Back to Journals » Infection and Drug Resistance » Volume 16
Carbapenem-Resistant Pseudomonas aeruginosa Spondylodiscitis Treated with Ceftazidime-Avibactam: A Case Report with Literature Review
Authors Danda GJDN , Franco AC, Gomes EAP , Montanaro VVA , Martins BJAF, Viana Bonan de Aguiar V
Received 15 May 2023
Accepted for publication 3 August 2023
Published 14 August 2023 Volume 2023:16 Pages 5309—5317
DOI https://doi.org/10.2147/IDR.S421209
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Guilherme José da Nóbrega Danda,1 Andreia Craveiro Franco,1 Elisangela Ana Paula Gomes,2 Vinícius Vianna Abreu Montanaro,3 Bernardo José Alves Ferreira Martins,4 Vitor Viana Bonan de Aguiar5
1Department of Internal Medicine, SARAH Network of Rehabilitation Hospitals, Brasília, Federal District, Brazil; 2Department of Microbiology, SARAH Network of Rehabilitation Hospitals, Brasília, Federal District, Brazil; 3Department of Neurology, SARAH Network of Rehabilitation Hospitals, Brasília, Federal District, Brazil; 4Department of Radiology, SARAH Network of Rehabilitation Hospitals, Brasília, Federal District, Brazil; 5Department of Neurosurgery, SARAH Network of Rehabilitation Hospitals, Brasília, Federal District, Brazil
Correspondence: Guilherme José da Nóbrega Danda, Hospital Sarah Brasília, SMHS 501 Bloco A, Brasília, DF, 70335-901, Brazil, Tel +55613199-1111, Email [email protected]
Abstract: Pyogenic spondylodiscitis (PS) is a highly morbid and potentially fatal bacterial infection with an increasing incidence in recent decades. Its diagnosis and treatment are challenging, especially with the expansion of multidrug- or extensively drug-resistant bacteria. We report a rare case of PS caused by carbapenem-resistant Pseudomonas aeruginosa (CRPA) that was treated with ceftazidime-avibactam (C/A). The choice of C/A therapy was based on the patient’s bacterial sensitivity profile and intolerance to the initial therapeutic regimen (polymyxin B and meropenem). The total antimicrobial treatment time was seven weeks. The evolution of the clinical course met the cure criteria, which was characterized by remission of signs and symptoms, normalization of inflammatory markers, and radiological improvement over 18 months of clinical follow-up. This is a rare case of CRPA spondylodiscitis that responded to C/A treatment.
Keywords: antibiotic, β-lactam/β-lactamase inhibitor, resistance, vertebral osteomyelitis, bone and joint infection
Introduction
Pyogenic spondylodiscitis (PS), also called vertebral osteomyelitis or discitis, is a bacterial infection of the spine that is more common in men aged 50 years and older.1–5 Despite its relatively rare incidence (0.4 to 7.4 cases per 100,000 persons/year), population studies have indicated its expansion in recent decades, probably due to a combination of population aging, advances in diagnostic methods, and an increase in invasive spinal procedures.1–4,6–13
The most common causative agent of PS is Staphylococcus aureus (20–84%), followed by Gram-negative bacilli (GNB) (7–33%) and coagulase-negative Staphylococcus spp. (5–16%).5,12,13 Pseudomonas aeruginosa is a rare cause of PS (up to 6%) that has been classically reported in patients with diabetes mellitus, surgical wounds, immunosuppressive therapy, or in intravenous drug abusers.1,10,11 When empiric antimicrobial therapy is necessary (eg in cases showing hemodynamic instability, sepsis, or severe neurologic symptoms), guidelines recommend a combination of broad-spectrum antibiotics (eg intravenous vancomycin and a third- or fourth-generation cephalosporin) that provide coverage against staphylococci, including methicillin-resistant Staphylococcus aureus (MRSA), streptococci and GNB.12
Despite the growing importance of PS, the optimal management of this infection remains controversial, and patients continue to show severe clinical outcomes (death, chronic pain, and permanent motor or sensory deficits), especially elderly patients with multiple comorbidities or with a disseminated infectious process.7,12,14–16 This epidemiological profile is even more concerning considering the current worldwide scenario of expansion of multidrug-resistant (MDR) or extensively drug-resistant (XDR) bacteria, especially carbapenemase-producing GNBs, which have few antibiotic options with proven efficacy.17–20
Despite the increased incidence of these microorganisms in clinical practice, the literature only contains a few published cases of PS caused by MDR/XDR-GNBs. In addition, treatment of bone infections with ceftazidime-avibactam (C/A), a new antibiotic combination that acts against non-metallo-β-lactamase-producing GNB, is also limited to a few reports.17–19
Thus, the present study describes a rare case of PS caused by carbapenem-resistant Pseudomonas aeruginosa (CRPA) acquired after severe COVID-19 wherein the patient was clinically cured by treatment with C/A. We have also provided a narrative review of this topic.
Case Presentation
A 51-year-old Caucasian man with hypertension who had experienced severe COVID-19 illness in September 2020 was admitted on February 25, 2021, to a quaternary Brazilian public service specializing in neurological and musculoskeletal rehabilitation.
During the acute phase of COVID-19, the patient experienced acute respiratory distress syndrome and required mechanical ventilation. While undergoing intensive care, he showed the following major clinical complications: ventilator-associated pneumonia due to carbapenem-resistant Acinetobacter baumannii treated with ampicillin-sulbactam and polymyxin B (two-week therapy); central line-related bloodstream infection due to CRPA treated with two-week meropenem and polymyxin B therapy; sacral pressure injury; and critical illness polyneuropathy/myopathy. The total duration of hospitalization was 57 days.
Two months after hospital discharge, after the resolution of the acute complications of COVID-19, the patient developed insidious pain in the lumbar region and right hip. His condition progressively worsened, impairing his ambulation and leading to his referral to our hospital. He reported no fever or sphincter symptoms during this period.
On admission, the patient presented with severe pain on palpation and mobilization of the lumbar spine and moderate pain in the right hip. Muscle strength, reflexes, and sensitivity were globally normal. Laboratory tests showed a hemoglobin level of 12.1 g/dL, total leukocyte count of 8700 cells/mm³, erythrocyte sedimentation rate (ESR) of 120 mm/hr (normal range, 0–15 mm/hr), and C-reactive protein (CRP) level of 1.5 mg/dL (normal range, 0–0.5 mg/dL).
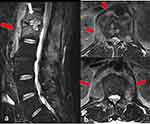
Computed tomography (CT) of the right hip showed periarticular calcifications typical of heterotopic ossifications. Magnetic resonance imaging (MRI) of the lumbar spine demonstrated bone edema in the T12 and L1 vertebral bodies associated with irregularity of the vertebral endplate with involvement of the psoas muscles, compatible with T12-L1 spondylodiscitis associated with psoitis (Figure 1). Two sets of blood cultures obtained after the MRI showed negative results.
On the basis of a hypothesis of PS with negative blood cultures, we performed a CT-guided bone biopsy on March 25, 2021, and sent the specimen for microbiological culture. Bacteria were identified using the Vitek® 2 equipment (bioMérieux, Marcy-l’Étoile, France). Antimicrobial-susceptibility testing was performed using the broth microdilution method with POLICIMBAC® (Probac, São Paulo, Brazil) for polymyxin B and the Etest® gradient strip (bioMérieux, Marcy-l’Étoile, France) for the other antimicrobials tested. Susceptibility minimum inhibitory concentration (MIC) breakpoints were determined using the Brazilian version (BrCast) of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) in force in 2021 (http://brcast.org.br).
The microbiological analysis revealed the growth of Pseudomonas aeruginosa sensitive only to polymyxin B (MIC = 2 mg/L) and C/A (MIC = 2 mg/L) and resistant to meropenem (MIC ≥ 16 mg/L), imipenem (MIC ≥ 16 mg/L), cefepime (MIC ≥ 64 mg/L), ceftazidime (MIC ≥ 64 mg/L), ciprofloxacin (MIC ≥ 4 mg/L), piperacillin-tazobactam (MIC ≥ 128 mg/L), tigecycline (MIC ≥ 8 mg/L), and amikacin (MIC ≥ 64 mg/L).
Since the patient’s condition was clinically stable, we decided to initiate antimicrobial treatment only after the result of the culture. Thus, on April 19, 2021, antibiotic therapy was initiated with polymyxin B (15,000 IU/kg IV every 12 hours) and meropenem (2 g IV every 8 hours in a 3-hour extended infusion). However, due to a widespread cutaneous rash associated with polymyxin B, on May 5, 2021, we switched the antimicrobial therapy to C/A (2.0 + 0.5 g IV every 8 hours) alone, and the dermatological lesions disappeared.
After starting C/A, the patient showed a progressive improvement in inflammatory markers and pain and progressive tolerance to rehabilitation exercises. No adverse events associated with C/A were observed. The total antimicrobial treatment time was seven weeks, ending on June 6, 2021, based on clinical and laboratory evaluations. For the heterotopic calcification of the right hip, we chose a conservative treatment based on nonsteroidal anti-inflammatory drugs and physical therapy, which resulted in improvement of hip pain.
The patient made a complete return to work and is being followed-up as an outpatient. The most recent clinical evaluation was performed on December 15, 2022. At the time, he was asymptomatic and tested negative for inflammatory markers. MRI of the lumbar spine revealed healing of the spondylodiscitis (Figure 2). Figure 3 presents the trends in inflammatory markers (ESR and CRP) throughout the clinical follow-up period.
 |
Figure 3 The curve of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) values during clinical follow-up. |
Discussion
We present a rare case and a narrative review of PS caused by CRPA treated with C/A. When the Medline database was searched on February 19, 2023, using the terms “ceftazidime-avibactam”, “discitis”, and “Pseudomonas aeruginosa”, we found no articles. When we searched the database only with “Pseudomonas aeruginosa” and “discitis”, we found ten references: of these, eight reported 91 cases of infectious spondylodiscitis, but none were caused by CRPA.21–28
PS is a challenging disease to diagnose and treat and is associated with high rates of morbidity and the potential for severe sequelae.1,2,6,9,12 In the present case, early diagnosis and pathogen-directed antimicrobial treatment based on microbiological test results were essential for the patient’s cure. Delays in diagnosing PS are common in medical practice, particularly in low- and middle-income countries, and have a negative impact on morbidity and lethality.1,29,30 In general, as observed in this case, infective spondylodiscitis should be considered in patients with back pain associated with risk factors (eg a history of previous infection) and an elevated ESR or CRP level.12 Fever associated with new neurological symptoms should also raise this suspicion.12
This report highlights a central line-related bloodstream infection caused by CRPA that occurred during the previous hospitalization (two months before the onset of spondylodiscitis symptoms) as a risk factor for PS. In the national and international scenarios, infections by carbapenem-resistant Gram-negative bacteria are emerging causes of healthcare-associated infections (HAIs) that represent a public health threat owing to their increasing incidence and higher lethality.20,31
Particularly in CRPA infections, a meta-analysis indicated that patients with CRPA bacteremia are three-fold more likely to die than those with carbapenem-sensitive Pseudomonas aeruginosa infection.32 Studies have also indicated that the prevalence of CRPA infection is higher in Latin America and Asia than in Europe, the United States, and Canada, possibly because of failures in infection control practices.32–34 These data, together with the findings in our case (CRPA in the bone fragment culture), indicate that the acquisition route of PS involved hematogenous spread from a central line-related bloodstream infection acquired during hospitalization for COVID-19 treatment.
In cases involving clinical suspicion for PS, guidelines recommend immediate imaging assessments. MRI is the modality of choice and can identify patients at risk of unfavorable clinical outcomes.1,12,35–38 In the present report, the absence of abscesses adjacent to the infected spine was a good prognostic factor favoring the response to antimicrobial treatment alone. Other factors associated with treatment failure described in the literature and absent in this case include patients aged 75 years or older, Staphylococcus aureus infection, diabetes mellitus, extra foci of osteomyelitis, fever, and persistence of pain or elevated inflammatory markers throughout therapy.2,12,38
For diseases with high morbidity rates that require prolonged antibiotic therapy, rapid identification of the etiologic agent is critical for successful management, especially in the current scenario of increasing infections by MDR/XDR bacteria, as illustrated in this case.1,36 Thus, this report highlights the value of CT-guided percutaneous spinal biopsy as an essential tool for microbiological diagnosis with a good yield and low adverse event rates.12,39 This procedure is considered the first invasive diagnostic step in the etiological investigation of patients suspected of having PS with negative blood culture results.12,39
Regarding the therapeutic aspects of PS, conservative treatment based on antimicrobial therapy is effective in most cases, and surgical interventions are reserved for patients with progressive neurological deficits, spinal instability, progressive deformities, and persistent pain despite appropriate antibiotic use.1,12,29 Remarkably, several surgical approaches have been described in the literature, all without high-quality evidence.1,12,29
In our patient, owing to the absence of neurological complications or other surgical indications, we opted for conservative treatment with antibiotics against Pseudomonas aeruginosa isolated from bone samples. In the management of PS, the antibiotic regimen should be tailored on the basis of the culture results, the ability of the antibiotics to penetrate the bone, and the patient’s tolerability profile.12,40,41 In the present case, we first chose a therapeutic regimen involving polymyxin B because of its increased clinical experience in treating MDR/XDR Gram-negative bacteria. However, the patient showed an allergic reaction, and based on the results of antimicrobial-susceptibility testing, we had to switch the therapy to C/A, an antibiotic that combines ceftazidime (a third-generation cephalosporin) with avibactam (a new non-β-lactam β-lactamase inhibitor) acting against Ambler class A (including extended-spectrum β-lactamases [ESBL] and Klebsiella pneumoniae carbapenemases [KPC]), class C, and some class D (including OXA-48) β-lactamases.17,18
C/A exhibits in vitro bactericidal activity against Pseudomonas aeruginosa MDR and Enterobacteriaceae MDR strains. It has been approved by the US Food and Drug Administration for treating complicated urinary tract infections, complicated intra-abdominal infections, and hospital/ventilator-acquired pneumonia.17 Despite the diffusion of ceftazidime and β-lactamase inhibitors in bone appears to be sufficient to promote a clinical response, the efficacy and safety of C/A in bone infections are not yet well known.17,42 A recent study by Davido et al, who induced osteomyelitis using carbapenem-resistant Klebsiella pneumoniae (CR-KP) in rabbits, demonstrated the ability of C/A to significantly reduce bone bacterial burden, alone or especially in combination with gentamicin.43
In the clinical setting, the effective use of C/A in a patient with spondylodiscitis was first reported in 2018 by Cani et al in a 60-year-old woman with a history of renal transplantation after undergoing a spinal surgical procedure.18 The etiologic agent isolated was a CR-KP (blakpc-positive isolate). On the basis of the results of sensitivity tests, C/A was initiated at 2.5 g IV every 8 hours. After 72 hours, because of worsening back pain and leukocytosis, the patient was subjected to an operative surgical washout and started on extended-interval amikacin in addition to C/A. However, owing to the good clinical evolution and the absence of synergism in the time–kill study, the aminoglycoside was discontinued after 13 days. The total treatment duration with C/A was six weeks.18
In 2018, three other reports of clinical success with C/A for treating bone infections were published.19,44,45 De León-Borrás et al reported a case involving a 36-year-old man who presented with vertebral osteomyelitis with a prevertebral abscess and bilateral psoas pyomyositis in the context of refractory bacteremia caused by a CR-KP. After the failure of therapeutic schemes involving carbapenems, polymyxin B, and amikacin, the authors described resolution of the infection after six-week C/A therapy.19
Schimmenti et al documented a case of a 26-year-old man with osteomyelitis of the right distal femur from a prosthetic joint infection that was also caused by a CR-KP.44 The organism was identified as a class A (KPC) carbapenemase enzyme producer. After a poor response to surgical interventions and therapies involving fosfomycin, colistin, sulfamethoxazole-trimethoprim, and tigecycline, the patient achieved clinical response with additional surgical debridement and C/A (2.5 g three times daily) for two weeks.44
Another case reported in 2018 by Mittal et al involved a 42-year-old man with CR-KP osteomyelitis of the right elbow.45 The isolate was both a producer of New Delhi metallo-β-lactamase-1 and Oxacillinase type-181 carbapenemase. The patient showed favorable outcomes after surgical intervention and combination therapy with C/A (2.5 g every 8 hours) and aztreonam (2 g every 8 hours) for 44 days. Although avibactam is ineffective against metallo-β-lactamase (MBL), the combination of C/A and aztreonam in this case was based on positive synergy testing performed by the authors.45 The explanation of this synergism relies on the preservation of aztreonam activity by the effectiveness of avibactam against both ESBL and carbapenemase.45,46
The synergistic effect of C/A and aztreonam in the treatment of bone infections caused by MBL-producing bacteria has also been reported by Mularoni et al.46 The patient was a 78-year-old diabetic woman with MBL-producing Pseudomonas aeruginosa sternal osteomyelitis following aortic valve replacement. After surgical treatment, based on time–kill curve analysis, triple antibiotic therapy with C/A (2.5 g every 8 hours), aztreonam (2 g every 8 hours), and amikacin (15 mg/kg/day) was administered for three weeks and resulted in clinical improvement. However, at month +12, the patient exhibited a recurrence of infection by the same bacteria, which required surgical reoperation and a four-week therapy with C/A, aztreonam, and amikacin; she subsequently showed no recurrence.46
In 2021, Ji et al also successfully reported the use of C/A for treating osteomyelitis.47 The report involved a child with CR-KP septic arthritis and primary hematogenous osteomyelitis in the right shoulder following cardiac surgery. The patient received C/A (50 mg/kg every 8 hours) for two weeks and showed no adverse events.47
In a retrospective French study, Rempenault et al evaluated nine patients with bone infections treated with C/A and reported a cure rate of 77.8%.17 Most infections were polymicrobial (77.8%), involving Enterobacteriaceae (77.8%) and Pseudomonas aeruginosa (44.4%) in addition to Gram-positive cocci (55.5%) and anaerobes (10.0%). Only one case of PS was included. Adverse events were observed in two patients (altered consciousness). The authors concluded that C/A therapy may be a salvage option for treating bone infections caused by MDR/XDR-GNB.17
In the present case, C/A was an effective and safe option for PS treatment. The choice of C/A was based exclusively on the results of antimicrobial-susceptibility testing because of the unavailability of molecular diagnostic methods at that time. However, we could deduce that the causative organism was probably a carbapenemase-producing Pseudomonas aeruginosa (ie strains with class A carbapenemase production) or an ESBL or AmpC producer with OprD porin deficiency and endowed with multidrug efflux pump systems. We also decided to administer monotherapy with C/A instead of combination therapy (eg C/A with aztreonam) based on previous reports of the clinical success of C/A monotherapy for treating osteomyelitis, the lack of high-quality evidence to support combination therapy in this setting, the local unavailability of aztreonam, and the resistance profile of the Pseudomonas aeruginosa isolated.17–19,44,47
The total antimicrobial treatment time chosen for our patient was based on his favorable clinical laboratory evaluation and was in accordance with current evidence that does not demonstrate additional advantages with prolonged antimicrobial therapy (>12 weeks) for the treatment of PS.2,12 Clinical cure was verified by complete resolution of the signs and symptoms of infection, normalization of inflammatory markers, radiological improvement, return of the patient to work activities, and absence of infection recurrence over 18 months of clinical follow-up.
This study had some limitations, such as the fact that it was a narrative review and some literature may have been missed. In addition, the lack of a molecular diagnosis may have influenced some of the conclusions of this study.
Conclusion
To our knowledge, this is the first report describing the clinical success of C/A for the treatment of CRPA spondylodiscitis in Brazil and one of the few reports in the literature describing the clinical cure of bone infections with this antibiotic. Thus, C/A therapy may be an effective and safe salvage treatment option for CRPA spondylodiscitis. The current scenario of expanding MDR/XDR-GNB infections reinforces the crucial role of spinal biopsy in the management of PS, since it can enable rapid identification of the etiological agent and, therefore, facilitate the correct choice of targeted antibiotic therapy. Prospective studies are required to assess the role of C/A in the treatment of bone infections.
Abbreviations
C/A, ceftazidime-avibactam; CR-KP, carbapenem-resistant Klebsiella pneumonia; CRP, C-reactive-protein; CRPA, carbapenem-resistant Pseudomonas aeruginosa; CT, computed tomography; ESBL, extended-spectrum β-lactamase; ESR, erythrocyte sedimentation rate; GNB, Gram-negative bacilli; HAIs, healthcare-associated infections; IV, intravenous; KPC, Klebsiella pneumoniae carbapenemases; MBL, metallo-β-lactamase; MDR, multidrug-resistant; MIC, minimum inhibitory concentration; MRI, magnetic resonance imaging; MRSA, methicillin-resistant Staphylococcus aureus; PS, pyogenic spondylodiscitis; XDR, extensively drug-resistant.
Data Sharing Statement
The data used and analyzed in this report are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The present study was submitted to and approved by the Human Research Ethics Committee of the SARAH Network of Rehabilitation Hospitals under protocol CAAE: 69132123.3.0000.0022 and is in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration. The patient signed the informed consent form for publication of the case details and any accompanying images.
Acknowledgments
The authors are grateful to their colleagues for the management of the patient and to the subject for participating in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. 2016;117(1):121–138. doi:10.1093/bmb/ldw003
2. Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385(9971):875–882. doi:10.1016/S0140-6736(14)61233-2
3. Da LY, Wong CB, Tsai TT, et al. Appropriate duration of post-surgical intravenous antibiotic therapy for pyogenic spondylodiscitis. BMC Infect Dis. 2018;18(1):468–476. doi:10.1186/s12879-018-3377-1
4. Waheed G, Soliman MAR, Ali AM, Aly MH. Spontaneous spondylodiscitis: review, incidence, management, and clinical outcome in 44 patients. Neurosurg Focus. 2019;46(1):E10. doi:10.3171/2018.10.FOCUS18463
5. Fantoni M, Trecarichi EM, Rossi B, et al. Epidemiological and clinical features of pyogenic spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl. 2):2–7.
6. Chong BSW, Brereton CJ, Gordon A, Davis JS. Epidemiology, microbiological diagnosis, and clinical outcomes in pyogenic vertebral osteomyelitis: a 10-year retrospective cohort study. Open Forum Infect Dis. 2018;5(3):ofy037. doi:10.1093/ofid/ofy037
7. Issa K, Diebo BG, Faloon M, et al. The epidemiology of vertebral osteomyelitis in the United States from 1998 to 2013. Clin Spine Surg. 2018;31(2):E102–E108. doi:10.1097/BSD.0000000000000597
8. Kehrer M, Pedersen C, Jensen TG, Lassen AT. Increasing incidence of pyogenic spondylodiscitis: a 14-year population-based study. J Infect. 2014;68(4):313–320. doi:10.1016/j.jinf.2013.11.011
9. Akiyama T, Chikuda H, Yasunaga H, Horiguchi H, Fushimi K, Saita K. Incidence and risk factors for mortality of vertebral osteomyelitis: a retrospective analysis using the Japanese diagnosis procedure combination database. BMJ Open. 2013;3(3):e002412. doi:10.1136/bmjopen-2012-002412
10. Costa J, de Andrade N, Arcangelo J, Pedrosa C, Figueira P. Espondilodiscite piogênica em adultos-diagnóstico e tratamento [Pyogenic spondylodiscitis in adults-diagnosis and treatment]. Rev Port Ortop e Traumatol. 2015;23(3):225–235.
11. Graña D, Gutiérrez MI, Torres D, Perendones M, Dufrechou C. Espondilodiscitis bacteriana inespecífica: una afección con incidencia creciente [Non Specific Bacterial Spondylodiscitis: an increasingly frequent condition]. Arch Med Interna. 2014;36(2):55–59.
12. Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26–e46. doi:10.1093/cid/civ482
13. Raghavan M, Lazzeri E, Palestro CJ. Imaging of spondylodiscitis. Semin Nucl Med. 2017;48(2):131–147. doi:10.1053/j.semnuclmed.2017.11.001
14. Lener S, Hartmann S, Barbagallo GMV, Certo F, Thomé C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir. 2018;160(3):487–496. doi:10.1007/s00701-018-3467-2
15. Boody BS, Tarazona DA, Vaccaro AR. Evaluation and management of pyogenic and tubercular spine infections. Curr Rev Musculoskelet Med. 2018;11(4):643–652. doi:10.1007/s12178-018-9523-y
16. Park K-H, Cho OH, Lee JH, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis. 2016;62(10):1262–1269. doi:10.1093/cid/ciw098
17. Rempenault C, Pagis V, Noussair L, et al. Treatment of bone and joint infections by ceftazidime/avibactam and ceftolozone/tazobactam: a cohort study. J Glob Antimicrob Resist. 2021;25:282–286. doi:10.1016/j.jgar.2021.04.003
18. Cani E, Moussavi F, Ocheretyaner E, Sharma R, Brown C, Eilertson B. Carbapenem-resistant Klebsiella pneumoniae vertebral osteomyelitis in a renal transplant recipient treated with ceftazidime-avibactam. Transpl Infect Dis. 2018;20(2):e12837. doi:10.1111/tid.12837
19. De León-Borrás R, Álvarez-cardona J, Vidal JA, Guiot HM. Ceftazidime/Avibactam for refractory bacteremia, vertebral diskitis/osteomyelitis with pre-vertebral abscess and bilateral psoas pyomyositis secondary to Klebsiella pneumoniae Carbapenemase-Producing Bacteria (KPC). P R Health Sci J. 2018;37(2):128–131.
20. Corbella L, Boán J, San-Juan R, et al. Effectiveness of ceftazidime-avibactam for treatment of infections due to Pseudomonas aeruginosa. Int J Antimicrob Agents. 2022;59(2):106517. doi:10.1016/j.ijantimicag.2021.106517
21. Menon KV, Sorour TMM. Epidemiologic and demographic attributes of primary spondylodiscitis in a Middle Eastern population sample. World Neurosurg. 2016;95:31–39. doi:10.1016/j.wneu.2016.07.088
22. Tonziello G, Valentinotti R, Stacul F, Giacomazzi D, Luzzati R. Spinal lesions by infectious spondylodiscitis and hepatocellular carcinoma presenting as spinal metastasis in an HIV-HCV co-infected patient. Infez Med. 2015;23(2):187–191.
23. Kdous M, Kraiem NE, Zhioua F, Ferchiou M. Pyogenic spondylodiscitis after laparoscopic sacral colpopexy with staples. Gynecol Obstet Fertil. 2015;43(6):478–480. doi:10.1016/j.gyobfe.2015.03.013
24. Dalwai R, Menon KV, Kumar RJ. Pyogenic diskitis of the L5-S1 disk space following inadvertent placement of a sacrocolpopexy screw. Int J Gynaecol Obstet. 2010;111(3):268–269. doi:10.1016/j.ijgo.2010.07.012
25. Nourbakhsh A, Garges KJ. Spondylodiscitis after vertebral fracture in the thoracic spine. Am J Orthop. 2009;38(10):E166–E169.
26. Koulaouzidis A, Christie E, Mitchell T, Bhat S. Spontaneous infectious spondylodiscitis (SIS) or pseudo-Pott’s disease. J Pak Med Assoc. 2007;57(9):474.
27. Gisserot O, Cremades S, Leyral G, Brisou P, de Jaureguiberry JP. Pseudo-Pott’s disease due to Pseudomonas aeruginosa. Presse Med. 2003;32(11):523–524.
28. Takeuchi H, Sakuma T, Fukuda Y, Harada J, Tada S. MR imaging of infected spondylodiskitis: interval signal change in vertebral body (including enhanced image of vertebral body). Nihon Igaku Hoshasen Gakkai Zasshi. 1995;55(8):555–561.
29. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl. 3):11–24. doi:10.1093/jac/dkq303
30. Mackenzie AR, Laing RBS, Smith CC, Kaar GF, Smith FW. Spinal epidural abscess: the importance of early diagnosis and treatment. J Neurol Neurosurg Psychiatry. 1998;65(2):209–212. doi:10.1136/jnnp.65.2.209
31. Allegranzi B, Tomczky S, Grayson ML, et al. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities. World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
32. Zhang Y, Chen XL, Huang AW, et al. Mortality attributable to carbapenem- resistant Pseudomonas aeruginosa bacteremia: a meta-analysis of cohort studies. Emerg Microbes Infect. 2016;5(3):e27. doi:10.1038/emi.2016.22
33. Gales AC, Jones RN, Turnidge J, Rennie R, Ramphal R. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY antimicrobial surveillance program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S146–S155. doi:10.1086/320186
34. Martins AF, Rosa GS, Saldanha GZ, et al. Epidemiologia dos microrganismos multirresistentes [Epidemiology of multidrug-resistant microorganisms]. In: Martins AF, Barros LS, editors. Prevenção de infecções por microrganismos multirresistentes em serviços de saúde [Prevention of infections by multidrug-resistant microorganisms in health services]. Brasília: Agência Nacional de Vigilância Sanitária; 2021:10–24.
35. Mchenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342–1350. doi:10.1086/340102
36. Park K-H, Cho OH, Jung M, et al. Clinical characteristics and outcomes of hematogenous vertebral osteomyelitis caused by gram-negative bacteria. J Infect. 2014;69(1):42–50. doi:10.1016/j.jinf.2014.02.009
37. Lemaignen A, Ghout I, Dinh A, et al. Characteristics of and risk factors for severe neurological deficit in patients with pyogenic vertebral osteomyelitis. Medicine. 2017;96(21):e6387. doi:10.1097/MD.0000000000006387
38. De Graeff JJ, Pereira NRP, Van Wulfftenpalthe OD, Nelson SB, Schwab JH. Prognostic factors for failure of antibiotic treatment in patients with osteomyelitis of the spine. Spine. 2017;42(17):1339–1346. doi:10.1097/MD.0000000000006387
39. Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218(1):211–214. doi:10.1148/radiology.218.1.r01ja06211
40. Falavigna A, Ferraz FAP. Espondilodiscite cervical espontânea causada por Salmonella typhi em paciente imunocompetente [Immunocompetent patient with spontaneous cervical spondylodiscitis caused by Salmonella typhi]. Arq Neuropsiquiatr. 2002;60(4):1034–1037. doi:10.1590/S0004-282X2002000600029
41. Feki A, Akrout R, Masmoudi K, et al. Infectious spondylodiscitis: a twenty-year experience from a single tertiary referral center. Egypt Rheumatol. 2018;41(3):231–235. doi:10.1016/j.ejr.2018.07.006
42. Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis. 2019;81:128–136. doi:10.1016/j.ijid.2019.02.005
43. Davido B, Crémieux AC, Vaugier I, et al. Efficacy of ceftazidime-avibactam in various combinations for the treatment of experimental osteomyelitis due to Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2023;61(1):106702. doi:10.1016/j.ijantimicag.2022.106702
44. Schimmenti A, Brunetti E, Seminari E, Mariani B, Cambieri P, Orsolini P. Prosthetic joint infection from carbapenemase-resistant Klebsiella pneumoniae successfully treated with ceftazidime-avibactam. Case Rep Infect Dis. 2018;2018:1854805. doi:10.1155/2018/1854805
45. Mittal J, Szymczak WA, Guo Y, et al. Two for the price of one: emerging carbapenemases in a returning traveller to New York City. BMJ Case Rep. 2018;2018:bcr–2018225440. doi:10.1136/bcr-2018-225440
46. Mularoni A, Mezzatesta ML, Pilato M, et al. Combination of aztreonam, ceftazidime-avibactam and amikacin in the treatment of VIM-1 Pseudomonas aeruginosa ST235 osteomyelitis. Int J Infect Dis. 2021;108:510–512. doi:10.1016/j.ijid.2021.05.085
47. Ji Z, Sun K, Li Z, Cheng W, Yang J. Carbapenem-resistant Klebsiella pneumoniae osteomyelitis treated with ceftazidime-avibactam in an infant: a case report. Infect Drug Resist. 2021;14:3109–3113. doi:10.2147/IDR.S32005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.