giving informed consent. Of these, 42 participants were randomized to six possible treatment sequences, with each participant acting as her/his own control. All randomized participants completed the study and were included in the analysis (Figure 1).
Baseline characteristics were balanced across the six treatment sequences (data not shown). After each allergen challenge, TNSS scores returned to their baseline values at the start of the following treatment period, excluding a potential carry-over effect from one treatment period into the subsequent one (ANOVA model, p-value>0.05) (Table 1).
 |
Table 1 Demographic and Clinical Characteristics of the Patients at Baseline (Intention-to-Treat Population) |
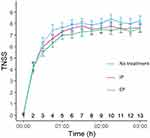
All participants suffered from increasing nasal symptoms (mean change from baseline in TNSS: untreated 6.96 ± 2.30; IP 6.59 ± 1.93; CP 6.34 ± 1.77) and a 40–45% reduction in nasal airflow during the 3 hr they were exposed to grass pollen. The increase in TNSS following allergen exposure over 3 hr was slightly lower with IP treatment than without treatment (∆=0.37, 95% CI −0.17 to 0.91; p=0.17). Our results obtained with CP showed a similar trend but did not reach statistical significance either (∆=0.61, 95% CI −0.17 to 0.91; Figure 2).
Post-hoc analysis evaluating TNSS at the end of the pollen challenge reaffirmed the IP effect on the relief of allergy symptoms. At the end of the three-hour allergen challenge, participants receiving IP nasal spray showed significantly lower TNSS than those receiving no treatment (∆=0.60; p=0.028) (Table 2). All individual nasal symptoms contributed to the IP effect, but rhinorrhea (∆=0.26; p=0.013) and nasal congestion (∆=0.17; p=0.076) to a larger extent. Fifty percent (50%) of the IP-treated participants showed a TNSS of 8 or less compared with 8.5 or less in the untreated group. The top 25% most affected participants experienced a TNSS of 9 or more in the IP-treated group and 10 or more in the untreated group (Table 3). Participants treated with CP did not show a reduction in TNSS when compared to those receiving no treatment at 3 hr after the onset of the allergen challenge (data not shown).
 |
Table 2 Mean TNSS, its Individual Nasal Symptoms, and Nasal Secretion Weight at 3 hr. Difference from “No Treatment” Control is Given as Mean Treatment Difference with Associated 95% CIs and P-values |
 |
Table 3 Population Analysis of TNSS Scores |
The mean nasal secretion over the entire duration of the 3-hr exposure to the allergen challenge was assessed as an objective parameter to complement the subjective self-assessment of the participants. Mean weight of nasal secretions was reduced by 13% after IP treatment compared to no treatment, but the difference did not reach statistical significance: mean within-participant changes in gram: untreated 2.85 ± 2.63; IP 2.48 ± 2.14 (∆=0.37; 95% CI −0.10 to 0.84; p=0.119) (Table 4).
 |
Table 4 Mean Nasal Secretion [G] Ø(0:30–3h) for the Full Analysis Set (FAS) |
None of the secondary endpoint analyses (TOSS, TRSS, and nasal airflow) revealed any treatment differences in allergy symptoms. The mean TOSS over the 3-hr allergen exposure was 1.63 ± 1.74 after IP and 1.61 ± 1.71 after no treatment (p=0.676 by Wilcoxon-test). The mean TRSS over the 3-hr allergen exposure was 1.16 ± 1.44 after IP and 1.16 ± 1.52 after no treatment (p=0.949 by Wilcoxon-test).
The mean nasal airflow over the 3-hr allergen exposure was 288.37 ± 127.07 mL/s after IP and 282.49 ± 146.41 mL/s after no treatment (p=0.696 by paired t test). In the safety population, a total of 11 adverse events occurred in 6 participants during the trial (Table 5). The only adverse event reported more than once was headache. Importantly, the incidence of adverse events was comparable among treatment groups, and no AE was deemed related to the study treatment. None of these events was serious or led to treatment withdrawal. Laboratory blood results did not reveal any clinically significant abnormalities. Mean systolic blood pressure, mean diastolic blood pressure, mean pulse rates and mean respiratory rates were stable during the trial. Results of physical examinations at screening and follow-up were normal.
 |
Table 5 Safety Profile. All Adverse Events Started During a Washout Phase and Were Rated as Non-Related to the Treatment |
Discussion
In this study, we evaluated the clinical performance of IP in reducing allergic rhinitis symptoms in participants with grass pollen allergy when compared to a “no-treatment” control. Our results show a trend towards decreased nasal symptoms and nasal secretion during the whole challenge period following a single application of IP. Although the effect did not reach statistical significance in the prespecified analysis, it did so in a post-hoc analysis evaluating nasal symptoms at the end of the challenge period. There were no adverse reactions in participants in any of the treatment groups.
Single prophylactic treatment with IP reduced patient reported allergic nasal symptoms by 0.6 symptom points at 3 hr. In a clinical study investigating the pharmaceutical effect of different treatments on patient reported allergic nasal symptoms (TNSS 0–12 point scale), Gross et al defined a threshold of 0.23 units as minimum clinically important difference.20 Accordingly, a TNSS reduction by 0.6 units, achieved with a single application of IP, is a remarkable and clinically relevant improvement.
In terms of population percentiles, 50% of the patients treated with IP showed a TNSS of 8 or less whereas untreated participants revealed a TNSS of 8.5 or less. Moreover, the top 25% most affected participants experienced a TNSS of 9 or more in the IP group compared to 10 or more in the untreated group. These data indicate that most of the allergic patients had a benefit of prophylactic IP treatment.
The reduction of nasal secretion is integrated into the TNSS score by the individual symptom “rhinorrhea” and is also mirrored by objective weight determination of nasal secretions, which was reduced by 13% over 3 hr in IP-treated individuals. Even though this reduction was not statistically significant, it shows the same trend. Nasal obstruction, a typical late reaction that takes place up to 6 hr after exposure,7 showed no difference across treatment groups when assessed by rhinomanometry. The IP effect, as evidenced in nasal symptoms, was not observed in eye or respiratory symptoms, which is unsurprising considering the product is acting locally on the nasal mucosal surface.
CP was chosen as a comparator because it is indicated as an add-on treatment for stuffy nose due to a cold or allergy. In a meta-analysis study, saline nasal irrigation produced a 28% improvement in nasal symptoms and a 62% reduction in medicine consumption in AR patients when performed daily over a period of up to 7 weeks.21 In our study, CP improved nasal symptoms somewhat, but not significantly. CP works by flushing out allergens from the nasal mucosa, while IP forms a long-lasting protective barrier on the nasal mucosa and thus is more appropriate as a prophylactic treatment.
Other barrier-forming nasal sprays that prevent allergen contact are currently on the market or under development for the treatment of AR. Tested substances include cellulose derivatives,22 clay mineral bentonite,23 thixotropic gel,24 petrolatum-based ointment,25 and lipid-based ointment.26 Of these, a hydroxypropyl methylcellulose powder (HPMC) nasal spray on sale since 1994 has been backed up by over 20 clinical studies. In a study conducted in a natural setting – during pollen season – patients were asked to apply nasal puffs and document their symptoms daily. A 4-week therapy with an HPMC powder nasal spray reduced TNSS by 26% compared to placebo.27 Other researchers evaluated the effect of a single application on TNSS using two different experimental allergen challenges: either by direct instillation of an allergen into the nasal cavities or through an environmental challenge chamber (such as the one used in our study). Results showed that a single application of an HPMC nasal spray before an intranasal dust mite challenge reduced TNSS by 41% over 4.5 hr in comparison to placebo.28 In contrast, Nehrig et al found that the same treatment before a 4-hr exposure to grass pollen in an environmental challenge chamber reduced TNSS by 12% over 4 hr in comparison to a “no treatment” control.23 This difference in effect size suggests that the continuous allergen challenge is much harsher than the single intranasal allergen challenge normally used for investigating antiallergic nasal sprays. The extent of TNSS reduction by a single application of HPMC nasal spray following a continuous allergen challenge is similar to that observed in our study with IP. Therefore, a more pronounced effect, similar to the one seen after multiple applications of HPMC (26% reduction in TNSS), can be expected with IP when it is used regularly in real-world settings during the pollen season. As IP’s main ingredient, iota-carrageenan, has an excellent safety profile and a long history of intranasal long-term applications also in sensitive populations (children, pregnant women, elderly), IP can be used regularly without any safety concerns. The long-lasting barrier develops its protective effect immediately after application and lasts up to 3 hr, as shown by an immediate reduction of allergy symptoms, which reaches significance at the end of the allergen challenge. Previous data have shown that carrageenan forms a protective layer on the mucosa, which remains intact for about 4 hr.29 During that time, allergen contact with the mucosa and consequently induction of the allergic reaction are reduced. This protective effect may multiply over time, as an initially less irritated mucosa is less prone to further provocation. Hence, during a constant allergen challenge, the difference in the total amount of pollen penetrating the mucosa and eliciting the allergic immune response in IP treated versus untreated participants becomes larger over time.
As IP acts only locally without any pharmacological effect, nasal spray represents an attractive alternative treatment option for mild-to-moderate allergic rhinitis or as a complementary therapy for moderate-to-severe allergic rhinitis. Safety, tolerability, and efficacy have previously been established in a pediatric population.30 Based on the physical, non-pharmacologic effect of the IP, safety and efficacy are generally expected to be similar across different populations and make the product suitable also for vulnerable populations like pregnant women, infants and children or patients with co-morbidities.
Strengths of this study include a crossover design, in which each patient acts as her/his own control, a random assignment to minimize possible effects from the order of treatment, and a zero-dropout rate. The 7-day washout period was considered sufficient to eliminate any effects of previous exposure to the allergen, as TNSS returned to baseline values at the beginning of each treatment period. A possible limitation of this study is the comparison of IP to CP and a “no treatment” group, which does not allow complete blinding. However, the use of a fully blinded comparator allows for an unbiased interpretation and confirms the findings of this study.
Conclusion
In conclusion, prophylactic treatment with the iota-carrageenan nasal spray IP alleviated nasal allergic symptoms and reduced nasal secretion in adults with grass pollen-induced allergic rhinitis. Based on adverse events and efficacy data, IP nasal spray can provide an effective, safe, and well-tolerated non-pharmacologic alternative for managing allergic rhinitis symptoms.
Data Sharing Statement
Data related to this manuscript can be made available from the corresponding author upon reasonable request.
Ethics Statement
The study was conducted in accordance with the ethical principles set forth in the Declaration of Helsinki and approved by the Ethics Committee of the City of Vienna (protocol code CAL_19_01, EK 19-276-1219).
Acknowledgments
This research was sponsored by Marinomed. Medical writing and editorial support were provided by Joana Enes (Gouya Insights).
Disclosure
NU, MM, and EP are employees of Marinomed. In addition, EP has a patent W02010000437 licensed to Sigmapharm Arzneimittel GmbH. PZ reports personal fees from ALK Abello, Allergopharma, Bencard, Leti, MadX, Roxall, Stallergenes, Thermo Fisher Scientific, outside the submitted work. MS reports having worked as a statistical consultant for Marinomed Biotech AG. The authors have no other competing interests in this work.
References
1. Bateman ED, Bousquet J, Keech ML, Busse WW, Clark TJH, Pedersen SE. The correlation between asthma control and health status: the GOAL study. Eur Respir J. 2007;29(1):56–63. doi:10.1183/09031936.00128505
2. Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nature Reviews Disease Primers. 2020;6(1):95. doi:10.1038/s41572-020-00227-0
3. Bauchau V, Durham SR. Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J. 2004;24(5):758–764. doi:10.1183/09031936.04.00013904
4. Bousquet J, Fokkens W, Burney P, et al. Important research questions in allergy and related diseases: nonallergic rhinitis: a GA2LEN paper. Allergy. 2008;63(7):842–853. doi:10.1111/j.1398-9995.2008.01715.x
5. D’Amato G, Cecchi L, Bonini S, et al. Allergenic pollen and pollen allergy in Europe. Allergy. 2007;62(9):976–990. doi:10.1111/j.1398-9995.2007.01393.x
6. Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49(1):32–40. doi:10.1373/49.1.32
7. Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. doi:10.1002/alr.22073
8. Schoenwetter WF, Dupclay L, Appajosyula S, Botteman MF, Pashos CL. Economic impact and quality-of-life burden of allergic rhinitis. Curr Med Res Opin. 2004;20(3):305–317. doi:10.1185/030079903125003053
9. Wise SK, Damask C, Roland LT, et al. International consensus statement on allergy and rhinology: allergic rhinitis - 2023. Int Forum Allergy Rhinol. 2023;13(4):293–859. doi:10.1002/alr.23090
10. Simons FER, Simons KJ. H1 antihistamines: current status and future directions. World Allergy Organ J. 2008;1(9):145–155. doi:10.1186/1939-4551-1-9-145
11. Scadding GK. Corticosteroids in the treatment of pediatric allergic rhinitis. J Allergy Clin Immunol. 2001;108(1 Suppl):S59–64. doi:10.1067/mai.2001.115568
12. Andersson M, Greiff L, Ojeda P, Wollmer P. Barrier-enforcing measures as treatment principle in allergic rhinitis: a systematic review. Curr Med Res Opin. 2014;30(6):1131–1137. doi:10.1185/03007995.2014.882299
13. Pacheco-Quito E-M, Ruiz-Caro R, Veiga M-D. Carrageenan: drug delivery systems and other biomedical applications. Mar Drugs. 2020;18(11):583. doi:10.3390/md18110583
14. Schütz D, Conzelmann C, Fois G, et al. Carrageenan containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol. 2021;320:L750–L756. doi:10.1152/ajplung.00552.2020
15. Große M, Ruetalo N, Businger R, et al. Evidence that quinine exhibits antiviral activity against SARS-CoV-2 infection in vitro; 2020.
16. Hebar A, Koller C, Seifert J-M, et al. Non-clinical safety evaluation of intranasal iota-carrageenan. PLoS One. 2015;10(4):e0122911. doi:10.1371/journal.pone.0122911
17. Mono chem-pharm Produkte GmbH. VisAlpin Instruction for Use. Available from: https://visalpin.at/wp-content/uploads/2018/03/Gebrauchsanweisung_Schnupfenspray_D-EN_12102020.pdf.
18. Day JH, Briscoe MP, Rafeiro E, Ellis AK, Pettersson E, Akerlund A. Onset of action of intranasal budesonide (Rhinocort aqua) in seasonal allergic rhinitis studied in a controlled exposure model. J Allergy Clin Immunol. 2000;105(3):489–494. doi:10.1067/mai.2000.104550
19. Devillier P, Le Gall M, Horak F. The allergen challenge chamber: a valuable tool for optimizing the clinical development of pollen immunotherapy. Allergy. 2011;66(2):163–169. doi:10.1111/j.1398-9995.2010.02473.x
20. Gross GN, Berman G, Amar NJ, Caracta CF, Tantry SK. Efficacy and safety of olopatadine-mometasone combination nasal spray for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2019;122(6):630–638.e3. doi:10.1016/j.anai.2019.03.017
21. Hermelingmeier KE, Weber RK, Hellmich M, Heubach CP, Mösges R. Nasal irrigation as an adjunctive treatment in allergic rhinitis: a systematic review and meta-analysis. Am J Rhinol Allergy. 2012;26(5):e119–25. doi:10.2500/ajra.2012.26.3787
22. Nasaleze International Ltd. Clinical research on Nasaleze; 2023. Available from: https://nasaleze.mk/wp-content/uploads/2020/10/Allergyl-Clinical-Trials-ENG.pdf.
23. Nehrig J, Grosse N, Hohenfeld IP, Hohlfeld JM, Badorrek P. Efficacy and safety of a drug-free, barrier-forming nasal spray for allergic rhinitis: randomized, open-label, crossover noninferiority trial. Int Arch Allergy Immunol. 2023;184(2):111–121. doi:10.1159/000526423
24. Stoelzel K, Bothe G, Chong PW, Lenarz M. Safety and efficacy of Nasya/Prevalin in reducing symptoms of allergic rhinitis. Clin Respirat J. 2014;8(4):382–390. doi:10.1111/crj.12080
25. Schwetz S, Olze H, Melchisedech S, Grigorov A, Latza R. Efficacy of pollen blocker cream in the treatment of allergic rhinitis. Arch Otolaryngol Head Neck Surg. 2004;130(8):979–984. doi:10.1001/archotol.130.8.979
26. Geisthoff UW, Blum A, Rupp-Classen M, Plinkert P-K. Lipid-based Nose Ointment for Allergic Rhinitis. Otolaryngol Head Neck Surg. 2005;133(5):754–761. doi:10.1016/j.otohns.2005.06.026
27. Aberg N, Dahl A, Benson M. A nasally applied cellulose powder in seasonal allergic rhinitis (SAR) in children and adolescents; reduction of symptoms and relation to pollen load. Pediatr Allergy Immunol. 2011;22(6):594–599. doi:10.1111/j.1399-3038.2011.01182.x
28. Emberlin JC, Lewis RA. A double blind, placebo-controlled cross over trial of cellulose powder by nasal provocation with Der p1 and Der f1. Curr Med Res Opin. 2007;23(10):2423–2431. doi:10.1185/030079907X231144
29. Graf C, Bernkop-Schnürch A, Egyed A, Koller C, Prieschl-Grassauer E, Morokutti-Kurz M. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. Int J Gen Med. 2018;11:275–283. doi:10.2147/IJGM.S167123
30. Fazekas T, Eickhoff P, Pruckner N, et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement Altern Med. 2012;12:147. doi:10.1186/1472-6882-12-147
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.