Back to Journals » Nutrition and Dietary Supplements » Volume 15
Cannabidiol (CBD) Upregulates Vitamin D3 Receptors (VDRs) Expression That Modulates Cytokines (TNF-α, IL-6), Tissue Elasticity, Cellular Senescence, and Mitochondrial ATP Generation in Human and Rodent Cell Lines
Authors Trivedi MK, Branton A, Trivedi D, Mondal S, Jana S
Received 14 August 2023
Accepted for publication 28 October 2023
Published 6 November 2023 Volume 2023:15 Pages 91—100
DOI https://doi.org/10.2147/NDS.S435447
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Mahendra Kumar Trivedi,1 Alice Branton,1 Dahryn Trivedi,1 Sambhu Mondal,2 Snehasis Jana2
1Trivedi Global, Inc, Research & Development, Henderson, Nevada, USA; 2Trivedi Science Research Laboratory Pvt. Ltd, Research & Development, Thane (W), Maharashtra, India
Correspondence: Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd, Thane (W), Maharashtra, India, Tel +91-022-25811234, Email [email protected]
Background: Cannabidiol (CBD) is a non-psychoactive cannabinoid derived from Cannabis sativa L. with very low toxicity for human and a wide variety of therapeutic uses as medicine. The study objective is to evaluate the impact of CBD on vitamin D3 receptor (VDR) protein expressions, tissue elasticity, anti-inflammatory, and anti-senescence activity in human and rodent cell lines.
Methods: Cell viability was estimated by MTT assay. Relative quantification (RQ) of VDR protein expression was measured by RT-PCR. Tissue elasticity was measured by atomic force microscopy (AFM). Cellular senescence and ATP were performed using trypan blue exclusion test and colorimetric assay, respectively.
Results: Cell viability assay data showed CBD was safe and nontoxic upto 7.5 μM. The VDR protein expression was significantly increased by 109.71% (p = 0.013), 236.96% (p ≤ 0.001), 170% (p ≤ 0.001), 100% (p = 0.019), 80% (p = 0.021), 427.27% (p ≤ 0.001), 366.67% (p ≤ 0.001), 56.31% (p = 0.016), and 63.84% (p ≤ 0.001) in MG-63, MDA-MB-231, SH-SY5Y, HEK-293, HT-29, EaHy-926, HepG2, A-549, and C2C12 cells, respectively compared to the normal control group. CBD treatment significantly reduced the levels of TNF-α (46.58%; p ≤ 0.049) and IL-6 (43.61%; p ≤ 0.001) at CBD-5 μM compared to the vehicle control group. Tissue elasticity was significantly (p ≤ 0.001) increased by 37.09% and 49.49% in CBD-2.5 and CBD-5 μM, respectively, compared to the vehicle control group. Significantly (p ≤ 0.001) reduced senescence cells by 39.22% in CBD-5 μM than the vehicle control group. The level of ATP was significantly (p ≤ 0.001) increased by 90.55, 117.06, and 153.54% in CBD-1, CBD-2.5, and CBD-5 μM, respectively, compared to the vehicle control group.
Conclusion: Overall, data suggest that CBD considerably improved VDR protein expression, inflammation, cell growth, tissue elasticity, and enhanced mitochondrial bioenergetics in multiple cell lines. In this study, for the first time, we showed the evidence suggesting that the VDR plays a critical and multifaceted role in various types of human cells.
Keywords: cannabidiol, inflammation, elasticity, VDR expression, senescence, ATP
Graphical Abstract:
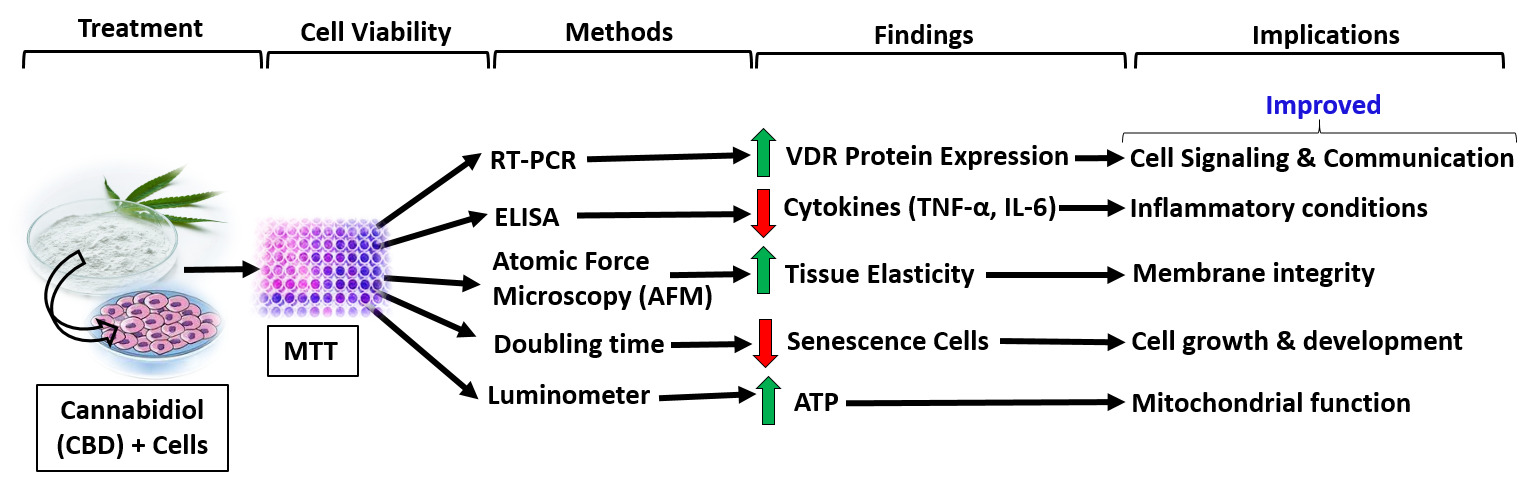
Introduction
Cannabidiol (CBD) is extensively used for various health problems and marketed as a dietary and wellness product. It does not cause intoxication or euphoria-like Δ9-tetrahydrocannabinol (THC). This favorable safety profile recently increased massive interest in using CBD products to manage various disorders.1 Various diseases like myocardial infarction, sickle cell anemia, diabetes, myocardial ischemia, peripheral artery disease, and cerebral ischemia occur due to abnormalities of blood viscosity, elasticity, and RBC morphology. One of the clinical trial data reported that CBD increased red blood cell viscosity and elasticity.2 CBD has potent anti-inflammatory,3 immunomodulatory, and analgesic effects through activating cannabinoid-1 and − 2 (CB1 and CB2) receptors. CBD treatment also reduced the production of cytokines in an animal model of chronic asthma, inflammatory lung diseases, etc.4 The preclinical animal experiment showed that CBD treatment decreases the levels of Th1 cytokines (TNF-α and IL-6) and modulates the cytokine storm.5 An in vitro experiment reported that CBD can reduce inflammatory cytokine production.6 Abnormal mitochondrial function leads to the development of a wide variety of neurodegenerative, diabetes mellitus, and cardiovascular disorders. CBD modulates mitochondrial activities through voltage-dependent anion channel-I, which functions to exchange important metabolites involved in energy metabolism (ie, ADP and ATP) across the outer mitochondrial membrane.7 Further, existing study data mentioned that prolonged treatment of 10 μM CBD can induce cellular senescence.8 Cellular senescence has harmful effects as it can hinder tissue repair and regeneration due to the accumulation of senescent cells, depletion of stem/progenitor cell compartments, and secretion of senescence-associated secretory phenotype (SASP).9 Additionally, CBD increases cell growth in a dose-dependent manner, while significantly decreasing cellular senescence as measured by beta-galactosidase activity within the range of 0.5 μM to 2.0 μM.10,11 Thomas et al (2007) reported that CBD has the ability to behave like a CB2 receptor inverse agonist and may contribute to its documented anti-inflammatory properties.12 Researcher also reported that in-house CB2 receptor inverse agonists are prone to bind with vitamin D receptors (VDRs).13,14 Moreover, CBD antagonizes the action of CB1 and CB2 receptor agonists and is suggested to act as an inverse agonist and a negative allosteric modulator of these receptors. CBD also inhibits fatty acid amide hydrolase (FAAH), increasing anandamide levels.15 VDR plays a critical and multifaceted role in human health and well-being.
Based on the above facts and correlation, authors hypothesized that CBD treatment might be one of the rescue mechanisms to induce VDR expression. To support its relevance, an in vitro experiment was performed to see the VDR expressions, inflammatory cytokines (TNF-α, IL-6), tissue elasticity, cellular senescence, and ATP level using various concentrations of CBD in multiple human and rodent cell lines.
Materials and Methods
Chemicals and reagents
CBD powder (99.9%) was obtained from Standard Hemp Company, USA. DMEM, DMSO, LPS, FBS, EDTA, and MTT were procured from Genexlife, Protaq Biomedical, Sigma-Aldrich, Genexlife, Parshuram, and Parshuram traders, respectively. H9C2 (rat cardiomyoblast), C2C12 (mouse myoblast cells), HaCaT (human keratinocytes), and SH-SY5Y (human neuroblastoma cells) cell lines were procured from NCCS, Pune. MG-63 (human osteosarcoma-like cells), HEK-293 (human embryonic kidney cells), MDA-MB-231 (human breast adenocarcinoma), A549 (human lung adenocarcinoma), HepG2 (human hepatocarcinoma cells), WI-38 (human lung fibroblasts), HT-29 (human colorectal adenocarcinoma), EA.hy926 (human endothelial cells), and THP-1 (human leukemia cells) cell lines were procured from ATCC, USA.
MTT Assay
Non-cytotoxic concentration was determined by exposing cells to different concentrations of cannabidiol (CBD). The respective vehicle control in the assay was DMSO/H2O2 with LPS. The number of viable cells was estimated based on the conversion of MTT to formazan dye using a mitochondrial enzyme. The effect of CBD on the cell viability of multiple cell lines was determined with the help of the following equation:
% Cell viability = 100- % cytotoxicity.
Where, % Cytotoxicity = {(O.D. of vehicle control cells – O.D. of cells treated with CBD)/OD of vehicle control cells}*100.
VDR Protein Expression
The single-cell suspension of all the cells was prepared in 10% FBS along with its specific medium. The cells were counted using a hemocytometer and plated in 35 mm culture dishes. The cells were incubated overnight under growth conditions to allow cell recovery and exponential growth. The above cells were subjected to serum starvation in DMEM + 10% FBS after overnight incubation. The cells were then treated with different concentrations of CBD in DMEM. Each set was then incubated for 24 hours in a CO2 incubator at 37°C, 5% CO2, and 95% humidity. The cells were harvested by scrapping and washed with PBS. The cell pellets obtained were analyzed for VDR gene expression using human VDR-specific primers such as forward: 5’-GCTGACCTGGTCAGTTACAGCA-3’, and reverse: 5’-CACGTCACTGACGCGGTACTT-3’. The VDR gene expression was normalized using Internal Control (IC) reference. Relative quantification (RQ) of VDR gene in CBD treated cells was calculated with respect to the normal cells using the following formula.16
RQ = 2−∆∆Ct
Where, CT: Threshold cycle.
Cytokines assay
The effect of CBD on the production of TNF-α and IL-6 was measured by the ELISA method using culture supernatants using a Biotek reader (SIAFRT/Synergy HT multimode reader). For the estimation of TNF-α and IL-6 in LPS (10 μg/mL) induced in a human monocytic cell line (THP-1) were exposed to CBD at selected nontoxic concentrations (1, 2.5, and 5 μM). After 48h of incubation, supernatants were analyzed for the secreted levels of cytokines using ELISA kits for TNF-α (Cat. DTA00C, R&D Systems) and IL-6 (Cat. D6050, R&D Systems) as per the manufacturer’s instructions.17
Estimation of Cellular Elasticity
The single-cell suspension of H9C2 cells was prepared in DMEM and 10% FBS using a hemocytometer. The cells were seeded density of 30,000 cells/well/0.5 mL in 48-well plates and incubated in a CO2 incubator for 24 hours and 95% humidity. The cells were centrifuged to obtain the pellet. After removing supernatants, the cells were resuspended in DMEM with 10% FBS. After treatment in all the experimental test groups, cells were incubated for 72 hours in 5% CO2. The culture supernatants from each well were collected after 24 hours of incubation and stored at below −20°C. The cells were fixed using 1% glutaraldehyde for 20 minutes and processed for elasticity measurement by atomic force microscopy (AFM). Young modulus was calculated from the force/distance indentation curves generated during AFM analysis using XEI data processing and analysis software.
Cellular senescence
The single-cell suspension of human lung fibroblasts (WI-38) was prepared in EMEM and 10% FBS using a hemocytometer. The initial density of cells was recorded (N (0)-0.2 million cells/well). The cells were incubated in a CO2 incubator at 95% humidity for 24 h. The cells were centrifuged to obtain the pellet. The supernatants were removed and the cells were reconstituted in medium EMEM with 10% FBS. After treatment with the selected concentration of CBD, cells were incubated as per condition then treated with 300 μM H2O2 in serum-free medium for 30 min. We determined cell count by trypan blue after trypsinizing cells for 48 h. The cell number was recorded as N(t) for each sample. The calculation of cell doubling time was calculated as follows using growth rate (amount of doubling in one unit of time):
Growth rate: ln{N(t)/N (0)}/t.
N(t) is the number of cells at the time of harvesting, N (0) is the number of cells at time 0 at the time of seeding, t is defined as the time (in hours), and doubling time was calculated as ln (2)/growth rate.
Measurement of ATP
Intracellular ATP levels were measured using an ATP assay kit (Cat. A22066; Invitrogen) as per the manufacturer's instruction. MG63 cells were harvested with lysate buffer and centrifuged at 1000g for 2 min at 4°C. A total of 50 μL ATP extract was then mixed with 50 μL of the luciferase reagent included in the kit. The fluorescence intensity at 562 nm was measured using a luminometer Synergy 2 multimode microplate reader. A standard curve for a series of defined ATP concentrations was prepared, and ATP content was calculated using the following formula: ATP content = ATP concentration/protein concentration.18
Statistics
The obtained data are shown (mean ± standard error of the mean) and subjected to statistical analysis using Sigma-Plot (V11.0). One-way analysis of variance (ANOVA) followed by post-hoc analysis by Tukey’s test was performed. F values p < 0.05 were regarded as statistically significant.
Results
Cell Viability Assay
The cytotoxic effect of CBD was evaluated on rat cardiomyoblast cell line (H9C2), human monocytic cell line (THP-1), human osteoblast (MG-63), human lungs fibroblasts (WI-38), MDA-MB-231, SH-SY5Y, HEK-293, HaCaT, human colorectal adenocarcinoma (HT-29), human endothelial cells (EA.hy926), human hepatocarcinoma cells (HepG2), human lung adenocarcinoma (A-549), and mouse myoblast cells (C2C12) with a concentration ranges from 0.25, 0.5, 1, 2.5, 5, 7.5 and 10 µM using MTT assay. The effect on the viability of cells was determined after 24 hours of treatment. Experimental data showed cell viability was observed more than 80% upto 7.5 µM test concentration. Hence, 1, 2.5, and 5 µM were selected for further studies.
Impact of CBD on VDR Protein Expression
Tukey’s post hoc analysis showed that relative quantification (RQ) of vitamin D3 receptor (VDR) protein expression was significantly increased by 87.38% (F(3,8) = 10.497, p = 0.042) and 109.71% (F(3,8) = 10.497, p = 0.013) at CBD-2.5 and CBD-5 µM, respectively in MG-63 cells compared to control group. The RQ value of VDR protein expression was significantly increased by 131.52% (F(3,8) = 23.787, p = 0.012) and 236.96% (F(3,8) = 23.787, p ≤ 0.001) at CBD-2.5 and CBD-5 µM, respectively in MDA-MB-231 cells compared to control group. Moreover, RQ value of VDR protein expression was significantly increased by 90% (F(3,8) = 17.932, p = 0.021), 110% (F(3,8) = 17.932, p = 0.007), and 170% (F(3,8) = 17.932, p ≤ 0.001) at CBD-1, CBD-2.5, and CBD-5 µM, respectively in SH-SY5Y cells than control group. Tukey’s post hoc analysis showed that RQ value of VDR protein expression was significantly increased by 100% (F(3,8) = 5.062, p = 0.019) and 80% (F(3,8) = 6.240, p = 0.021) at CBD-5 µM in HEK-293 and HT-29 cells, respectively with respect to control group. Additionally, RQ value of VDR protein expression was significantly increased by 290.91% (F(3,8) = 104.519, p ≤ 0.001) and 427.27% (F(3,8) = 104.519, p ≤ 0.001) at CBD-2.5 and CBD-5 µM, respectively in EaHy926 cells compared to control group. Further, RQ value of VDR protein expression was significantly increased by 208.33% (F(3,8) = 78.611, p ≤ 0.001) and 366.67% (F(3,8) = 78.611, p ≤ 0.001) at CBD-2.5 and CBD-5 µM, respectively in HepG2 cells compared to control group. Tukey’s post hoc analysis showed that RQ value of VDR protein expression was significantly increased by 56.31% (F(3,8) = 7.101, p = 0.016) and 63.84% (F(3,8) = 38.719, p ≤ 0.001) at CBD-5 µM in A-549 and C2C12 cells, respectively compared to the control group (Table 1).
 |
Table 1 The effect of cannabidiol (CBD) on vitamin D3 receptor (VDR) expression in terms of relative quantification (RQ) in multiple cell lines |
Anti-inflammatory activity of CBD
The level of TNF-α in the normal control (NC) group was 52.3 ± 4.10 pg/mL, and it was significantly increased by 148.41% in the vehicle control (VC) group (DMSO 0.05%) under the influence of lipopolysaccharides (LPS-10 µg/mL). Furthermore, the level of TNF-α was significantly decreased by 34.79% (F(4,10) = 6.608, p = 0.009) and 46.58% (F(4,10) = 6.608, p = 0.049) in the CBD-2.5 and CBD-5 µM, respectively compared to the VC group. Besides, the level of IL-6 was significantly increased by 231.83% in the VC group (DMSO 0.05%) under the influence of LPS than the NC group (73.2 ± 2.49 pg/mL). Moreover, the level of IL-6 was significantly decreased by 23.92% (F(4,10) = 99.377, p ≤ 0.001), 35.57% (F(4,10) = 99.377, p ≤ 0.001), and 43.61% (F(4,10) = 99.377, p ≤ 0.001) in the CBD-1, CBD-2.5, and CBD-5 µM, respectively with respect to the VC group (Figure 1).
Effect of CBD on Tissue Elasticity
The mean Young Modulus (YM) was slightly increased in the vehicle control group compared to normal control group. A decrease in YM is reciprocal to an increase in tissue elasticity. Moreover, YM was significantly reduced by 10.96% (F(4,10) = 130.627, p = 0.015), 37.09% (F(4,10) = 130.627, p ≤ 0.001), and 49.49% (F(4,10) = 130.627, p ≤ 0.001) in the CBD-1, CBD-2.5, and CBD-5 µM, respectively compared to the vehicle control (0.05% DMSO) group in rat cardiomyoblast cell line (H9C2) (Figure 2).
Anti-Senescence Activity of CBD
The cell doubling time was significantly (p ≤ 0.001) increased in the H2O2-treated group by 239.2% compared to the normal control group. Further, doubling time was significantly reduced by 19.38% (F(3,8) = 42.226, p = 0.042), 28.37% (F(3,8) = 42.226, p = 0.004), and 39.22% (F(3,8) = 42.226, p ≤ 0.001) in the CBD-1, CBD-2.5, and CBD-5 µM, respectively compared to H2O2-treated group in human lungs fibroblasts (WI-38) cell line (Figure 3).
Effect of CBD on Mitochondrial Function - ATP Synthesis
The level of ATP was significantly increased by 90.55% (F(3,8) = 48.350, p ≤ 0.001), 117.06% (F(3,8) = 48.350, p ≤ 0.001), and 153.54% (F(3,8) = 48.350, p ≤ 0.001) in CBD-1, CBD-2.5, and CBD-5 µM, respectively compared to the vehicle control group (Figure 4).
Discussion
CBD has low addictive, no hallucinogenic, and fewer side effects with very low affinity on CB1 and CB2 receptors. Animal and clinical studies reported that CBD can act as an antagonist/inverse agonist at certain concentrations,19 and are prone to bind with vitamin D receptors (VDRs).13,14 In this experiment, we found that CBD significantly increased VDR expression in multiple cell lines, which might be due to the binding with VDRs or some other receptors. In vivo studies show that CBD predominately reduced the levels of pro-inflammatory cytokines, TNF-α and IL-6.20 CBD extract showed significant reduction in pro-inflammatory cytokines (TNF-α and IL-1β) in human-derived PBMCs, neutrophils, and T cells as well as in vivo systemically inflamed mouse model.5,21 Another study (in vitro and in vivo) also reported that CBD reduced pro-inflammatory cytokines such as TNF-α, IFN- γ, IL-1, IL-2, and IL-6 through Janus kinase 2/signal transducer and activator of transcription 3 (JAK/STAT) signaling pathways.22 Our results provide evidence that CBD significantly reduced the secretion of pro-inflammatory cytokines in the human monocytic cell lines (THP-1) under LPS-induced inflammatory conditions, which might be helpful for the management of various inflammatory disorders.
Another in vitro experiment reported that CBD extract in combination with THC significantly increased the elasticity of blood cells ie, RBC morphology and membrane integrity.2 Moreover, clinical trial data had shown that topical application of CBD-enriched ointment without THC significantly improved skin elasticity.23 Our results showed that CBD significantly improved elasticity in rat cardiomyoblast cell line (H9C2) at various concentrations. The possible mechanism is that due to the presence of essential fatty acid in CBD, it may inhibit enzyme 5-α-reductase that improve the elasticity.24 Additionally, CBD modulates the membrane elasticity, which might be due to the amphiphilic property of CBD, which hyperpolarize the voltage-gated sodium (Nav) channel inactivation curves without changing the voltage dependence of activation.25
According to Li et al, prolonged treatment of CBD at 10 µM induced cellular senescence by inhibition of cell proliferation and simultaneous activation of senescence-associated β-galactosidase (SA-β-gal) and also increased another senescence biomarker, p16 in primary human sertoli cells,8 and in zebrafish.11 Another research finding reported that CBD protects amyloid-beta (Aβ)-induced senescence ie, anti-senescence activity through Parkin-dependent mitophagy in human astrocyte cells.26 Here, H2O2-induced senescence cells level through arresting cell cycle process. Our study data (Figure 3) showed that the cell doubling time is higher in the H2O2-induced group per se as compared to the normal control group without H2O2. CBD treatment at various concentrations significantly reduced the doubling time, which might be due to the inhibition of cell cycle arrest.
Study indicated that CBD altered cellular ATP production at both sub-cytotoxic and lethal concentrations in a dose-dependent and time-sensitive manner.27 Mitochondria are the principal source of adenosine triphosphate (ATP), produced through oxidative phosphorylation. Some studies have suggested mitochondria as targets for cannabinoids. Valvassori et al indicated that CBD can regulate intracellular Ca2+ via the mitochondrial Na+/Ca2+-exchanger pathway, which increases NADH availability and enhances the flow of electrons down the respiratory chain, thus increasing ATP synthesis.28 In this study, CBD significantly increased ATP levels at the tested concentration (1 to 5 µM) in human osteoblasts (MG-63), which might help to regulate and homeostasis the mitochondrial functions.
Recently, Lu et al showed that VDR is necessary for the optimal function of various human cells.29 In the present study, we found a strong correlation and association of VDR expression in cells and cells growth/function. It can be hypothesized that the VDR signaling is crucial for the maintenance of cell growth. Moreover, the expression of VDR expression in enterocytes and colonocytes within the gastrointestinal tract points toward the strong necessity of the VDR pathway to maintain gut epithelial integrity which is in line with previous findings.30 Our findings suggest that CBD treatment significantly increased VDR expression in colorectal cell lines. Mechanism action of the potential anti-inflammatory activity of CBD is likely mediated via the CB2 receptor. The ability of CBD to behave as a CB2 receptor inverse agonist may contribute to its documented anti-inflammatory properties. However, the exact mechanistic bases of the effects of CBD are still unknown.19 The mechanisms leading to the changes in VDR expression and CBD treatment are presently unknown. However, this can conceivably originate via multiple mechanisms, likely triggered initially via activation of CB2 cannabinoid receptors by CBD. It is known that the CBD and VDR systems might interact through nuclear receptor pathways at different levels. The effects of CBD were found due to the interactions and modulation by endogenous cannabinoids (eCBs) via the CB2 receptor, followed by interaction between the two systems at the receptor level, notably between VDR and CB2R.31–33 More in-depth studies are required to investigate the mechanism underlying CBD’s role in VDR binding affinity and its expression.
Conclusion
Our data clearly show that CBD significantly increased VDR expression in human and rodent cell lines. CBD treatment also exerts anti-inflammatory effect, improved tissue elasticity, anti-senescence activity, and enhanced mitochondrial activity. In this study, for the first time, we showed the evidence suggesting that the VDR plays a critical and multifaceted role in various types of human cells.
Abbreviations
CBD, Cannabidiol; THC, Δ9-tetrahydrocannabinol; CB, Cannabinoid; SASP, Senescence associated secretory phenotype; VDRs, Vitamin D3 receptors; FAAH, Fatty acid amide hydrolase; IC, Internal control; RQ, Relative quantification; CT, Threshold cycle; Nav, Voltage gated sodium; JAK/STAT, Janus kinase 2/signal transducer and activator of transcription 3.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
The authors extend their sincere thanks and gratitude to Dabur Research Foundation, India, for providing the facilities and support that enabled the successful completion of the work.
Funding
This research received no funding.
Disclosure
Mr Mahendra Kumar Trivedi, Ms Alice Branton, and Mrs Dahryn Trivedi are employees of Trivedi Global Inc. Mr Sambhu Mondal and Dr Snehasis Jana are employees of Trivedi Science Research Laboratory Pvt Ltd. The authors report no other conflicts of interest in this work.
References
1. Rapin L, Gamaoun R, El Hage C, Arboleda MF, Prosk E. Cannabidiol use and effectiveness: real-world evidence from a Canadian medical cannabis clinic. J Cannabis Res. 2021;3(1):19. doi:10.1186/s42238-021-00078-w
2. James TR, Richards AA, Lowe DA, Reid WA, Watson CT, Pepple DJ. The in vitro effect of delta-9-tetrahydrocannabinol and cannabidiol on whole blood viscosity, elasticity and membrane integrity. J Cannabis Res. 2022;4(1):15. doi:10.1186/s42238-022-00126-z
3. Khodadadi H, Salles EL, Jarrahi A, et al. Cannabidiol modulates cytokine storm in acute respiratory distress syndrome induced by simulated viral infection using synthetic RNA. Cannabis Cannabinoid Res. 2020;5(3):197–201. doi:10.1089/can.2020.0043
4. Vuolo F, Abreu SC, Michels M, et al. Cannabidiol reduces airway inflammation and fibrosis in experimental allergic asthma. Eur J Pharmacol. 2019;843:251–259. doi:10.1016/j.ejphar.2018.11.029
5. Vuolo F, Petronilho F, Sonai B, et al. Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediators Inflamm. 2015;2015:538670. doi:10.1155/2015/538670
6. Turner S, Barker VD, Adams AA. Effects of cannabidiol on the in vitro lymphocyte pro-inflammatory cytokine production of senior horses. J Equine Vet Sci. 2021;103:103668. doi:10.1016/j.jevs.2021.103668
7. Chan JZ, Duncan RE. Regulatory effects of cannabidiol on mitochondrial functions: a review. Cells. 2021;10(5):1251. doi:10.3390/cells10051251
8. Li Y, Li X, Cournoyer P, et al. Cannabidiol-induced transcriptomic changes and cellular senescence in human sertoli cells. Toxicol Sci. 2023;191(2):227–238. doi:10.1093/toxsci/kfac131
9. Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. 2021;9:645593. doi:10.3389/fcell.2021.645593
10. Gerasymchuk M, Robinson GI, Groves A, et al. Phytocannabinoids stimulate rejuvenation and prevent cellular senescence in human dermal fibroblasts. Cells. 2022;11(23):3939. doi:10.3389/fcell.2021.645593
11. Pandelides Z, Thornton C, Faruque AS, Whitehead AP, Willett KL, Ashpole NM. Developmental exposure to cannabidiol (CBD) alters longevity and health span of zebrafish (Danio rerio). Geroscience. 2020;42(2):785–800. doi:10.1007/s11357-020-00182-4
12. Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–623. doi:10.1038/sj.bjp.0707133
13. Cheng F Polypharmacology analysis of anti-osteoporosis agents and cannabinoid 2 receptor inverse agonists for osteoporosis drug research. [Master’s Thesis]. University of Pittsburgh; 2014. Available from: http://d-scholarship.pitt.edu/id/eprint/21179.
14. Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell. 2000;5(1):173–179. doi:10.1016/s1097-2765(00)80413-x
15. Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abílio VC. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9:482. doi:10.3389/fphar.2018.00482
16. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2^-DDCT Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
17. Trivedi MK, Mondal SC, Gangwar M, Jana S. Effect of a novel ashwagandha-based herbomineral formulation on pro-inflammatory cytokines expression in mouse splenocyte cells: a potential immunomodulator. Pharmacogn Mag. 2017;13(Suppl 1):S90–S94. doi:10.4103/0973-1296.197709
18. Bao Z, Dai X, Wang P, Tao Y, Chai D. Capsaicin induces cytotoxicity in human osteosarcoma MG63 cells through TRPV1-dependent and -independent pathways. Cell Cycle. 2019;18(12):1379–1392. doi:10.1080/15384101.2019.1618119
19. An D, Peigneur S, Hendrickx LA, Tytgat J. Targeting cannabinoid receptors: current status and prospects of natural products. Int J Mol Sci. 2020;21(14):5064. doi:10.3390/ijms21145064
20. Henshaw FR, Dewsbury LS, Lim CK, Steiner GZ. The effects of cannabinoids on pro- and anti-inflammatory cytokines: a systematic review of in vivo studies. Cannabis Cannabinoid Res. 2021;6(3):177–195. doi:10.1089/can.2020.0105
21. Aswad M, Hamza H, Pechkovsky A, et al. High-CBD extract (CBD-X) downregulates cytokine storm systemically and locally in inflamed lungs. Front Immunol. 2022;13:875546. doi:10.3389/fimmu.2022.875546
22. Peyravian N, Deo S, Daunert S, Jimenez JJ. The anti-inflammatory effects of cannabidiol (CBD) on acne. J Inflamm Res. 2022;15:2795–2801. doi:10.2147/JIR.S355489
23. Palmieri B, Laurino C, Vadalà M. A therapeutic effect of CBD-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin Ter. 2019;170(2):e93–e99. doi:10.7417/CT.2019.2116
24. Dobrev H. Clinical and instrumental study of the efficacy of a new sebum control cream. J Cosmet Dermatol. 2007;6(2):113–118. doi:10.1111/j.1473-2165.2007.00306.x
25. Ghovanloo MR, Ruben PC. Cannabidiol and sodium channel pharmacology: general overview, mechanism, and clinical implications. Neuroscientist. 2022;28(4):318–334. doi:10.1177/10738584211017009
26. Wang Z, Zheng P, Nagaratnam N, Solowij N, Huang X. Parkin mediates cannabidiol prevention of amyloid-beta-induced senescence in human astrocytes. Cannabis and Cannabinoid Res. 2023. doi:10.1089/can.2022.0186
27. Gross C, Ramirez DA, McGrath S, Gustafson DL. Cannabidiol induces apoptosis and perturbs mitochondrial function in human and canine glioma cells. Front Pharmacol. 2021;12:725136. doi:10.3389/fphar.2021.725136
28. Valvassori SS, Bavaresco DV, Scaini G, et al. Acute and chronic administration of cannabidiol increases mitochondrial complex and creatine kinase activity in the rat brain. Braz J Psychiatry. 2013;35(4):380–386. doi:10.1590/1516-4446-2012-0886
29. Lu R, Zhang YG, Xia Y, et al. Paneth cell alertness to pathogens maintained by vitamin D receptors. Gastroenterology. 2021;160(4):1269–1283. doi:10.1053/j.gastro.2020.11.015
30. Matos C, Mamilos A, Shah PN, et al. Downregulation of the vitamin D receptor expression during acute gastrointestinal graft versus host disease is associated with poor outcome after allogeneic stem cell transplantation. Front Immunol. 2022;13:1028850. doi:10.3389/fimmu.2022.1028850
31. Trivedi MK, Mondal S, Jana S. Cannabidiol improves thyroid function via modulating vitamin D3 receptor in vitamin D3 deficiency diet-induced rat model. J Food Sci Technol. 2022;59(8):3237–3244. doi:10.1007/s13197-022-05492-3
32. Navarrete F, García-Gutiérrez MS, Aracil-Fernández A, Lanciego JL, Manzanares J. Cannabinoid CB1 and CB2 receptors, and monoacylglycerol lipase gene expression alterations in the basal ganglia of patients with Parkinson’s disease. Neurotherapeutics. 2018;15(2):459–469. doi:10.1007/s13311-018-0603-x
33. Navarro G, Carriba P, Gandı´a J, et al. Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. Sci World J. 2008;8:1088–1097. doi:10.1100/tsw.2008.136
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




