Back to Journals » Drug Design, Development and Therapy » Volume 17
CAR T Cell Therapy: Remedies of Current Challenges in Design, Injection, Infiltration and Working
Authors Zhu Y , Feng J , Wan R, Huang W
Received 20 March 2023
Accepted for publication 6 June 2023
Published 14 June 2023 Volume 2023:17 Pages 1783—1792
DOI https://doi.org/10.2147/DDDT.S413348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Yuxuan Zhu,1,2 Jianguo Feng,2 Rongxue Wan,2,3 Wenhua Huang2– 4
1The First Clinical Medical School, Southern Medical University, Guangzhou, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Digital Medicine and Biomechanics, National Key Discipline of Human Anatomy, School of Basic Medical Sciences, Southern Medical University, Guangzhou, People’s Republic of China; 3Affiliated Hospital of Guangdong Medical University, Zhanjiang, People’s Republic of China; 4Guangdong Engineering Research Center for Translation of Medical 3D Printing Application, Guangzhou, People’s Republic of China
Correspondence: Rongxue Wan; Wenhua Huang, Email [email protected]; [email protected]
Abstract: Chimeric antigen receptor (CAR) T cell therapy, as an innovative immunotherapy, plays a huge role in current cancer therapy. Although CAR T cell therapy has demonstrated therapeutic effects in some subtypes of B cell leukemia or lymphoma, there are many challenges that limit the therapeutic efficacy of CAR T cells in solid tumors. And how to efficiently transport CAR T cells to tumor tissues is a continuing concern for us. In this review, experiments have been extensively studied and compared. We finally compared the influence of different injection methods on therapeutic efficacy. We also carefully explored the difficulties of designing, homing, and working of CAR T cells, and ultimately came up with better solutions for each process to help CAR T cells reach tumor tissue more efficiently and quickly. These results will have significant implications for guiding CAR T cell therapy in cancer treatment.
Keywords: immunotherapy, CAR T cells, metastasis, infiltration, tumor microenvironment
Introduction
Chimeric antigen receptor (CAR) T cell therapy is a landmark immunotherapy in tumor therapy, since it has a very effective and lasting therapeutic effect and broad clinical application prospects. The CAR is a synthetic receptor consisting of an antigen recognition fraction and a T cell signal transduction domain that can redirect lymphocytes to identify and eliminate cells expressing specific target antigens.1 CAR T cell therapy is currently the most widely used form of this technology. CAR T cell therapy targeting the B cell marker CD19 has shown clear efficacy in a variety of hematologic malignancies such as B cell lymphoma and the lymphoblastic of acute lymphoblastic leukemia according to clinical trials.2–4 As early as 2017, the US Food and Drug Administration (FDA) launched the CAR T cell preparation for refractory large B lymphoma, and then various CAR T cell products for hematological tumors have been launched.2 On June 23, 2021, the CAR T cell therapy product relma‐cel (JWCAR029) was approved for marketing in China, becoming the first drug in China for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy.5 At the same time, scientists are developing new CAR engineering strategies to extend the clinical success of CAR T cells to patients with other malignancies.6 Studies have confirmed that CAR T cell therapy has a significant effect on the treatment of lung cancer and triple-negative breast cancer.7,8
Despite the remarkable success in treating hematologic tumors, CAR T cell therapy still faces many challenges in the treatment of solid tumors due to the difficulty in reaching and infiltrating the tumor.9 Theoretically, the modified T cells have a poor homing ability to the tumor sites, and a hostile tumor microenvironment (TME) containing many immunosuppressive cells. The other suppressors may impair the migrating CAR T cells’ cytotoxic.10 Helping CAR T cells homing more efficiently and quickly is a challenge that all current trials must address. Therefore, a comprehensive understanding of the various conditions that CAR T cells can encounter during their migration to the tumor site is absolutely necessary to advance the application of CAR engineering in cancer immunology. This review briefly describes a series of difficulties in transporting CAR T cells to the tumor site, including the specific mode of recognition / administration / exhaustion of CAR T cells. A comparative analysis of the efficacy of various injection routes is conducted in the article. In addition, we further explored the methods to optimize CAR structure / improve tumor microenvironment and genetic screening, so as to further promote the treatment of CAR T cells for solid tumors.
Recognition of Tumor Cell Surface Antigens by CAR T Cells
CAR T cell therapy is an emerging strategy for treating tumors by isolating and extracting T cells from human blood, modifying them in vitro by genetic engineering to increase their anti-tumor activity, at last infusing them back into patients after expansion. The first generation of CARs consisted of the extracellular antigen recognition single chain fragment variable (scFv), merging the transmembrane region and the intracellular signaling domain of the T cell receptor CD3ζ molecule.11 However, due to the slow expansion and poor persistence of the first generation CAR T cells, the scientists added an ITAM region from the co-stimulatory molecule CD28/CD137 to the intracellular segment of the first generation CAR T cells. After the extracellular antigen recognition region was combined with the target antigen, the T cells obtained both antibody-stimulating and co-stimulatory signals.12,13 It exerts direct tumor-killing effect and releases cytokines to recruit more immune cells. Currently, there are widely used bispecific T cell compound such as CD3 / CD19, CD19/CD20, CD5/CD7, etc.14–18 This dual recognition mechanism makes CAR T cell therapy more specific, better tumor-killing efficacy, and greatly reduces the risk of off-target toxicity.
The selection of tumor surface-specific antigens plays a pivotal role in the design of CAR T cells. That is, we need to find as much as possible an antigen only expressed on the surface of the tumor. In the early days, the site of this study mostly relied on databases and literature searches for these specific antigens. With the rapid development of gene sequencing technologies, as well as bioinformatics, WES and RNA sequencing of tumor tissues as well as normal tissues to contrasting the tumor-specific mutation sites are diffusely performed. Then, we can select for the predominant sequences with a strong affinity, as determined by HLA typing and the interaction with the specific antigen-TCR. Finally, the gene fragment with the strongest T cell activation was selected in vitro.19 At present, there have been corresponding clinical validation trials, including specific antigen sites for glioma NCT04749641, melanoma NCT03715985, and NCT03558945 in pancreatic cancer, which are widely conducted. Moreover it has been reported that the target PVRL4 of epithelial cancer can be obtained by suppression subtractive hybridization.20 Now, thanks to the in-depth study of representational difference analysis / suppression subtractive hybridization and continuity analysis of gene expression, scientists can isolate differentially expressed genes and thus discover gene segments closely related to malignancy.21–23
The Effects of Different Injection Methods on CAR T Cell Infiltration
It is not difficult to find that scientists are now investigating the effects of different injection methods on the efficacy of CAR T cell immunotherapy. In a study on the treatment of atypical teratoma and rhabdomyoma, the researchers used B7-H3.BB.z-CAR T cells to inject into the tumor (IT), ventricle (ICV) or caudal vein (IV) in order to treat the tumor.24 Finally ICV eradicated all formed tumors, with a very significant effect compared to other controls, whereas intravenous administration of the same dose of B7-H3.BB.z-CAR T cells did not cure any mice.24 Meanwhile, the study found that the delivery of CAR T cells by the IT and ICV pathway did not cause the elevation of systemic inflammatory cytokines. However, systemic inflammatory cytokines increased after IV delivery. Since intravenously injected CAR T cells activated monocytes in the circulatory system, it leads to the massive release of inflammatory cytokines IL-1 and IL-6 from monocytes and cause a cytokine storm (CRS).25–27 This will potentially cause severe necrosis of the tissue, so that CAR T cell transport is also closely related to the levels of systemic inflammatory cytokines. Different modes of administration may also therefore have a huge impact on the body’s immune function. In order to more clarify the impact of different injection methods on the treatment effect, firstly scientists need to clarify the approximate path of CAR T cells to the tumor under the three different administration methods, as shown in Figure 1.
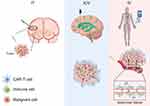 |
Figure 1 Transport paths of CAR T cells under different injection modes. Abbreviations: IV, intravenous injection; IT, intratumoral injection; ICV, intra-cerebroventricular injection. Note: By Figdraw. |
CAR T cells that are injected into the body via vein travel in the human vascular system. When it recognizes the specific receptors expressed by vascular endothelial cells, it will purposefully migrate out of the circulatory system and into the tissues. In the tissue, CAR T cells can look for traces of tumor everywhere. Once it recognizes the tumor mass, it can further increase in value, activate and finally break through the tumor envelope into the tumor.28 According to Figure 1, compared with the tumor area injection, the blood brain barrier is an additional obstacle for CAR T cells to reach the local tumor tissue of the central nervous system.29 It has been shown that CAR T cells first anchor activated white blood cell adhesion molecules on endothelial cells of the blood-brain barrier. CAR T cells then successfully cross the blood-brain barrier by sensing secondary waves, which are mostly mediated by intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, on the endothelial cells.30 When CAR T cells break through the blood brain barrier, they can reach the tumor site in the cerebrospinal fluid under the guidance of chemokines and try to penetrate the tumor surface capsule into the tumor tissue, and then overcome the role of immunosuppressive factors in the harsh tumor microenvironment, playing a tumor killing effect and trying to recruit more immune cells to achieve the effect of eliminating tumors. In recent years, it has been found that the migration of CAR T cells in the blood system leads to the loss of a large number of CAR T cells.24 The way of intratumoral injection has attracted extensive attention. It not only avoids the loss of CAR T cells caused by long-distance migration, but also helps to reduce the systemic toxicity. Some tumors in other special parts can also be injected through the third space, such as the cerebrospinal fluid of central nervous system tumors and the pleural cavity of lung tumors.24,31 In all dosing regimens, CAR T cells may encounter the following common problems in the above path:1 Because the tumor tissue surface is coated with the extracellular matrix, the first step of CAR T cells infiltration is to penetrate this layer of barrier.32 It has been found that expressing heparanase in CAR T cells can cleave the main component of heparan sulfate chain in the extracellular matrix, thus breaking through the tumor surface envelope, and it does not affect the active of CAR T cells.2,33,34 Abnormal vasculature and high interstitial hydraulic pressure will reduce the ability of CAR T cells to play an anti-tumor effect in view of the adverse environment in the tumor. The experiment with a method of photothermal ablation in tumor tissue can use near infrared light irradiation to produce effective heat to burn tumor cells and can improve the curative effect of CAR T cell tumor killing.35 Another nanoengineering strategy through CAR T cell biohybridization not only improves the internal environment of tumor tissue to retain the original function of CAR T cells, but also has the ability of fluorescent tracing and microenvironment remodeling, which allows CAR T cells to perform within tumor tissue.36
Having defined the pathway of CAR T cells into the tumor, attention should be paid to trying to compare the advantages and disadvantages of various injection modalities in order to find a much more suitable regimen for CAR T cell therapy. Some of the current clinical studies were collated and as shown in Table 1.
 |
Table 1 Comparison of the Advantages and Disadvantages of Different Injection Methods Within Various Tumors |
According to Table 1, CAR T cells need to travel further away to reach the tumor when systemic i. v. On the one hand, this process will inevitably produce some quantity and time consumption. To achieve the same treatment, intravenous often takes several times or more doses and longer time to work.24,31,37 On the other hand, when the CAR T cells pass through the normal tissues that coexpress the surface antigen with the tumor, they may also exert a killing effect, leading to systemic toxicity outside of the target.24,39 But it also has the advantage that it can be more comprehensive in tumor removal (NCT03146234). So sometimes we need to weigh the pros and cons of choosing the injection method. Furthermore, intravenous administration can also cause a cytokine release syndrome, which may be fatal to the body.24,41
For intracranial tumors, compared to intraventricular injection, intratumor injection limitation is strong, that is to say, intratumor injection may only have killing effect on local tumor and for some distant tumor tissue killing effect is weak, since in intraventricular injection we can also find it for tumor cells in the spine can also have the effect of clearing.37 Both of them are regional injections, which can not only achieve effective treatment at the minimum dose but also avoid systemic toxicity and generate immune memory to maintain the effect.24,37
In addition to comparisons in theoretical efficacy, we should also focus on the practical operational difficulties of these injection pathways. Intravenous administration requires intravenous catheter administration, and intraventricular administration requires ventricular puncture. These operations often require clinicians to perform in places with certain requirements, so the requirements for location and operators are higher.24,37 Regional delivery of high pressure and low pressure requires a series of regulation of pressure.40 Moreover, the way difficulty of intratumoral in situ injection is also greatly increased due to the complexity of the intratumoral vasculature. In contrast, for common primary tumors, regional administration is a more ideal way of administration for both therapeutic effects and adverse effects (NCT03198546).
Strategies for Optimizing CAR T Cell Homing
Promoting the Expression of Chemokines
Chemokines act as a similar tour guide in directing the CAR T cells to the tumor site, providing a forward direction for the CAR T cells. Different tumor cells may secrete different chemokine.42 When it interacts with the chemokine receptors on the CAR T cells, it can recruit the CAR T cells into the tumor. We have now attempted to develop CAR T cells coexpressing chemokine receptors to promote the efficient progression of CAR T cells towards the tumor site.43–45 Malignant pleural mesothelioma highly secretes the chemokine CCL2. The transduction of the chemokine receptor CCR2b into mesoCAR T cells using lentiviral vectors can significantly increase the number of CAR T cells at tumor sites and significantly enhance the anti-tumor efficacy, capable of treating established mesothelin-expressing tumor.46 A variety of tumor cells, such as pancreatic and ovarian cancers, can secrete IL-8 and can be detected in the circulation of the mouse models. IL-8 can act on the G protein-coupled receptor CXCR2 to recruit downstream signaling proteins and activate second messenger-mediated signalling. CXCR2 can chemotaxis a range of biological effects such as wandering and degranulation of neutrophils and lymphocytes. This biological effect plays a huge role in multiple inflammatory responses, cell development and the proliferation of tumor cells. Therefore, IL-8 and CXCR2 are very important for the construction of malignant tumor microenvironment.47 CAR T cells using targeted αvβ 6 integrins co-expressing CXCR1 and CXCR2 were able to migrate more efficiently toward IL-8 maintaining cytolytic activity, and significantly improved therapeutic efficacy in pancreatic and ovarianed xenograft models.48
Enhancing the Expression of Vascular Endothelin Receptor
Vascular endothelial selectin receptors play a sentinel-like role in the homing of CAR T cells distributed in circulation in specific paths, directing it to the specific sites by binding to vascular endothelial selectin ligands expressed by CAR T cells.49,50 Circulating cells have been found to transport to the bone marrow when the tetra-sugar sialic acid-Lewis X-modified E (sLeX) glycosylated) binds to E-selectin receptors expressed in the vascular endothelium.51 When the CAR T cell-autonomous sLeX expression/E-selectin binding capacity is enhanced, it can be bound to E-selectin receptors in vascular endothelin, thus enabling it to transport to the bone marrow in the circulating blood.
Transforming the Tumor Microenvironment
Although various targets can be artificially created from CAR T cells to the tumor site to guide CAR T cells as far as possible, the inhibitory effect of tumor microenvironment is a great obstacle for CAR T cells.52 Specific performance is: The tumor site secretion of transforming growth factor β (TGF β) or IL-10 immune suppressive cytokines, such as regulatory T cells (Tregs) or myeloid suppressive cells and PD-L1 on the cell surface expression and immunosuppressed metabolic environment may be on CAR T cells in the tumor site aggregation and exert a powerful inhibitory effect.53 To overcome this limitation, we reviewed strategies: (1) directly counteracting immunosuppressive factors, such as transgene expression of IL-15 in cytotoxic T cells counteracting Treg-mediated immunosuppression.54 (2) improving the metabolic environment of T cells in TME, such as timely supplementation of amino acids critical for T cell function, such as arginine and glutamine.55,56 (3) The pretreatment method was used to promote the colonization of CAR T cells.57 It has been shown that pretreatment with oxaliplatin (Ox) and cyclophosphamide (Cy) contributes to CAR T cells accumulate within the tumor and enhances the disease response to immune checkpoint suppression. The reason behind this may be that oxaliplatin induced immunogenic tumor cell death, which promotes the local inflammatory process, while oxaliplatin reverses STAT6-mediated immunosuppression, which upregulates the susceptibility of cytotoxic T cells and eventually leads CAR T cells into the tumor site. But it should be noted that the combinatorial approach of pretreatment drugs is a key for the success of this pathway.58
However, the tertiary lymphatic structure in the field of immunity has attracted much attention. The tertiary lymphoid structure is composed of T cell regions with endothelial system. It can recruit various immune cells and induce differentiation of mature T cells and B cells, presenting antigen to play antitumor effects. The presence of an additional tertiary lymphatic structure has a clear predictive effect on the prognosis of cancer. According to the current study, it can be found in various solid tumors, such as hepatocellular carcinoma and renal cell carcinoma et all.59,60 Studies have confirmed that the tertiary lymphoid structure contributes to the infiltrating of CAR T cells.61 It promotes the infiltration of CAR T cells in the following aspects: (1) it is rich in immune cells for presenting antigens and a stable endothelial system. This suggests that it can support CAR T cells for survival in the tumor microenvironment. (2) TLS can promote the continuous influx of naive immune cells to maintain immunity. This would greatly expand the effect of CAR T cells.62 We can stimulate the maturation of tertiary lymphatic structures to enhance CAR T cell infiltration and cooperate with CAR T cells to clear tumor tissue. Multiple approaches have been developed to stimulate the generation of tertiary lymphatic structures, including promoting vascular normalization in the therapeutic melanoma microenvironment to promote tertiary lymphatic structure formation through treatment with STING agonists.63 It is also noteworthy that the application of a scaffold of a large number of biomaterials can induce the formation of tertiary lymphatic structures by allowing the infiltration of immune cells, including artificial collagen matrix and hydrogel.64,65 In conclusion, the tertiary lymphoid structures are a trusted ally of CAR T cells.
Improving Cytokine Release and Immune Memory
In the process of tumor killing, the ability of locally infiltrating CAR T cells alone may not be enough to fully eliminate, so CAR T cells are required to express more cytokines to recruit more immune cells (such as dendritic cells, T cells, etc.) into the tumor for common cooperation. Currently, the cytokines that can be successfully expressed are IL-7 / CCL19 / CCL21 / IL-12 / CXCL11 and CCR2b.66–71
Temporary drug action can lead to substantial tumor reduction. But the residence time of drugs in the human body is limited. Failure of the CAR T cells is also inevitable Clearing tumor tissue in the short term is not our ultimate goal. Some scientists are trying to study the methods to give CAR T cells endogenous immunity by analyzing the exhaustion mechanism of CAR T cells.72 Currently the immune memory capacity can be achieved by delivering immunostimulants such as RN7SL1, STING, ect.73–75 They activate endogenous RNA and promote CAR T cell proliferation and effector memory differentiation. This can enhance the effect of the drug in the body and help maintain the anti-tumor effect.
Conclusion
CAR T cell therapy has been a great success in some clinical trials against hematological malignancies, but the degree of aggregation and activity in solid tumor sites is a huge challenge. In order to improve the efficacy of CAR T cell therapy, feasible solutions were proposed in detail from the aspects of design, injection, infiltration and working of CAR T cells. For the infiltration degree of CAR T cells, we can guide CAR T cells to the target aggregation more often by finding more specific and abundant surface antigens. Secondly, we need to help CAR T cells overcome the immunosuppression of the local tumor microenvironment to facilitate the smooth entry of CAR T cells into the tumor interior. The most advanced approach is to construct tertiary lymphatic structures. In addition, we can better guide CAR T cells into the tumor by promoting the expression of chemokines, etc. In our careful study, we found that the efficacy and side effects of CAR T cell therapy varied with different injection methods in the current clinical studies. However, there are few comparative analyses on the specific efficacy of each injection route. In this context, we also focused on analyzing the impact of various injection methods on CAR T cell transport, which is inspired by the understanding of the CAR T cell transport path. Every step of the research we do is designed to contribute to improving anti-tumor efficacy. Although the challenges remain, we want newer strategies and solutions to create a safer and more effective treatment modality.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the National Natural Science Foundation of China (NO.82101930, to R.W.), National Natural Science Foundation of China (NO. 61427807, to W.H.) and Discipline construction project of Guangdong Medical University (G622280009, 4SG22260G to W.H). This work was also supported by the China Postdoctoral Science Foundation (No. 2019M662985, to Jianguo Feng).
Disclosure
The authors report no conflicts of interest in this work.
References
1. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73.
2. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–2544.
3. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448.
4. Park JH, Rivière I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449–459.
5. Ying Z, Yang H, Guo Y, et al. Relmacabtagene autoleucel (relma-cel) CD19 CAR-T therapy for adults with heavily pretreated relapsed/refractory large B-cell lymphoma in China. Cancer Med. 2021;10(3):999–1011.
6. Tian Y, Li Y, Shao Y, Zhang Y. Gene modification strategies for next-generation CAR T cells against solid cancers. J Hematol Oncol. 2020;13(1):54.
7. Jie Y, Liu G, Feng L, et al. PTK7-Targeting CAR T-Cells for the Treatment of Lung Cancer and Other Malignancies. Front Immunol. 2021;12:665970.
8. Yang P, Cao X, Cai H, et al. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell Immunol. 2021;360:104262.
9. Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139.
10. Lindo L, Wilkinson LH, Hay KA. Befriending the Hostile Tumor Microenvironment in CAR T-Cell Therapy. Front Immunol. 2020;11:618387.
11. Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398.
12. Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32(7):689–702.
13. Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21(3):145–161.
14. Goebeler ME, Bargou R. Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk Lymphoma. 2016;57(5):1021–1032.
15. Löffler A, Kufer P, Lutterbüse R, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95(6):2098–2103.
16. Robinson HR, Qi J, Cook EM, et al. A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood. 2018;132(5):521–532.
17. Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol Res. 2016;4(6):498–508.
18. Dai Z, Mu W, Zhao Y, et al. T cells expressing CD5/CD7 bispecific chimeric antigen receptors with fully human heavy-chain-only domains mitigate tumor antigen escape. Signal Transduction Targeted Therapy. 2022;7(1):85.
19. Zhang Q, Jia Q, Zhang J, Zhu B. Neoantigens in precision cancer immunotherapy: from identification to clinical applications. Chin Med J. 2022;135(11):1285–1298.
20. Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016;76(10):3003–3013.
21. Sasheva P, Grossniklaus U. Differentially Methylated Region-Representational Difference Analysis (DMR-RDA): a Powerful Method to Identify DMRs in Uncharacterized Genomes. Methods Mol Biol. 2017;1456:113–125.
22. Xu K, Zhang YY, Han B, et al. Suppression subtractive hybridization identified differentially expressed genes in colorectal cancer: microRNA-451a as a novel colorectal cancer-related gene. Tumour Biol. 2017;39(5):1010428317705504.
23. Castellanos-Rueda R, Di Roberto RB, Schlatter FS, Reddy ST. Leveraging Single-Cell Sequencing for Chimeric Antigen Receptor T Cell Therapies. Trends Biotechnol. 2021;39(12):1308–1320.
24. Theruvath J, Sotillo E, Mount CW, et al. Locoregionally administered B7-H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med. 2020;26(5):712–719.
25. Majzner RG, Ramakrishna S, Yeom KW, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603(7903):934–941.
26. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55.
27. Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021;56(3):552–566.
28. June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365.
29. Huang X, Hussain B, Chang J. Peripheral inflammation and blood-brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. 2021;27(1):36–47.
30. Kanda K, Hayman GT, Silverman MD, Lelkes PI. Comparison of ICAM-1 and VCAM-1 expression in various human endothelial cell types and smooth muscle cells. Endothelium. 1998;6(1):33–44.
31. Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151.
32. Li M, Wang Y, Li M, Wu X, Setrerrahmane S, Xu H. Integrins as attractive targets for cancer therapeutics. Acta pharmaceutica Sinica B. 2021;11(9):2726–2737.
33. de Mestre AM, Staykova MA, Hornby JR, Willenborg DO, Hulett MD. Expression of the heparan sulfate-degrading enzyme heparanase is induced in infiltrating CD4+ T cells in experimental autoimmune encephalomyelitis and regulated at the level of transcription by early growth response gene 1. J Leukoc Biol. 2007;82(5):1289–1300.
34. Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21(5):524–529.
35. Chen Q, Hu Q, Dukhovlinova E, et al. Photothermal Therapy Promotes Tumor Infiltration and Antitumor Activity of CAR T Cells. Adv Mater. 2019;31(23):e1900192.
36. Chen Z, Pan H, Luo Y, et al. Nanoengineered CAR-T Biohybrids for Solid Tumor Immunotherapy with Microenvironment Photothermal-Remodeling Strategy. Small. 2021;17(14):e2007494.
37. Priceman SJ, Tilakawardane D, Jeang B, et al. Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2(+) Breast Cancer Metastasis to the Brain. Clin Cancer Res. 2018;24(1):95–105.
38. Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proce National Acad Sci. 2009;106:3360–3365.
39. Nellan A, Rota C, Majzner R, et al. Durable regression of Medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J Immunother Cancer. 2018;6(1):30.
40. Chai LF, Hardaway JC, Heatherton KR, et al. Antigen Receptor T Cells (CAR-T) Effectively Control Tumor Growth in a Colorectal Liver Metastasis Model. J Surgical Res. 2022;272:37–50.
41. Hu Y, Sun J, Wu Z, et al. Predominant cerebral cytokine release syndrome in CD19-directed chimeric antigen receptor-modified T cell therapy. J Hematol Oncol. 2016;9(1):70.
42. Mollica Poeta V, Massara M, Capucetti A, Bonecchi R. Chemokines and Chemokine Receptors: new Targets for Cancer Immunotherapy. Front Immunol. 2019;10:379.
43. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38.
44. Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75.
45. Song DG, Ye Q, Poussin M, Harms GM, Figini M, Powell DJ. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood. 2012;119(3):696–706.
46. Moon EK, Carpenito C, Sun J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17(14):4719–4730.
47. Liu K, Wu L, Yuan S, et al. Structural basis of CXC chemokine receptor 2 activation and signalling. Nature. 2020;585(7823):135–140.
48. Whilding LM, Halim L, Draper B, et al. CAR T-Cells Targeting the Integrin αvβ6 and Co-Expressing the Chemokine Receptor CXCR2 Demonstrate Enhanced Homing and Efficacy against Several Solid Malignancies. Cancers. 2019;11(5):54.
49. Mondal N, Silva M, Castano AP, Maus MV, Sackstein R. Glycoengineering of chimeric antigen receptor (CAR) T-cells to enforce E-selectin binding. J Biol Chem. 2019;294(48):18465–18474.
50. Watson HA, Durairaj RRP, Ohme J, et al. L-Selectin Enhanced T Cells Improve the Efficacy of Cancer Immunotherapy. Front Immunol. 2019;10:1321.
51. Sánchez-Martínez D, Gutiérrez-Agüera F, Romecin P, et al. Enforced sialyl-Lewis-X (sLeX) display in E-selectin ligands by exofucosylation is dispensable for CD19-CAR T-cell activity and bone marrow homing. Clin Transl Med. 2021;11(2):e280.
52. Boulch M, Cazaux M, Loe-Mie Y, et al. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci Immunol. 2021;6:57.
53. Iwahori K. Cytotoxic CD8(+) Lymphocytes in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1224:53–62.
54. Hurton LV, Singh H, Najjar AM, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A. 2016;113(48):E7788–e97.
55. Martín-Otal C, Navarro F, Casares N, et al. Impact of tumor microenvironment on adoptive T cell transfer activity. Int Rev Cell Mol Biol. 2022;370:1–31.
56. Nachef M, Ali AK, Almutairi SM, Lee SH. Targeting SLC1A5 and SLC3A2/SLC7A5 as a Potential Strategy to Strengthen Anti-Tumor Immunity in the Tumor Microenvironment. Front Immunol. 2021;12:624324.
57. Nian Z, Zheng X, Dou Y, et al. Rapamycin Pretreatment Rescues the Bone Marrow AML Cell Elimination Capacity of CAR-T Cells. Clin Cancer Res. 2021;27(21):6026–6038.
58. Davies DM, Maher J. Crosstown Traffic: lymphodepleting Chemotherapy Drives CAR T Cells. Cancer Cell. 2021;39(2):138–140.
59. Calderaro J, Petitprez F, Becht E, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70(1):58–65.
60. Meylan M, Petitprez F, Becht E, et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022;55(3):527–41.e5.
61. Johansson-Percival A, Ganss R. Therapeutic Induction of Tertiary Lymphoid Structures in Cancer Through Stromal Remodeling. Front Immunol. 2021;12:674375.
62. Gago da Graça C, van Baarsen LGM, Mebius RE. Tertiary Lymphoid Structures: diversity in Their Development, Composition, and Role. J Immunol. 2021;206(2):273–281.
63. Chelvanambi M, Fecek RJ, Taylor JL, Storkus WJ. STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment. J Immunother Cancer. 2021;9(2):856.
64. Suematsu S, Watanabe T. Generation of a synthetic lymphoid tissue-like organoid in mice. Nat Biotechnol. 2004;22(12):1539–1545.
65. Löwenberg C, Balk M, Wischke C, Behl M. Shape-Memory Hydrogels: evolution of Structural Principles To Enable Shape Switching of Hydrophilic Polymer Networks. Acc Chem Res. 2017;50(4):723–732.
66. Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol. 2018;36(4):346–351.
67. Pang N, Shi J, Qin L, et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14(1):118.
68. Luo H, Su J, Sun R, et al. Coexpression of IL7 and CCL21 Increases Efficacy of CAR-T Cells in Solid Tumors without Requiring Preconditioned Lymphodepletion. Clin Cancer Res. 2020;26(20):5494–5505.
69. Agliardi G, Liuzzi AR, Hotblack A, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun. 2021;12(1):444.
70. Gao Q, Wang S, Chen X, et al. Cancer-cell-secreted CXCL11 promoted CD8(+) T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC. J Immunother Cancer. 2019;7(1):42.
71. Li G, Zhang Q, Han Z, et al. IL-7 and CCR2b Co-Expression-Mediated Enhanced CAR-T Survival and Infiltration in Solid Tumors. Front Oncol. 2021;11:734593.
72. Pietrobon V, Todd LA, Goswami A, Stefanson O, Yang Z, Marincola F. Improving CAR T-Cell Persistence. Int J Mol Sci. 2021;22(19):123.
73. Johnson LR, Lee DY, Eacret JS, Ye D, June CH, Minn AJ. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell. 2021;184(19):4981–95.e14.
74. Xu N, Palmer DC, Robeson AC, et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. 2021;218(2):64.
75. Ji F, Zhang F, Zhang M, et al. Targeting the DNA damage response enhances CD70 CAR-T cell therapy for renal carcinoma by activating the cGAS-STING pathway. J Hematol Oncol. 2021;14(1):152.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
