Back to Journals » Infection and Drug Resistance » Volume 12
Candida isolates causing refractory or recurrent oropharyngeal candidiasis in 11 hospitals in China
Authors Yu SY, Zhang L, Chen S, Kong F, Xiao M, Wang H, Hou X, Zhou ML , Zhang G, Zhang JJ, Duan SM, Kang W, Xu YC
Received 24 December 2018
Accepted for publication 19 March 2019
Published 18 April 2019 Volume 2019:12 Pages 865—875
DOI https://doi.org/10.2147/IDR.S199359
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Shu-Ying Yu,1–3 Li Zhang,1,3 Sharon Chen,4 Fanrong Kong,4 Meng Xiao,1,3 He Wang,1,3 Xin Hou,1–3 Meng-Lan Zhou,1–3 Ge Zhang,1,3 Jing-Jia Zhang,1,3 Si-Meng Duan,1,3 Wei Kang,1,3 Ying-Chun Xu1,3
1Department of Clinical Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing 100730, People’s Republic of China; 2Graduate School, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing 100730, People’s Republic of China; 3Beijing Key Laboratory for Mechanisms Research and Precision Diagnosis of Invasive Fungal Diseases (BZ0447), Beijing, People’s Republic of China; 4Centre for Infectious Diseases and Microbiology Laboratory Services, ICPMR –New South Wales Health Pathology, The University of Sydney, Westmead, New South Wales, Australia
Introduction: We studied the species distribution and antifungal susceptibilities of Candida isolates causing refractory or recurrent oropharyngeal candidiasis (OPC) in a multicenter study in China (2013–2016).
Methods: Species identification was performed using the Bruker Biotyper (Bruker Daltonics, Germany) matrix-assisted laser desorption/ionization time of flight mass spectrometry system supplemented by internal transcribed spacer sequencing as required. Antifungal susceptibilities were determined by the Clinical and Laboratory Standards Institute document (CLSI) M27-A3 broth microdilution methodology.
Results: A total of 558 non-duplicate Candida isolates comprising 10 species were obtained from 535 patients. Candida albicans was the most common species (89.6%), followed by C. glabrata (5.2%), C. tropicalis (2.9%), and C. parapsilosis (0.7%). Azoles were active against C. albicans with susceptibility rates of 96% and 95.8% for fluconazole and voriconazole, respectively. MIC50 values of C. albicans to fluconazole, voriconazole, itraconazole, and miconazole were 1, 0.03, 0.25 and 0.12 μg/mL, respectively, higher than those in previous studies of which OPC patients (corresponding MIC50 values of 0.25 , 0.015 , 0.06 , and 0.03 μg/mL). Except for itraconazole, the MIC50 and MIC90 values of 58 non-C. albicans to other azoles were two to threefold higher than C. albicans. Miconazole, amphotericin B, nystatin, and 5-flucytosine had good in vitro antifungal activity for all isolates.
Conclusion: The study provides valuable data on the species distribution and antifungal susceptibility of oropharyngeal Candida isolates from geographically diverse areas of China. C. albicans remains the most common species but with increasing rates of azoles resistance.
Keywords: oral candidiasis, Candida, identification, antifungal susceptibility
Introduction
In healthy humans, the oral cavity is colonized by numerous microbial species.1,2 Candida species are commensals that can be found in the oral tract of 15.2–75% of healthy individuals.2–5 However, a number of external and internal factors can lead to host-fungus unbalance, which results in yeast overgrowth and potential oropharyngeal candidiasis (OPC). OPC is frequently observed in patients with HIV/AIDS, malignancy, diabetes mellitus, and in patients with solid organ transplants or those who are prescribed antibiotics. Hence, immunocompromised patients are predisposed to developing refractory and recurrent episodes of OPC, which may be severe and be associated with disease progression.2,3,5–9
Many of such patients with refractory and recurrent OPC are exposed to repeated courses of antifungal therapy which poses a risk for emergence of drug-resistant yeast isolates. Candida species can cause a variety of lesions in the oral cavity including pseudomembranous and erythematous lesions, angular cheilitis and median rhomboid glossitis. These can be irritative and painful and impact digestion and absorption of food, with subsequent systemic infection, especially in immunocompromised patients.10–12 Candida albicans remains the most causative pathogen, although non-C. albicans Candida species, such as C. tropicalis, C. krusei, C. parapsilosis, and C. glabrata, have played an increasing role.2,9
OPC is treated with either topical or systemic antifungal agents with polyenes and azole antifungal agents being the most frequently used. Hence, in settings where for example, azole resistance is emergent, selection of antifungal therapy is challenging.1,2,13,14 In Africa, the resistance rate of Candida isolates to azoles in Ethiopian HIV-infected patients with OPC has ranged from 1.3% to 12.3% depending on the azole antifungal agent.2
Local data including species distribution and the antifungal susceptibility profile of fungal species causing OPC is essential as these data cannot be generalized across countries. In China, data on the relative frequency of C. albicans vs non-albicans Candida species as etiologic agents of OPC and that on drug resistance of OPC isolates of C. albicans to the currently available antifungals are few.11,15 The present multicenter study aimed to determine the trend of the species distribution and antifungal susceptibility patterns of yeast isolates obtained from refractory and recurrent OPC collected from a large number of hospitals in China.
Materials and methods
Ethics statement
The study was approved by the Human Research Ethics Committee of Peking Union Medical College Hospital (PUMCH) (No. S-263). Written informed consent was obtained from all patients in the study for permission to study the isolates cultured from them for scientific research.
Clinical isolates
A total of 558 non-duplicate Candida isolates from 535 patients with refractory or recurrent OPC were included over the study period, October 1, 2013 to April 30, 2016. Patients were from eleven different hospitals in seven provinces in China. The definition of refractory or recurrent OPC was infection that has recurred or persisted after at least one treatment course with an appropriate antifungal agent.16
Laboratory procedures
Species identification
At each study site, specimens were obtained by swabbing the oropharyngeal mucosa with a sterile swab, which was then were plated onto Sabouraud’s dextrose agar (SDA) (bioMérieux, France) and incubated at 37°C for 48 hrs. Isolates were also inoculated onto CHROMagar Candida medium (CHROMagar, Paris, France). The initial species identification result was obtained by morphological appearance on CHROMagar Candida medium (CHROMagar) and by using the VITEK 2® compact system (BioMérieux, Marcyl’ Etoile, France). Species identification (final identification result) was confirmed at a reference mycology facility at the PUMCH, Beijing, China using Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectroscopy (MALDI-TOF MS) system (Biotyper version 3.1 software, Bruker Daltonics, Billerica, USA) supplemented by DNA sequencing of the fungal internal transcribed spacer (ITS) region,17 where MALDI-TOF MS identification produced a log score of <2.0.
In brief, for ITS sequencing, DNA extraction was performed using beating protocol and amplification of the ITS region was carried out with the primer pairs ITS1 (5ʹ-TCC GTA GGT GAA CCT GCG G-3ʹ) and ITS4 (5ʹ-TCC TCC GCT TAT TGA TAT GC-3ʹ) as described by Zhang et al.18 The PCR products were purified with QIAquick PCR Purification Kit (QIAGEN, Germany), then sequenced in both directions using corresponding PCR amplification primer pairs at Ruibiotech Co. Ltd. (Beijing, China) using the DNA analyzer ABI 3730XL system (Applied Biosystems, Foster City, CA). Species identification was performed by comparing the obtained sequences against GenBank database with nucleotide Basic Local Alignment Search Tool (BLASTn,
Antifungal susceptibility testing
Candida species will be allowed antifungal susceptibility testing after 24 hrs of incubation in the ambient air atmosphere at 35°C in SDA. In vitro susceptibility testing of all isolates to fluconazole, voriconazole, itraconazole, miconazole, ketoconazole, amphotericin B, nystatin and 5-flucytosine were performed by broth microdilution methodology according to the Clinical and Laboratory Standards Institute (CLSI) M27-A3 protocol.19 The minimum inhibitory concentration (MIC) for amphotericin B and nystatin was read as the lowest concentration that resulted in no discernible growth following 24 hrs of incubation. For 5-flucytosine and the azoles, approximately 50% reduction in growth relative to the drug-free growth control was considered as the MIC endpoint. C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were the quality control strains for each test run.19 The final range of concentrations tested was: 0.015–16 μg/mL for amphotericin B, miconazole, itraconazole, voriconazole, nystatin and ketoconazole, 0.06–64 μg/mL for 5-flucytosine and 0.125–128 μg/mL for fluconazole. All experiments were performed in duplicate and on two separate occasions.
MIC results were interpreted by species-specific clinical breakpoints (CBPs) as recommended by the CLSI M60 method.20 With regard to species for which there are no CBPs, we used epidemiological cutoff values (ECVs) to differentiate wild-type (WT) from non-WT isolates according to CLSI M59 method21 and ECVs from the study by Pfaller et al.22 MIC results in miconazole, nystatin, and ketoconazole were defined using an arbitrary breakpoint as previously described.23–25 The CBPs and ECV against common Candida species used in this study summarized in Table S1.
Results
Species distribution and patient characteristics
A total of 558 Candida isolates comprising ten species were confirmed as causative pathogens at the reference laboratory. C. albicans was the most frequently isolated species accounting for 500 (89.6%) of isolates, followed by C. glabrata, 29 (5.2%), C. tropicalis, 16 (2.9%), C. parapsilosis, 4 (0.7%), C. krusei, 2 (0.4%), and other Candida species, 7 (1.2%).
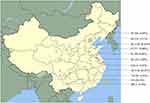
In the present study, 76% of isolates were isolated from females and 24%, in males. Isolation of Candida isolates varied with age which increased from a frequency of 8.7% (patients aged 20–39 years) to 54.3% (patients aged≥60 years). Table 1 summarizes the patient characteristics, and the geographical distribution of infection is presented in Figure 1.
 | Table 1 Clinical characteristics of patients with refractory and recurrent oropharyngeal candidiasis and species distribution of the Candida isolates |
Twenty-one patients suffered mixed oropharyngeal yeast infections with C. albicans cultured in almost all (95.2%) mixed infections, except in one episode caused by C. guilliermondii and C. parapsilosis. Mixed infections caused by three yeasts were those of C. albicans with C. glabrata and C. tropicalis in two patients. Amongst infections caused by two yeasts, C. albicans featured in eleven patients with C. glabrata, in two patients with C. tropicalis and in one patient with C. parapsilosis, C. guilliermondii, C. carpophila, C. lusitaniae, and C. norvegensis (see Table S2 for summary).
Agreement between initial and final identification results
The agreement of identification results for yeasts obtained from participating hospitals and the reference laboratory is showed in Table 2. Overall agreement was 91.2% (509/558 isolates) with the highest agreement observed for C. albicans (486/500, 97.2% isolates). However, for other Candida species, the identification agreement was substantially lower (0–57.2%).
Incorrect identification to species level by conventional methods included those identified through the use of CHROMagar Candida medium and VITEK 2® compact system and varied with species of Candida: C. albicans (12/500, 2.4%), C. glabrata sensu stricto (12/28, 42.8%), C. tropicalis (12/16, 75%), C. parapsilosis (3/4, 75%) and six other species (7/9, 77.8%) including C. krusei (2/2), C. lusitaniae (0/2,100%), C. guilliermondii (0/2,100%), C. norvegensis (0/1,100%), C. carpophila (0/1,100%), and C. pelliculosa (0/1,100%). Among 12 C. albicans isolates, five isolates were misidentified to C. glabrata sensu stricto and seven, to C. tropicalis. Moreover, nine C. glabrata sensu stricto isolates and eleven C. tropicalis isolates were incorrectly identified as C. albicans. Almost all uncommon Candida species were incorrectly identified. Further, minor errors occurred in five isolates (0.9%) including four species (Table 2).
Antifungal susceptibilities
The susceptibility results for causative Candida species are shown in Table 3. Most azoles drugs were highly active against C. albicans. For miconazole, the MIC50 and MIC90 values were 0.125 and 0.5 μg/mL, respectively, and 488/500 isolates (97.6%) were susceptible to this drug. Fluconazole and voriconazole had comparable activity against C. albicans, where 96% and 95.8%, respectively were susceptible to these drugs. Two fluconazole-resistant C. albicans isolates were also resistant to voriconazole. For C. glabrata, the MIC50 of fluconazole was 16 μg/mL and all C. glabrata isolates were susceptible-dose dependent. Except for ketoconazole (with the percentage of non-wild type of 37.9%), all isolates of C. glabrata were susceptible to the other antifungal drugs. One of 16 isolates (6.2%) of C. tropicalis was resistant to fluconazole, voriconazole, and miconazole, with MIC of 128, 8, and 16 μg/mL, respectively. Only one (25%) C. parapsilosis isolate was resistant to all the azole drugs, C. albicans showed a high non-susceptibility rate (67.2%) against itraconazole, which was much higher than that of other Candida species with the percentage of wild type from 75% to 100%.
 | Table 3 Antifungal susceptibility results of 558 isolates to eight antifungal agents |
 | Table 4 Review about the species distribution of yeast species obtained from OPC and IFI patients |
The MIC50 values of uncommon Candida species were at least three folds higher than those of C. albicans for fluconazole, voriconazole, miconazole, and ketoconazole; for itraconazole the MIC50 was only one dilution higher. Compared to the azoles, amphotericin B, nystatin and 5-flucytosine had greater in vitro activity against these species. The susceptibility rates to these three drugs reached up to 100% for C. glabrata, C. tropical, and C. parapsilosis isolates. However, three C. albicans isolates were considered resistant to amphotericin B and one isolate had non-WT MICs to 5-flucytosine. Of 58 non-C. albicans Candida isolates, the MIC50 and MIC90 values of fluconazole, voriconazole, ketoconazole, and miconazole were two to three folds as high as of those of C. albicans. In comparison, MIC50 values for amphotericin B, nystatin and 5-flucytosine were very similar between non-C. albicans species and C. albicans. Ketoconazole had the lowest MIC50 and MIC90 values, with nearly two folds lower values than those of itraconazole and miconazole in C. albicans although miconazole had higher susceptibility rate.
Discussion
Refractory or recurrent episodes of OPC result in increased morbidity and reduced quality of life. Their targeted management relies on accurate epidemiological surveillance data to determine whether current empirical treatments are appropriate. However, no contemporary large-scale surveillance studies of OPC have been performed in China to determine species distribution and if there are any variations in antifungal susceptibilities of causative yeast species, particularly in patients who are repeatedly exposed to antifungal agents.
Our finding of C. albicans as the predominant species (89.6%), and C. glabrata (5.2%) and C. tropicalis (2.9%) as the next most common Candida species, are consistent with the assessments made in similar studies of the in vitro susceptibility of oropharyngeal Candida isolates from UK patients and Tanzanian HIV-infected patients.26,27 The main Candida species were C. albicans (521 isolates, 84.3%), C. glabrata (59 isolates, 9.5%), and C. tropicalis (13 isolates, 2.1%) in the UK study and C. albicans (250 isolates, 84.5%), C. glabrata (20 isolates, 6.8%), and C. tropicalis (8 isolates, 2.7%) in the Tanzanian study.26,27The frequency of C. albicans isolates in our study is also similar to that found among hospitalized patients in Kunming (82.2%) in a prior study,15 but conversely, much lower (54.3%) than that compared to another study conducted in Hainan.11 In addition, it has been observed the frequencies of C. albicans in three large-scale studies of invasive fungal infections (IFIs) studies were much lower than our study’s with infection rate from 34.6% to 65.3%.28–30 Thus, our study suggests that C. albicans still remains the predominant species in OPC patients. The species distribution of yeast species obtained from OPC and IFI patients are summarized in Table 4. 15,26–31
Incorrect species identification results by the CHROMagar Candida medium and the VITEK 2® compact system not only occurred with the uncommon Candida isolates (1.3%), but also amongst common Candida species (7.5%). It is well known that CHROMagar Candida medium and the VITEK 2® compact system have limitations for the identification of certain Candida species. CHROMagar Candida medium can only identify a few Candida species including C. albicans (colored green), C. tropicalis (dark blue), and C. krusei (pink and downy appearance).32 Likewise, certain common yeasts cannot be identified by the Vitek 2® compact system (biomerieux).33 Moreover, mixed infections such as that in the present study, can pose a challenge in both the diagnosis and treatment of refractory and recurrent OPC. Incorrect identification will greatly affect the choice of antifungal agent, so accurate identification by MALDI-TOF MS and/or ITS sequencing is essential to confirm any unusual species identifications or where more than one species may be present.
Of note, compared with previous studies which have mainly included OPC patients who had not received previous antifungal treatment, in our study, we have observed reduced susceptibility to all antifungal agents for the majority Candida species.11,15,26,27 For the predominant species, C. albicans, the MIC50 values of fluconazole, voriconazole, itraconazole, miconazole, and ketoconazole were 1 , 0.03 , 0.25 , 0.12 , and 0.03 μg/mL, respectively. These values are higher than those reported by Kuriyama et al, which found for patients who had not received previous antifungal therapy, MIC50 values for fluconazole, voriconazole, itraconazole, miconazole, and ketoconazole of 0.25 , 0.015 , 0.06 , 0.03 , and 0.03 μg/mL, respectively. Because of different breakpoints or ECVs employed in our, and the above study, we are not able to compare the rates of resistance or frequency of non-WT isolates. The MIC50 values in our study cohort suggest that prior exposure to azoles and other antifungal agents, predisposes to the risk of reduced susceptibility to antifungal agents as observed before.26,27
Itraconazole is an important antifungal agent for OPC treatment in China. In a previous study,34 long-term itraconazole prophylaxis was associated with reduction in susceptibility to itraconazole and cross-resistance to fluconazole in mucosal C. albicans from patients with AIDS. This may explain why the Candida isolates showed reduced susceptibility to azoles in our study. According to the ESCMID guidelines for management of OPC,35 fluconazole remains the preferred antifungal drug. Miconazole, itraconazole, voriconazole are potential alternatives to fluconazole but itraconazole has a higher incidence of unreliable oral bioavailability and drug–drug interactions compared with fluconazole. The use of itraconazole may also be complicated by cross-resistance to fluconazole.36 Therefore, regular monitoring of serum drug levels is required.
Even though miconazole has a higher MIC breakpoint in comparison with other azoles,23 it still was the most active azole for C. albicans. Miconazole may be administered as a topical agent. Various topical formulations including miconazole buccal tablets, miconazole chewing gum, miconazole oral gel, and miconazole lacquer have been used to treat oral candidiasis. Hence, miconazole may be considered alternate regimen for OPC in countries where topical miconazole is used.13
Of note, 1.4% C. albicans isolates were resistant to fluconazole, slightly higher than the rate of resistance found in the China Hospital Invasive Fungal Surveillance Net study (resistance rate 0.5%). Based on our data, itraconazole and miconazole established great antifungal activity to C. glabrata with 100% susceptibility rate, while the susceptibility rate of voriconazole and ketoconazole was relatively low. We also found one of 16 isolates of C. tropicalis and one out of four isolates of C. parapsilosis to be resistant to all azoles. Azole resistance amongst Candida spp. are usually attributed to selection pressure caused by prior exposure antifungal agents and cumulative doses of azoles.27 Whilst there are no interpretative breakpoint criteria for amphotericin B, nystatin and 5-flucytosine, the present study has shown that MICs for miconazole, amphotericin B, nystatin and 5-flucytosine were within a narrow range consistent with a previous study.27
Two main limitations of this study are mentioned herein. Firstly, we did not collect data on patient underlying disease, drug history, antifungal treatment and outcomes of refractory, and recurrent OPC. Further, the present survey focused only on centers located in seven provinces of China. Extending coverage of the study to the whole country would be significant for continued surveillance.
In conclusion, the present study provides valuable surveillance data on the species distribution and antifungal susceptibility of a large number of oropharyngeal yeast isolates collected from geographically diverse areas of China. Significantly reduced susceptibility to all the azoles was observed form patients with recurrent OPC. C. albicans is still the most frequently isolated species but has elevated MICs to the azoles. In contrast, miconazole, amphotericin B, nystatin, and 5-flucytosine have retained high antifungal activity. For adequate therapy, efforts must be maintained to carry out accurate and timely identification and antifungal susceptibility testing.
Acknowledgments
We thank all the participants in the present study. The isolates analyzed in this study were isolated from eleven hospitals (hospital name, abbreviation, and location): Shanghai Ninth People’s Hospital, JD, Shanghai; Stomatological Hospital of the Fourth Military Medical University, JY, Shanxi; Qilu Hospital of Shandong University, QL, Shandong; Nanjing Stomatological Hospital Medical School of Nanjing University, NJ, Jiangsu; Taihe Hospital, TH, Hubei; Sichuan Provincial People’s Hospital, SR, Sichuan; Stomatological Hospital of Jiangsu Province, NY, Jiangsu; Peking University Hospital of Stomatology, BD, Beijing; West China School/Hospital of Stomatology Sichuan University, HX, Sichuan; Beijing Stomatological Hospital, SY, Beijing; Shanghai Public Health Clinical Center, GW, Shanghai.
This work was supported by National Nature Science Foundation of China (81572057), the Fundamental Research Funds for the Central Universities (3332018035) and the Innovation Fund of Peking Union Medical College (No. 2018-1002-01-02).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work
Disclosure
Professor Sharon Chen reports grants from MSD Australia, outside the submitted work. The authors report no further conflicts of interest in this work.
References
1. Brito GN, Inocencio AC, Querido SM, Jorge AO, Koga-Ito CY. In vitro antifungal susceptibility of Candida spp. oral isolates from HIV-positive patients and control individuals. Braz Oral Res. 2011;25(1):28–33.
2. Moges B, Bitew A, Shewaamare A. Spectrum and the in vitro antifungal susceptibility pattern of yeast isolates in Ethiopian HIV patients with oropharyngeal candidiasis. Int J Microbiol. 2016;2016:3037817. doi:10.1155/2016/3037817
3. Sun H, Chen Y, Zou X, et al. Occurrence of oral Candida colonization and its risk factors among patients with malignancies in China. Clin Oral Investig. 2016;20(3):459–467. doi:10.1007/s00784-015-1524-2
4. Mohammadi R, Ataei B. Candidiasis in pediatrics; identification and in vitro antifungal susceptibility of the clinical isolates. Iran J Ped Hematol Oncol. 2016;6(1):43–51.
5. Jayachandran AL, Katragadda R, Thyagarajan R, et al. Oral candidiasis among cancer patients attending a Tertiary Care hospital in Chennai, South India: an evaluation of clinicomycological association and antifungal susceptibility pattern. Can J Infect Dis Med Microbiol. 2016;2016:8758461. doi:10.1155/2016/8758461
6. Berberi A, Noujeim Z, Aoun G. Epidemiology of oropharyngeal candidiasis in human immunodeficiency virus/acquired immune deficiency syndrome patients and CD4+ counts. J Int Oral Health. 2015;7(3):20–23.
7. Silva-Rocha WP, de Brito Lemos VL, Ferreira MR, et al. Effect of the crude extract of Eugenia uniflora in morphogenesis and secretion of hydrolytic enzymes in Candida albicans from the oral cavity of kidney transplant recipients. BMC Complement Altern Med. 2015;15:6. doi:10.1186/s12906-015-0522-x
8. Minea B, Nastasa V, Kolecka A, et al. Etiologic agents and antifungal susceptibility of oral candidosis from Romanian patients with HIV-infection or type 1 diabetes mellitus. Pol J Microbiol. 2016;65(1):123–129.
9. Das PP, Saikia L, Nath R, Phukan SK. Species distribution & antifungal susceptibility pattern of oropharyngeal Candida isolates from human immunodeficiency virus infected individuals. Indian J Med Res. 2016;143(4):495–501. doi:10.4103/0971-5916.184288
10. Clark-Ordonez I, Callejas-Negrete OA, Arechiga-Carvajal ET, Mourino-Perez RR. Candida species diversity and antifungal susceptibility patterns in oral samples of HIV/AIDS patients in Baja California, Mexico. Med Mycol. 2016. doi:10.1093/mmy/myw069
11. Wu J, Guo H, Yi G, et al. Prevalent drug resistance among oral yeasts from asymptomatic patients in Hainan, China. Mycopathologia. 2014;177(5–6):299–307. doi:10.1007/s11046-014-9747-3
12. Geusau A, Antoniewicz L, Poitschek C, Presterl E, Willinger B. In vitro susceptibility of Candida isolates from organ transplant recipients to newer antifungals. Mycopathologia. 2014;177(3–4):143–156. doi:10.1007/s11046-014-9738-4
13. Zhang LW, Fu JY, Hua H, Yan ZM. Efficacy and safety of miconazole for oral candidiasis: a systematic review and meta-analysis. Oral Dis. 2016;22(3):185–195. doi:10.1111/odi.12380
14. Marcos-Arias C, Eraso E, Madariaga L, Carrillo-Munoz AJ, Quindos G. In vitro activities of new triazole antifungal agents, posaconazole and voriconazole, against oral Candida isolates from patients suffering from denture stomatitis. Mycopathologia. 2012;173(1):35–46. doi:10.1007/s11046-011-9460-4
15. Li YY, Chen WY, Li X, et al. Asymptomatic oral yeast carriage and antifungal susceptibility profile of HIV-infected patients in Kunming, Yunnan Province of China. BMC Infect Dis. 2013;13:46. doi:10.1186/1471-2334-13-46
16. Garcia-Cuesta C, Sarrion-Perez MG, Bagan JV. Current treatment of oral candidiasis: a literature review. J Clin Exp Dent. 2014;6(5):e576–e582. doi:10.4317/jced.51798
17. Wang H, Fan YY, Kudinha T, et al. A comprehensive evaluation of the Bruker Biotyper MS and Vitek MS Matrix-Assisted laser desorption ionization-time of flight mass spectrometry systems for identification of yeasts, part of the National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study, 2012 to 2013. J Clin Microbiol. 2016;54(5):1376–1380. doi:10.1128/JCM.00162-16
18. Zhang L, Xiao M, Wang H, et al. Yeast identification algorithm based on use of the Vitek MS system selectively supplemented with ribosomal DNA sequencing: proposal of a reference assay for invasive fungal surveillance programs in China. J Clin Microbiol. 2014;52(2):572–577. doi:10.1128/JCM.02543-13
19.
20.
21.
22. Pfaller MA, Diekema DJ. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and laboratory standards institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50(9):2846–2856. doi:10.1128/JCM.00937-12
23. Blanco D, van Rossem K. A prospective two-year assessment of miconazole resistance in Candida spp. With repeated treatment with 0.25% miconazole nitrate ointment in neonates and infants with moderate to severe diaper dermatitis complicated by cutaneous candidiasis. Pediatr Dermatol. 2013;30(6):717–724. doi:10.1111/pde.12107
24. Paula SB, Morey AT, Santos JP, et al. Oral Candida colonization in HIV-infected patients in Londrina-PR, Brazil: antifungal susceptibility and virulence factors. J Infect Dev Ctries. 2015;9(12):1350–1359. doi:10.3855/jidc.6970
25. Razzaghi-Abyaneh M, Sadeghi G, Zeinali E, et al. Species distribution and antifungal susceptibility of Candida spp. isolated from superficial candidiasis in outpatients in Iran. J Mycol Med. 2014;24(2):e43–e50. doi:10.1016/j.mycmed.2014.01.004
26. Kuriyama T, Williams DW, Bagg J, Coulter WA, Ready D, Lewis MA. In vitro susceptibility of oral Candida to seven antifungal agents. Oral Microbiol Immunol. 2005;20(6):349–353. doi:10.1111/j.1399-302X.2005.00236.x
27. Hamza OJ, Matee MI, Moshi MJ, et al. Species distribution and in vitro antifungal susceptibility of oral yeast isolates from Tanzanian HIV-infected patients with primary and recurrent oropharyngeal candidiasis. BMC Microbiol. 2008;8:135. doi:10.1186/1471-2180-8-135
28. Xiao M, Sun ZY, Kang M, et al. Five-year national surveillance of invasive Candidiasis: species distribution and Azole susceptibility from the China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol. 2018;56(7). doi:10.1128/JCM.00577-18.
29. Guo LN, Xiao M, Cao B, et al. Epidemiology and antifungal susceptibilities of yeast isolates causing invasive infections across urban Beijing, China. Future Microbiol. 2017;12:1075–1086. doi:10.2217/fmb-2017-0036
30. Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48(4):1366–1377. doi:10.1128/JCM.02117-09
31. Kamikawa Y, Mori Y, Nagayama T, et al. Frequency of clinically isolated strains of oral Candida species at Kagoshima University Hospital, Japan, and their susceptibility to antifungal drugs in 2006-2007 and 2012-2013. BMC Oral Health. 2014;14:14. doi:10.1186/1472-6831-14-90
32. Stefaniuk E, Baraniak A, Fortuna M, Hryniewicz W. Usefulness of CHROMagar Candida Medium, Biochemical Methods–API ID32C and VITEK 2 compact and two MALDI-TOF MS systems for Candida spp. identification. Pol J Microbiol. 2016;65(1):111–114.
33. Chao QT, Lee TF, Teng SH, et al. Comparison of the accuracy of two conventional phenotypic methods and two MALDI-TOF MS systems with that of DNA sequencing analysis for correctly identifying clinically encountered yeasts. PLoS One. 2014;9(10):e109376. doi:10.1371/journal.pone.0109376
34. Goldman M, Cloud GA, Smedema M, et al. Does long-term itraconazole prophylaxis result in in vitro azole resistance in mucosal Candida albicans isolates from persons with advanced human immunodeficiency virus infection? Antimicrob Agents Chemother. 2000;44(6):1585–1587.
35. Lortholary O, Petrikkos G, Akova M, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infect. 2012;18(Suppl 7):68–77. doi:10.1111/1469-0691.12042
36. Cartledge JD, Midgley J, Petrou M, Shanson D, Gazzard BG. Unresponsive HIV-related oro-oesophageal candidosis – an evaluation of two new in-vitro azole susceptibility tests. J Antimicrob Chemother. 1997;40(4):517–523.
Supplementary materials
 | Table S1 The clinical breakpoints and epidemiological cutoff values (18−23) against common Candida species used in this study |
 | Table S2 The cases of 21 patients suffered mixed oropharyngeal yeast infections summarized |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.