Back to Journals » Infection and Drug Resistance » Volume 16
Candida parapsilosis-Caused Arthritis with Rice Body Formation: A Case Presentation and Literature Review
Authors Qi W , Ren Y, Wang H, Wan Y, Pan H, Yao J
Received 3 May 2023
Accepted for publication 15 June 2023
Published 26 June 2023 Volume 2023:16 Pages 4123—4135
DOI https://doi.org/10.2147/IDR.S416990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Weihui Qi,1,2,* Yanyun Ren,3,* Huang Wang,1,2 Yue Wan,3 Hao Pan,1,2 Jun Yao1,2
1Department of Orthopaedics, Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2Department of Orthopaedics, Hangzhou Ding Qiao Hospital, Hangzhou, People’s Republic of China; 3Department of Stomatology No. 903 Hospital of PLA, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Yao; Hao Pan, Department of Orthopaedics, Hangzhou TCM Hospital of Zhejiang Chinese Medical University (Hangzhou Hospital of Traditional Chinese Medicine), Hangzhou, Zhejiang Province, 310000, People’s Republic of China, Email [email protected]; [email protected]
Abstract: A 68-year-old male patient came to the orthopedics department because of swelling and pain in his left shoulder joint. He received more than 15 intraarticular steroid injections in the shoulder joint at a local private hospital. MRI showed that the synovial membrane of the joint capsule was thickened and swollen, and there were extensive “rice body-like” low T2 signal shadows filling. Arthroscopic removal of rice bodies and subtotal bursectomy were performed. The observation channel was placed through the posterior approach, and a large amount of rice bodies in yellow bursa fluid were observed to flow out. Rice bodies with a diameter of approximately 1– 5 mm filled the joint cavity were seen in the observation channel. The histopathological examination of the rice body showed that it was mainly composed of fibrin without a clear tissue structure. Bacterial and fungal cultures of synovial fluid suggested Candida parapsilosis infection, so the patient received antifungal treatment. However, the shoulder swelled again after three weeks, MRI revealed that there was significant fluid accumulation in the subacromial-subdeltoid region with necrotic synovial tissue floating and ultrasound examination showed joint cavity effusion, synovial hyperplasia, and some synovium looked like “floating weeds”. After 2 weeks, there were recurrent rice bodies in the articular cavity. Arthroscopic surgery was performed again to clean the joint and a catheter was placed for irrigation and drainage, and a large amount of necrotic synovial tissue floating as seen in ultrasound. Finally, patient received sensitive antifungal treatment and did not relapse within 6 months. During the recurrence in the current case, we recorded the process of rice body formation, which has for the first time been reported.
Keywords: rice body formation, Candida parapsilosis, fungal arthritis, intraarticular injection, corticosteroids
Introduction
As a rare disease, rice body is considered a non-specific response to chronic inflammation and was first described as being associated with tuberculous arthritis.1 Current research suggests that rice body is most common in rheumatoid arthritis,2,3 but there are also a few reports suggesting that rice body formation is related to trauma,4 juvenile idiopathic arthritis,5 seronegative inflammatory arthritis,6 infection,7,8 graft reaction,9 and even osteoarthritis.10 Almost all rice bodies are formed in the joint bursa or tendon sheath, but there are also reports suggesting the formation of rice bodies may occur without any joint connection.2
Candida parapsilosis infections of the intraarticular region are also extremely rare, though they may occur in patients treated with systemic immunosuppressants or after joint replacement.11 The diagnosis of fungal arthritis strictly relies on fungal culture, and there is a certain rate of missed diagnosis, which leads to delayed treatment.12 In this article, we report a rare case of articular infection of Candida parapsilosis with the formation of a rice body after repeated intraarticular injections.
Case Report
A 68 years old male patient came to the orthopedics department because of pain and swelling of the left shoulder joint. The patient developed left shoulder joint pain without obvious incentive 6 months prior, and there was no obvious shoulder joint swelling or limited shoulder joint movement at that time. He received more than 15 irregular intraarticular steroid injections in the shoulder joint at a local private hospital. The pain was significantly relieved within 1 week after each injection, but it recurred. One month prior to presentation, the patient had aggravated pain in the left shoulder joint, and the joint gradually swelled with obvious limitations of abduction and internal rotation. He had a history of fracture in his left shoulder 20 years earlier. Due to the age of the injury, the details could not be traced. No surgical treatment was performed at that time, and he recovered well. Physical examination found local swelling and tenderness in the shoulder, with limited range of motion. The patient had no fever, rheumatic disease, tuberculosis, or immunosuppressive drugs. The blood test indicated a white blood cell count of 5.43*109/L, a C-reactive protein (CRP) level of 3.27 mg/L, an erythrocyte sedimentation rate of 17 mm/h, a rheumatoid factor less than 20 IU/mL, and positive results from T-spot and TB-Ab tests.
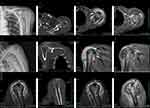
Plain radiography (Figure 1A and B) and CT scan (Figure 1D–F) showed bone erosion at the humeral head, the subchondral portion of the glenoid and the acromion. MRI (1.5) showed uneven signal increases in the humeral head, acromion and glenoid, articular cartilage wear, massive tears of the supraspinatus, subacromial space communication with the glenohumeral joint, and subdeltoid bursa communication with the glenohumeral joint cavity. The synovium of the joint was thickened and swollen, and there were extensive “rice body-like” low T2 signal shadows filling (Figure 1G–I). The enhanced gadopentetate with meglumine scan showed obvious enhancement of the synovium in the T1 sequence, but no enhancement was found in the rice body formation inside the bursa (Figure 1J–L).
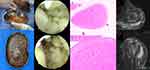
Considering that infectious diseases needed to be ruled out and discussions about surgical procedures such as rotator cuff repair and shoulder joint replacement would have to wait, arthroscopic removal of rice bodies and subtotal bursectomy were performed. The observation channel was placed through the posterior approach, and a lot of rice bodies in yellow bursa fluid were observed to flow out (Figure 2A and B). After the lens was placed, rice bodies with a diameter of approximately 1–5 mm filled the joint cavity (Figure 2C and D). Placing the shaver through the lateral approach, the field of vision became clear after removing most of the rice bodies. We found a spontaneous rupture of the long head tendon of the biceps brachii and a large rupture in the supraspinatus; the subacromial space was connected to the joint through this rupture. Three arthroscopic approaches (posterior approach, anterior approach and lateral approach) were used to monitor and operate and to fully explore the subacromial bursal, subdeltoid bursal, the periglenoid and the anterior spaces of the subscapularis. The rice body and denatured synovial tissue were fully shaved. Postoperative MRI showed that although the swelling around the joint was obvious, there was no remaining rice body (Figure 2G and H).
The histopathological examination of the rice body showed that it was mainly composed of fibrin without a clear tissue structure, with some small amount of tissue and cell coagulation (Figure 2E and F). A PCR test for the detection of Mycobacterium tuberculosis DNA suggested negative results, and bacterial and fungal cultures of synovial fluid suggested Candida parapsilosis infection performed by Vitek-2 (BioMerieux, France) automated system. However, peripheral blood culture showed no bacterial or fungal proliferation. Drug sensitivity test (DST) indicated that voriconazole, itraconazole, fluconazole, 5-flucytosine, and amphotericin are susceptible. Therefore, the patient received fluconazole (400 mg/qd) intravenously for 1 week, and then oral administration (200 mg/bid) to sequential therapy for 8 weeks as planned.
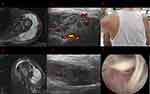
However, the shoulder swelled again after two weeks of oral administration (Figure 3E). MRI revealed a lot of fluid accumulation in the subacromial-subdeltoid region with necrotic synovial tissue floating (Figure 3A and B). In view of the large amount of fluid accumulation in the joint cavity, the joint cavity puncture was performed, and about 100 mL of yellow turbid liquid was extracted. The fungus culture showed that there was still Candida parapsilosis. Then the joint cavity was punctured for many times due to repeated swelling of the shoulder joint. Ultrasound examination showed joint cavity effusion, synovial hyperplasia, and some synovium looked like “floating weeds” (Figure 3C and D). In view of the recurring symptoms, arthroscopic surgery was performed again to clean the joint and a catheter was placed for irrigation and drainage (1 for irrigation and 1 for drainage). Under arthroscopy, there is no obvious rice body formation in the joint cavity, but a large amount of necrotic synovial tissue floating as seen in ultrasound (Figure 3F). A large amount of necrotic tissue flows out from the drainage tube and was pulled out due to blockage 10 days later. After operation, 400mg voriconazole was given intravenously, and 200mg bid was taken orally after three consecutive negative cultures. At present, the patient has received oral treatment for 8 weeks and change to the fluconazole (200 mg/bid) 4 weeks for the damage of liver function, without swelling again, and the subsequent culture is negative. The patient has been treated for 6 months and has not recurred.
Discussion and Review of the Literature
Rice body formations were described in tuberculosis joints by Riese in 1895 in the first time1 and have been observed in various chronic nonspecific inflammations of joints/bursae/tendon sheaths, such as systemic immune abnormalities, infection and degeneration. In our comprehensive literature review (Table 1), We found that rice bodies are most commonly seen in rheumatoid arthritis,2,3,13–15 it can occur in systemic juvenile rheumatoid arthritis,5,16 seronegative rheumatoid arthritis,6 injury,4 graft reaction,9 bacteria/fungi/mycobacterium tuberculosis infections,1,7,8,17,18 Milwaukee shoulder, radiotherapy damage,19 camptodactyly, osteoarthritis,10 and even in conditions of unknown etiology.20–23 Rice body formation in Candida parapsilosis-induced arthritis is very rare and has been reported only once.8 Fungal infections in the joints are extremely rare, and common risk factors may be systemic immunosuppression, such as immunosuppressive therapy or postoperative joint prosthesis.11 The report made by Jeong et al8 showed a case of Candida parapsilosis infection in an immunocompetent patient whose risk factors were old age and unexplained operation before 18 months. In this case, the patient had several corticosteroid injections into his shoulder, which might have caused the impairment of immunity. What is more, injectable corticosteroids contaminated with fungal strains will greatly increase the chances of infection. The New England Journal of Medicine disclosed the fungal infections associated with coordinated methylprednisolone injections with substantial morbidity and mortality in 2013.24 In addition, according to patient’s description, the sterile procedures were not strictly implemented in the process of configuration and injection which make it easier for patients to be inoculated with pathogens.
 |
Table 1 Clinical Characteristics of Patients with Rice Body Formation. |
The pathogenesis of rice body formation remains largely unknown and usually occurs secondary to nonspecific responses to synovial inflammation. Some researchers believe that the rice body is initially formed in synovial fluid and increases with fibrin aggregation.4,36 Other researchers believe that intra-articular synovitis, microinfarct after ischemia and necrosis, subsequent detachment of necrotic synovium into the articular cavity, and fibrin encapsulation in synovial fluid are the most likely reasons.18,37,38 In the recurrent process of this case, we found the process of synovial necrosis and shedding through MRI and ultrasound examination, which was not reported in the previous literatures, so we tended to be secondary pathogenesis process. After a literature review, we suggested that the above two mechanisms may exist at the same time or that there may be different mechanisms in different etiologies, because the performance of rice bodies is inconsistent in different reports. Some reports suggested that there are thousands of rice bodies,8,14,18 while some cases indicate fewer.3,39 Most of the rice bodies are small in diameter, while a few reports describe the rice bodies as being large in diameter.3,27 Most of the rice bodies were not visible on X-ray, while a few cases were revealed by X-ray.29,35 Moreover, the osteochondral erosion described in this article is relatively rare but was reported in a previous article that was also related to fungal infection.8 Almost all rice body formation is seen in a single joint, only Mutlu28 reported a case of simultaneous involvement of the shoulder and knee joints. The above differences may be related to the course and the location of the disease, but we believe that the most likely relationship is the etiological factor. However, due to the low incidence and currently unreproducible animal experiments, the above inferences cannot be scientifically proven.
Histopathologically, rice bodies are mostly composed of irregular and inhomogeneous fibrin material with inflammatory cell infiltration, but some observers have reported a more regular fibrin outer membrane. The ultrastructure of rice bodies under electron microscope is rarely mentioned. Muirhead et al4 reported that the ultrastructure of the electron dense material was fibrillary and exhibited an axial periodicity at high magnification, consistent with that of fibrin in rice bodies. The ultrastructure of the outside membrane was shown to be a mixture of collagen infiltrating fibroblasts, inflammatory cells and macrophages. Li-Yu et al10 reported that the rice body consists of coarse fibrin and fine fibrin with typical stripes. The existence of typical tiny apatite like crystals was confirmed by electron microscope.
Bone erosion can be observed on plain radiographs and CT scans, and if mineralized, rice bodies may also be noted. Ultrasound and MRI are very important imaging techniques for diagnosing rice body bursitis/synovitis due to the nonspecific symptoms of swelling, not necessarily pain.25,26,30,40 Huang et al30 suggested that sonography facilitates accurate preoperative diagnosis by explicit delineation of the dissection tract and the characteristic fried rice pattern of rice bodies. From the literature review, consistent with the current case, MRI was the most important examination in diagnosis of rice body. The MRI characteristics of the rice body have been clearly described. The low- and medium-signal nodules on T1-weighted images may not be clearly demarcated from the bursa fluid, and there is clear, relatively low signal filling on T2 images. The bursa was enhanced, while rice bodies were not after enhancement. Rice body formation needs to be distinguished from pigmented villonodular synovitis and synovial chondromatosis. The bodies of synovial chondromatosis contain cartilage components, so the T2 sequence shows a high signal, which can be distinguished from rice granules. Pigmented villonodular synovitis can be seen as diffuse hyperplasia of synovial villi, nodular changes and deposition of hemosiderin, usually showing a low signal in T1 and T2 sequences, and MRI different FE sequences can prove the presence of hemosiderin.41
In terms of treatment, surgical removal of rice bodies and diseased synovium is the current consensus, and almost all have achieved good results, except for a case of tendon rupture after surgery reported by Nagasawa.31 Depending on the surgical site, open surgery or arthroscopy can be selected, or a combination of the two.3,32,33,42 Rice bodies are a non-specific inflammatory response. In addition to symptomatic treatment of rice grains and synovium, the treatment of primary disease is also very important. Vyas et al34 reported a case of significant degeneration of the body during the treatment of rheumatoid arthritis with local steroids without any surgery because of the influence of COVID-19. In the current case, the primary disease was thought to be Candida parapsilosis infection, which was confirmed by fungal culture. Biofilm formation on the surface of medical devices or prostheses or in host tissues is the pathogenic factor and mechanism of most Candida infections. Compared to Candida albicans, the biofilm of Candida parapsilosis is thinner and less structured, consisting only of clumped budding spores, which may be due to its lower pathogenicity.43 Worldwide, resistance to antibiotics, including antifungals, is facing serious challenges, so drug sensitivity testing has tremendous significance in selecting the right antifungal drug.44 Voriconazole has good synovial fluid permeability and good bioavailability, and has less nephrotoxicity than amphotericin.45,46 In this case of recurrent Candida arthritis, lavage combined with voriconazole showed a good effect.
Conclusion
We report a rare case of arthritis with rice body formation caused by Candida parapsilosis infection in an immunocompetent patient and the synovium changes in the early stage of rice body formation were reported for the first time. MRI and fungal culture are important for diagnosing this rare disease. Debridement or lavage and antifungal therapy according to susceptibility testing are effective. It is worth mentioning that the contaminated corticosteroid and unstandardized practice of corticosteroid injection may cause serious complications.
Consent for Publication
Written informed consent was obtained from the patient for images and the details of this case. Details of the case have been approved by the IRB of the Hangzhou Hospital of Traditional Chinese Medicine affiliated to Zhejiang Chinese Medical University.
Disclosure
Weihui Qi and Yanyun Ren are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Riese H. Die Reiskörperchen in tuberculös erkrankten Synovialsäcken. Langenbecks Archiv Surg. 1895;42:1–99.
2. Matzer M, Carl HD, Swoboda B. Reiskorn-Riesenbursitis des Schulter-/Nackenbereichs bei langjähriger rheumatoider Arthritis ohne Gelenkverbindung [Giant bursitis with rice bodies of the shoulder/neck region in a patient with rheumatoid arthritis without joint-connection]. Z Rheumatol. 2007;66(5):430–433. German. doi:10.1007/s00393-007-0163-7
3. Subramaniam R, Tan JWL, Chau CYP, Lee KT. Subacromial bursitis with giant rice bodies as initial presentation of rheumatoid arthritis. J Clin Rheumatol. 2012;18(7):352–355. doi:10.1097/RHU.0b013e3182677023
4. Muirhead DE, Johnson EH, Luis C. A light and ultrastructural study of rice bodies recovered from a case of date thorn-induced extra-articular synovitis. Ultrastruct Pathol. 1998;22(4):341–347. doi:10.3109/01913129809103355
5. Rovenska E, Stvrtina S, Greguska O, Pravda L, Rovensky J. Conspicuous synovial lymphatic capillaries in juvenile idiopathic arthritis synovitis with rice bodies. Ann Rheum Dis. 2005;64(2):328–329. doi:10.1136/ard.2003.019984
6. Iyengar K, Manickavasagar T, Nadkarni J, Mansour P, Loh W. Bilateral recurrent wrist flexor tenosynovitis and rice body formation in a patient with sero-negative rheumatoid arthritis: a case report and review of literature. Int J Surg Case Rep. 2011;2(7):208–211. doi:10.1016/j.ijscr.2011.07.001
7. Bhat P, Khurana S, Fanaroff R, Adams SM, Rabinowitz RP. Rice body formation due to -associated chronic arthropathy. IDCases. 2021;23:e01030. doi:10.1016/j.idcr.2020.e01030
8. Jeong YM, Cho HY, Lee S-W, Hwang YM, Kim Y-K. Candida septic arthritis with rice body formation: a case report and review of literature. Korean J Radiol. 2013;14(3):465–469. doi:10.3348/kjr.2013.14.3.465
9. Barad SJ. Severe subacromial-subdeltoid inflammation with rice bodies associated with implantation of a bio-inductive collagen scaffold after rotator cuff repair. J Shoulder Elbow Surg. 2019;28(6):e190–e192. doi:10.1016/j.jse.2019.02.019
10. Li-Yu J, Clayburne GM, Sieck MS, et al. Calcium apatite crystals in synovial fluid rice bodies. Ann Rheum Dis. 2002;61(5):387–390. doi:10.1136/ard.61.5.387
11. Fidel PL, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80–96. doi:10.1128/CMR.12.1.80
12. Chen S, Chen Y, Zhou Y-Q, et al. Candida glabrata-induced refractory infectious arthritis: a case report and literature review. Mycopathologia. 2019;184(2):283–293. doi:10.1007/s11046-019-00329-8
13. Best C, Basu A, Sengupta M. Subacromial-subdeltoid bursal rice bodies causing shoulder pain. PM & R. 2015;7(9):1014–1016. doi:10.1016/j.pmrj.2015.04.014
14. Joshi PS. Severe sub-acromial bursitis with rice bodies in a patient with rheumatoid arthritis: a case report and review of literature. Malay Orthopaed J. 2018;12(2):52–55. doi:10.5704/MOJ.1807.010
15. Moreno S, Forcada P, Soria X, et al. Tenosynovitis with rice body formation presenting as a cutaneous abscess. J Cutan Pathol. 2014;41(7):602–605. doi:10.1111/cup.12316
16. Cuomo A, Pirpiris M, Otsuka NY. Case report: biceps tenosynovial rice bodies. J Pediatr Orthop B. 2006;15(6):423–425. doi:10.1097/01.bpb.0000228392.62678.df
17. Bayram S, Erşen A, Altan M, Durmaz H. Tuberculosis tenosynovitis with multiple rice bodies of the flexor tendons in the wrist: a case report. Int J Surg Case Rep. 2016;27:129–132. doi:10.1016/j.ijscr.2016.08.021
18. Kim R-S, Lee J-Y, Jung S-R, Lee K-Y. Tuberculous subdeltoid bursitis with rice bodies. Yonsei Med J. 2002;43(4):539–542. doi:10.3349/ymj.2002.43.4.539
19. Guo JJ, Wu K, Xu Y, Yang H. Hundreds of rice bodies in the subacromial-subdeltoid bursa: report of two cases and literature review. BMC Musculoskelet Disord. 2020;21(1):539. doi:10.1186/s12891-020-03563-0
20. Cegarra-Escolano M, Jaloux C, Camuzard O. Rice-body formation without rheumatic disease or tuberculosis in a “sausage” ring finger. Hand Surg Rehabil. 2018;37:255–258. doi:10.1016/j.hansur.2018.03.005
21. Chen A, Wong L-Y, Sheu C-Y, Chen B-F. Distinguishing multiple rice body formation in chronic subacromial-subdeltoid bursitis from synovial chondromatosis. Skeletal Radiol. 2002;31(2):119–121. doi:10.1007/s002560100412
22. Ergun T, Lakadamyali H, Aydin O. Multiple rice body formation accompanying the chronic nonspecific tenosynovitis of flexor tendons of the wrist. Radiat Med. 2008;26(9):545–548. doi:10.1007/s11604-008-0270-7
23. Forse CL, Mucha BL, Santos MLZ, Ongcapin EH. Rice body formation without rheumatic disease or tuberculosis infection: a case report and literature review. Clin Rheumatol. 2012;31(12):1753–1756. doi:10.1007/s10067-012-2063-8
24. Smith RM, Schaefer MK, Kainer MA, et al. Fungal infections associated with contaminated methylprednisolone injections. N Engl J Med. 2013;369(17):1598–1609. doi:10.1056/NEJMoa1213978
25. Griffith JF, Peh WC, Evans NS, Smallman LA, Wong RW, Thomas AM. Multiple rice body formation in chronic subacromial/subdeltoid bursitis: MR appearances. Clin Radiol. 1996;51(7):511–514. doi:10.1016/S0009-9260(96)80193-0
26. Spence LD, Adams J, Gibbons D, Mason MD, Eustace S. Rice body formation in bicipito-radial bursitis: ultrasound, CT, and MRI findings. Skeletal Radiol. 1998;27(1):30–32. doi:10.1007/s002560050331
27. Sugano I, Nagao T, Tajima Y, et al. Variation among giant rice bodies: report of four cases and their clinicopathological features. Skeletal Radiol. 2000;29(9):525–529. doi:10.1007/s002560000258
28. Mutlu H, Silit E, Pekkafali Z, et al. Multiple rice body formation in the subacromial-subdeltoid bursa and knee joint. Skeletal Radiol. 2004;33(9):531–533. doi:10.1007/s00256-004-0757-y
29. Matsumoto T, Fujita K, Fujioka H, et al. Massive nonspecific olecranon bursitis with multiple rice bodies. J Shoulder Elbow Surg. 2004;13(6):680–683. doi:10.1016/j.jse.2004.03.008
30. Huang -C-C, Ko S-F, Weng L-H, et al. Sonographic demonstration of hyperechoic fibrin coating of rice bodies in trochanteric bursitis: the “fried rice” pattern. J Ultrasound Med. 2006;25(5):667–670. doi:10.7863/jum.2006.25.5.667
31. Nagasawa H, Okada K, Senma S, Chida S, Shimada Y. Tenosynovitis with rice body formation in a non-tuberculosis patient: a case report. Ups J Med Sci. 2009;114(3):184–188. doi:10.1080/03009730902931408
32. Lui TH. Dorsalis pedis psuedoaneurysm: a complication followed extensor tendoscopy of the ankle in a non-tuberculosis patient with tenosynovitis with rice body formation. Foot Ankle Surg. 2016;22(2):e1–e5. doi:10.1016/j.fas.2015.12.003
33. Mohammed Reda F, Talal G, Moncef B, Reda-Allah B, Moulay Omar L, Mohammed Saleh B. Mass of the thenar eminence hiding idiopathic massive rice bodies formation with a compression of the median nerve: case report and review of the literature. Int J Surg Case Rep. 2018;50:28–31. doi:10.1016/j.ijscr.2018.07.025
34. Vyas S, Bhadu D, Goswami RP, Kumar U. Subacromial subdeltoid rice body bursitis in rheumatoid arthritis treated with local steroids. Int J Rheum Dis. 2022;25(5):627–629. doi:10.1111/1756-185X.14305
35. Haibo Z, Tianrui W, Wenlian S, et al. A case of rice body synovitis of the knee joint. Orthop Surg. 2022;14(3):628–632. doi:10.1111/os.13195
36. Popert AJ, Scott DL, Wainwright AC, Walton KW, Williamson N, Chapman JH. Frequency of occurrence, mode of development, and significance or rice bodies in rheumatoid joints. Ann Rheum Dis. 1982;41(2):109–117. doi:10.1136/ard.41.2.109
37. Cheung HS, Ryan LM, Kozin F, McCarty DJ. Synovial origins of Rice bodies in joint fluid. Arthritis Rheum. 1980;23(1):72–76. doi:10.1002/art.1780230112
38. McCarthy DJ, Cheung HS. Origin and significance of rice bodies in synovial fluid. Lancet. 1982;2(8300):715–716. doi:10.1016/S0140-6736(82)90735-8
39. Li W, Xiao D-M, Jiang C-Q, Zhang W-T, Lei M. Arthroscopic treatment of bony loose bodies in the subacromial space. Int J Surg Case Rep. 2015;11:101–103. doi:10.1016/j.ijscr.2015.02.004
40. Chau CLF, Griffith JF, Chan PT, Lui TH, Yu KS, Ngai WK. Rice-body formation in atypical mycobacterial tenosynovitis and bursitis: findings on sonography and MR imaging. AJR Am J Roentgenol. 2003;180(5):1455–1459. doi:10.2214/ajr.180.5.1801455
41. Barile A, Sabatini M, Iannessi F, et al. Pigmented villonodular synovitis (PVNS) of the knee joint: magnetic resonance imaging (MRI) using standard and dynamic paramagnetic contrast media. Report of 52 cases surgically and histologically controlled. Radiol Med. 2004;107(4):356–366.
42. Yamamoto D, Tada K, Suganuma S, Ikeda K, Tsuchiya H. Non-tuberculous mycobacterium or fungus induced chronic tenosynovitis with rice body of the hand. J Hand Surg Asian-Pacific Vol. 2017;22(3):337–342. doi:10.1142/S0218810417500393
43. Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002;70(2):878–888. doi:10.1128/IAI.70.2.878-888.2002
44. Lamoth F, Lewis RE, Kontoyiannis DP. Role and interpretation of antifungal susceptibility testing for the management of invasive fungal infections. J Fungi. 2020;7(1):17. doi:10.3390/jof7010017
45. Sebastian S, Malhotra R, Pande A, Gautam D, Xess I, Dhawan B. Staged reimplantation of a total hip prosthesis after co-infection with Candida tropicalis and Staphylococcus haemolyticus: a case report. Mycopathologia. 2018;183(3):579–584. doi:10.1007/s11046-017-0177-x
46. Sili U, Yilmaz M, Ferhanoglu B, Mert A. Candida krusei arthritis in a patient with hematologic malignancy: successful treatment with voriconazole. Clin Infect Dis. 2007;45(7):897–898. doi:10.1086/521253
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.