Back to Journals » International Journal of General Medicine » Volume 17
Cancer-Related Fatigue and Its Influencing Factors Among Colorectal Cancer Patients: A Generalized Linear Modeling Approach
Authors Wang S , Song Y, Zhang H, Song J, Guo X, Jiang X
Received 1 November 2023
Accepted for publication 5 February 2024
Published 16 February 2024 Volume 2024:17 Pages 579—595
DOI https://doi.org/10.2147/IJGM.S447697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Song Wang,1 Yuanyuan Song,2 Huaguo Zhang,3 Jing Song,4 Xiaoyan Guo,5 Xiaolian Jiang1
1West China School of Nursing, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 2Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, People’s Republic of China; 3Department of Nursing, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 4School of Stomatology, Bengbu Medical University, Bengbu, Anhui, People’s Republic of China; 5Department of Pharmacy, Dezhou Municipal Hospital, Dezhou, Shandong, People’s Republic of China
Correspondence: Xiaolian Jiang, West China School of Nursing, West China Hospital, Sichuan University, No. 37, Guoxue Lane, Wuhou District, Chengdu, Sichuan, 610041, People’s Republic of China, Email [email protected]; [email protected]
Purpose: This study aimed to improve cancer-related fatigue (CRF) and health outcomes of colorectal cancer patients by understanding the status quo of CRF, exploring the relations of coping, anxiety symptoms, depressive symptoms, body image perception and CRF, and also identifying the factors affecting CRF based on a generalized linear modeling approach.
Patients and Methods: An exploratory cross-sectional study was conducted on 370 colorectal cancer patients at two hospitals in Anhui Province, China, from July 2020 to February 2021. The data were collected by using general information questionnaire, cancer fatigue scale, simplified coping style questionnaire, generalized anxiety disorder-7 scale, patient health questionnaire-9, and body image scale. Descriptive statistics, t-tests, one-way analysis of variance, Pearson correlation analyses, and generalized linear model analyses were applied to analyze the data.
Results: The average CRF score of the patients was 21.612 (SD=6.160), with a prevalence rate of 69.4% for clinically relevant fatigue. The generalized linear model revealed that: In step 1, gender (female) (B=1.799, Waldχ2=7.506, p=0.006), per capita monthly income (1001– 3000 RMB) (B=− 1.673, Waldχ2=5.536, p=0.019) and treatment modalities (chemotherapy+others) (B=2.425, Waldχ2=8.211, p=0.004) were related to CRF. In step 2, depressive symptoms (B=1.223, Waldχ2=129.019, p< 0.001) and negative coping strategies (B=0.215, Waldχ2=11.347, p=0.001) exhibited significant positive correlations with CRF, positive coping strategies (B=− 0.319, Waldχ2=59.175, p< 0.001) showed significant negative correlations with CRF; While anxiety symptoms (B=0.162, Waldχ2=1.840, p=0.175) and body image perception (B=0.013, Waldχ2=0.048, p=0.826) had no correlations with CRF.
Conclusion: The prevalence of CRF was relatively high among colorectal cancer patients. Coping and depressive symptoms were the modifiable influencing factors of CRF. Tailored interventions dedicated to promoting positive coping behavior, diminishing negative coping behavior and reducing depressive symptoms may improve the CRF of patients with colorectal cancer. Healthcare providers working with these patients should receive corresponding education and training in these complementary treatments. Additionally, when developing non-pharmacological interventions, appropriate consideration of the patients’ gender, income condition and the type of anticancer treatment is also necessary.
Keywords: colorectal cancer, cancer-related fatigue, coping style, anxiety, depression, body image distress
Introduction
Colorectal cancer is the third most common malignancy around the world and the second most common in China, with standardized incidence rates of 19.5 per 100,000 and 23.9 per 100,000 in 2020, respectively.1,2 It is predicted that the number of new cases of colorectal cancer will reach 3.2 million worldwide in 2040.3 Colorectal cancer has brought a heavy disease burden to the current society and become a growing global public health issue. Therefore, it is necessary to raise public attention to the population of colorectal cancer.
With the continuous progress and development of diagnosis and treatment techniques for cancers, the overall survival for colorectal cancer has been prolonged and the survival rate has also increased.4–6 Although various screening programs and treatment modalities of colorectal cancer bring considerable benefits for patients, they also lead to a raft of symptom burdens, which have serious impact on the quality of life of patients. Cancer-related fatigue (CRF) is one of the most disabling and distressing symptoms reported by people diagnosed with cancer, especially among patients undergoing chemotherapy, radiotherapy, and biological therapy.7 CRF was defined as “a distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” by the National Comprehensive Cancer Network (NCCN).8 CRF was pervasive in cancer patients. The prevalence of CRF varied across different studies depending on the cancer types, clinical stages, treatment methods, assessment tools and measurement time, research design, sample size, etc. In a meta-analysis including 84 studies of patients with mixed types of cancer, the pooled prevalence of CRF was reported to be 52.0%,9 which was relatively high. The prevalence of CRF among short-term (within 5 years since diagnosis) and long-term survivors (>5 years since diagnosis) with colorectal cancer were 41% and 35%, respectively.10 CRF exerts profound detrimental effects on the physical, psychological, social functioning, and economic aspects for both patients and their primary family members; and may degrade quality of life, affect treatment compliance and future rehabilitation, and reduce overall survival of patients.11–14
As a symptom with high incidence and severe adverse outcomes, CRF is often neglected by patients and medical staff, it has been underestimated and inadequately managed in clinical settings.15,16 One of the main barriers to the effective management may be associated with a lack of evidence about the influencing factors and personalized interventions of CRF.17 Thus, early understanding and identifying the factors affecting CRF is of vital importance in developing a tailored intervention to alleviate this symptom for cancer populations.
Given the subjective and multidimensional nature of CRF, the influencing factors of CRF are complex and multifaceted, including demographic, cancer- or treatment-related, clinical, psychosocial, behavioral, physiological, genetic factors, and so on.18,19 Despite the emergence of a large number of studies on CRF, relevant studies regarding the influencing factors of CRF mainly concentrated on the patients with lung cancer or breast cancer, there was a paucity of literature that examined factors (especially some modifiable psychosocial factors) associated with CRF in colorectal cancer patients. Additionally, the relationships between CRF and its associated factors, such as gender, age, marital status, occupational status, anxiety and depressive symptoms, and other psychosocial factors, remain discrepant and inconclusive.9,18,20–22
From the perspectives of supportive care, understanding the modifiable or controllable psychosocial factors of CRF, early identifying the patients at a high risk of experiencing fatigue, and offering some tailored interventions are of great significance for improving the quality of life and positive health outcomes of cancer patients. Cancer itself and related treatments may also bring a series of psychosocial concerns to patients, such as various negative emotions (eg, anxiety, depression), changes in coping styles (eg, coping with avoidance or denial), changes to patients’ appearance (eg, body image concerns),23 etc. Up to now, the research on the psychosocial factors affecting CRF in colorectal cancer patients are relatively scarce. In addition, it is not clear if these existing psychological variables (symptoms of depression and anxiety) lead to CRF or are caused by CRF, these causal relationships need to be further clarified in subsequent studies. To our knowledge, the influence of different coping styles and body image perception on CRF among colorectal cancer patients has rarely been examined before. Two systematic reviews reported that body image distress had been linked to chronic fatigue among breast cancer patients;24,25 however, the relationship between body image and fatigue in patients with colorectal cancer is unknown. Therefore, the purpose of this study was to understand the relations of coping, anxiety symptoms, depressive symptoms, body image perception and CRF among colorectal cancer patients, particularly explore the impact of these psychosocial factors on CRF, construct a preliminary model on influencing factors, help healthcare providers develop effective interventions and promote CRF management for colorectal cancer population.
Patients and Methods
Design and Participants
This investigation was an exploratory cross-sectional study reported in compliance with the STROBE checklist (https://www.strobe-statement.org/). A convenience sample of 370 colorectal cancer patients undergoing postoperative adjuvant therapy was recruited from the oncology division, the radiation oncology division, and the gastrointestinal surgery division of two hospitals affiliated to a medical university in Anhui Province, China between July 2020 and February 2021. G*Power version 3.1.9.7 software (available from https://stats.oarc.ucla.edu/other/gpower/) was used to estimate the sample size.26 When the medium effect size f2 of multiple linear regression for F-test was 0.15, an α error probability=0.05, a power (1-β error probability)=0.80,27 the number of predictors in our study was 19, the minimum sample size should be 153. Considering a drop-out rate of 20%, the optimal number of participants was 184.
The inclusion criteria of the cases were as follows: (1) people diagnosed with colorectal cancer by histopathological examination; (2) age of 18 years old or more; (3)Karnofsky performance status score more than 60 points; (4) awareness of the illness diagnosis and undergoing postoperative adjuvant therapy at least once; and (5) willing to take part in the study and sign the informed consent. The exclusion criteria of the cases were as detailed below: (1) people within a month after surgery; (2) with primary malignancies in other sites; (3) with other serious life-threatening diseases; (4) with language or written communication difficulties; and (5) with cognitive impairment, or a history of psychiatric illness.
Study Process
The study was agreed by the Ethics Committee of the relevant hospitals (Approval Number: 2020#482). The investigators selected potential participants through the patient information platform (PIP), which mainly included a list of patient bed information and an electronic medical record system. The researchers first screened the patients diagnosed with colorectal cancer through a list of patient bed information, and then reviewed their medical records through an electronic medical record system to identify the eligible patients; finally, contacted and invited them to participate in our study. Before the formal investigation, the purposes, significance, and detailed procedures of the study were explained to the respondents who met all inclusion criteria using unified instructions. After signing the informed consent, the questionnaires were administered to the patients who were asked to fill in the self-reported questionnaires within 10–20 minutes. To avoid mutual interference between the patients in the same ward and protect their confidentiality or sensitive information, we placed them in a relatively separate and quiet room to complete the questionnaires. During the investigation, the patients independently completed the questionnaires according to their actual situations; If the patients had any questions about the contents of questionnaires, the investigator would assist in answering them. The researchers checked the integrity of the submitted questionnaires and informed patients to fill in any omissions. All data of the patients were confidential and anonymous. After each data was encoded, and then collated and analyzed. Any personal information about the patients would not appear in the literature. The data were input into statistical software and reviewed by two researchers.
Variables and Measurements
Sociodemographic and Disease-Related Data Questionnaire
The questionnaire was self-designed by the research group, including fourteen variables, eg, gender, age, educational level, marital status, primary residence, dwelling state, family per capita monthly income, and employment status; cancer site, clinical stages, treatment modalities, number of chemotherapy cycles, stoma, and other chronic diseases. The above data were collected from the patients’ medical records, when the information was incomplete, the investigators could obtain relevant data from the patients.
Cancer Fatigue Scale(CFS)
The CFS was designed by Okuyama to assess the fatigue severity of cancer patients.28 It is a 15-item self-rating scale, consisting of 3 domains: physical fatigue, affective fatigue, and cognitive fatigue. A Likert five-point rating method is used on a scale of 0(not at all) to 4(very much), the total score of CFS is 0 to 60 points, with higher scores representing more severe fatigue. The cut-off value of CFS is 18/19, the total score of CFS ≥18 points is defined as “clinical fatigue”.29 Clinical fatigue refers to the patient being disturbed by at least one of the following aspects: fatigue interfered with (a) walking ability, (b) sleep, (c) normal work, (d) mood, (e)relationships with others, (f) enjoyment of life, and (g) general activities of life, in the past 24 hours.29 Some good psychometric properties of CFS in cancer patients have been reported in a previous study.28 The Chinese version of CFS has also been proven to have satisfactory reliability and validity.30,31 The Cronbach’s alpha coefficient for this scale in the present sample was 0.93.
Simplified Coping Style Questionnaire(SCSQ)
The SCSQ is a self-reported questionnaire developed by Xie in 1998 for assessing coping patterns that individuals adopt when they encounter difficulties and/or setbacks.32 The scale includes 20 items and 2 dimensions: positive coping styles (items 1 to 12) and negative coping styles (items 13 to 20). Each item is scored on a four-point Likert scale ranging from 0(never) to 3(very often). The total scores of positive coping and negative coping range from 0 to 36 points, 0 to 24 points, respectively. Individuals with higher positive/negative coping scores were more likely to take positive/negative coping strategies.33 Positive coping strategies: such as relieving oneself through work, learning, or other meaningful activities; talking with others and disclosing inner troubles; trying to find the good side of things. Negative coping strategies: such as attempting to put problems aside through rest or vacation; eliminating troubles through smoking, drinking, eating, etc; believing that “time will change the status quo, the only thing to do is waiting”. The SCSQ (Chinese version) was widely used in patients with cancer, the Cronbach’s alpha coefficients for positive coping and negative coping subscales in our study were 0.86 and 0.83, respectively.
Generalized Anxiety Disorder-7 (GAD-7) Scale
The 7-item GAD scale was developed by Spitzer34 for screening the cases with generalized anxiety disorders and assessing the severity of anxiety symptoms, with Cronbach’s alpha coefficient of 0.92. The GAD-7 is scored on a four-point Likert-type scale ranging from 0(not at all) to 3(almost every day). The total score of GAD-7 is 0 to 21 points, the higher the score, the more severe the anxiety. A GAD-7 score of 0–4 indicates without anxiety, ≥5 indicates anxiety (5–9: “mild anxiety”; 10–14: “moderate anxiety”; and 15–21: “severe anxiety”).34 The GAD-7 scale has been verified to be reliable among Chinese inpatients, with Cronbach’s alpha coefficient of 0.90.35 In the current study of colorectal cancer patients, the Cronbach’s alpha coefficient for GAD-7 scale was 0.83.
Patient Health Questionnaire-9(PHQ-9)
The PHQ-9 developed by Kroenke36 is a reliable and valid 9-item self-reported measure of depression severity, with Cronbach’s alpha coefficient of 0.89. Each item of the PHQ-9 is scored on a four-point Likert scale ranging from 0(not at all) to 3(almost every day). The total score of PHQ-9 ranges from 0 to 27 points, the higher the score, the more severe the depression.37 A PHQ-9 score of 0–4 represents without depression, ≥5 represents depression (5–9: “mild depression”; 10–14: “moderate depression”; 15–27: “moderately severe to severe depression”). In the present study of colorectal cancer patients, the Cronbach’s alpha coefficient for PHQ-9 was 0.80.
Body Image Scale(BIS)
The BIS is a 10-item self-rating measure developed by Hopwood38 to assess participants’ feelings and changes about their bodies and appearances. Zhang revised the scale into Chinese version and applied it to Chinese cancer populations.39 The BIS has 3 dimensions: affective dimension (items 1, 2, 4, 6), behavioral dimension (items 5, 7), and cognitive dimension (items 3, 8, 9, 10). It uses a four-point response scale (0=“not at all” to 3=“very much”) and the total score ranges from 0 to 30 points, with higher scores indicating the higher levels of body image distress, the total score of the scale ≥10 is defined as body image disorder.39 The original BIS demonstrated satisfactory psychometric characteristics, with Cronbach’s alpha coefficient of 0.93.40 In the present survey, this measure also showed better reliability (Cronbach’s alpha coefficient=0.85).
Statistical Analyses Methods
Data processing and analysis were performed using IBM SPSS (Version 28.0) software and Jamovi software (Version 2.3.21, download from https://www.jamovi.org/download.html), along with Storm Statistical Platform (www.medsta.cn/software). Sociodemographic and colorectal cancer-related clinical characteristics of the patients were described by frequencies (n) and percentages (%). The scores of CRF, coping styles, anxiety, depression, and body image perception were presented using means, standard deviations (SDs), and 95% confidence intervals (CIs). Independent samples t-test, one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test were used to compare the differences of CFS scores in participants with different sample groups. Pearson’s correlation analyses were employed to test the relationships between coping styles, anxiety, depression, body image perception, and CRF. The results of correlation analyses were shown in a heatmap generated by Jamovi software (Version 2.3.21). A generalized linear model was constructed to identify the factors that affect CRF and its dimensions. Statistically significant independent variables in univariate analyses were included into generalized linear model analyses. A p-value of ≤0.05 was considered statistically significant.41
Results
Sociodemographic and Disease-Related Characteristics of the Sample
Initially, a total of 370 eligible patients with colorectal cancer were approached, of whom 363 returned complete and valid responses, 7 were excluded due to duplicated data, thus 363 were finally included for data analyses (effective response rate of 98.1%). Among these patients, 62.8% were men, 94.2% were middle-aged and elderly, 94.5% were married, 89.8% were unemployed; most patients had primary school or below education (45.7%), came from rural areas (57.3%), lived with their family members (97.2%), had an average monthly income of the family of ≤1000 RMB (47.1%). 51.2% were colon cancer patients, 79.1% had a III or IV cancer staging, 83.2% undergone chemotherapy only, 92.6% had ≤6 chemotherapy cycles, 76.9% had no stoma, and 63.4% had no other chronic diseases. Other information are shown in Table 1.
 |
Table 1 Sociodemographic and Disease-Related Characteristics of the Patients (n=363) |
CFS Scores in Patients with Colorectal Cancer
The average score of CFS was 21.612 (SD=6.160, range 8–39, 95% CI: 20.976–22.247), with a prevalence rate of 69.4% (252/363, 95% CI: 0.644–0.741) for clinical fatigue (CFS score ≥18). As to the three dimensions of CFS, the mean score of physical fatigue was 8.799 (SD=3.619, range 1–20, 95% CI: 8.425–9.172); the mean score of affective fatigue was 8.218 (SD=1.866, range 2–13, 95% CI: 8.025–8.410); the mean score of cognitive fatigue was 4.595 (SD=1.908, range 0–14, 95% CI: 4.398–4.792).
There were statistically significant differences in CFS scores among patients with different genders (t=−3.296, p=0.001), average family monthly incomes (F=3.568, p=0.029), clinical stages (t=−2.822, p=0.005), treatment modalities (t=−3.391, p=0.001), number of chemotherapy cycles (t=−2.469, p=0.014) groups (see Table 2); The mean scores of CFS were significantly higher among patients who were women, those with per capita monthly income of less than 1000 RMB, those with clinical stages III or IV, those who had received chemotherapy+ (targeted therapy/immunotherapy), those with number of chemotherapy cycles more than 6 times (see Figure 1). For the categorical variable of average monthly income of the family, Bonferroni post-hoc analysis was further conducted. The multiple comparisons test results showed that the CFS score of patients with per capita monthly income of less than 1000 RMB was higher than that of patients with per capita monthly income of 1001–3000 RMB, the difference was statistically significant, p<0.05. The information of other variables are shown in Table 2.
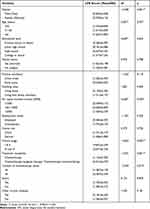 |
Table 2 Differences Analyses of CFS Scores in Different Sample Groups (n=363) |
The Scores of Psychosocial Variables (Coping Styles, Anxiety, Depression, and Body Image)
The scores of SCSQ, GAD-7, PHQ-9, and BIS are shown in Table 3. The mean scores of positive coping and negative coping were 16.653 (SD=5.508, 95% CI: 16.084–17.221) and 8.289 (SD=3.418, 95% CI: 7.937–8.642), respectively, which were relatively low compared to their total scores. The top five positive coping strategies for the mean score of items were: “Try to see the good side of things as much as possible” (Mean=1.750, SD=0.575), “Don’t take some problems too seriously” (Mean=1.630, SD=0.592), “Relieve oneself through work, learning, or other meaningful activities” (Mean=1.620, SD=0.754), “seeking support from others” (Mean=1.480, SD=0.641), “talking with others and disclosing inner distress or troubles” (Mean=1.400, SD=0.764); The top five negative coping strategies for the mean score of items were: self-consolation (Mean=2.020, SD=0.637), acceptance of reality (Mean=1.330, SD=0.750), fantasy (Mean=1.290, SD=0.796), relying on others to solve problems (Mean=1.220, SD=0.738), and trying to forget some unpleasant things (Mean=0.990, SD=0.889). Identifying the most frequent coping strategies used by colorectal cancer patients can inform the development of tailored interventions.
 |
Table 3 The Scores of SCSQ, GAD-7, PHQ-9, and BIS of the Patients (n=363) |
The mean score of anxiety was 2.488 with a prevalence of 23.4% and that of depression 3.468 with a prevalence of 28.4%. The percentages of different severity of anxiety and depression are shown in Figure 2, the degree of anxiety or depression was mainly mild. The mean score of BIS was 5.419 with an incidence of 11.8% for body image disorder.
 |
Figure 2 The percentages of different severity of anxiety and depression. |
Correlations between CRF and Psychosocial Variables
Pearson’s correlation analyses revealed that the scores of negative coping, anxiety, depression, and BIS were significantly positively correlated with CFS score (r=0.46, r=0.61, r=0.76, r=0.30, respectively; all p<0.001). Positive coping score was significantly negatively correlated with CFS score (r=−0.57, p<0.001). Besides, the levels of depression and CFS score yielded the highest correlation (r=0.76, p<0.001) (see Figure 3).
Generalized Linear Model Analysis
To identify independent factors affecting the participants’ CRF severity, the scores of CFS and its three dimensions were employed as the dependent variables respectively, the above-mentioned related variables with p<0.05 (gender, average monthly income of the family, cancer staging, treatment modalities, number of chemotherapy cycles, positive coping, negative coping, anxiety, depression, body image) in univariate analyses were used as the independent variables. A generalized linear model was established (see Table 4). The results showed that: In model 1, gender (female) (B=1.799, Waldχ2=7.506, p=0.006), per capita monthly income (1001–3000 RMB) (B=−1.673, Waldχ2=5.536, p=0.019) and treatment modalities (chemotherapy+others) (B=2.425, Waldχ2=8.211, p=0.004) exhibited significant correlations with CRF among colorectal cancer patients. In model 2, the psychosocial factors of depressive symptoms (B=1.223, Waldχ2=129.019, p<0.001), positive coping (B=−0.319, Waldχ2=59.175, p<0.001) and negative coping (B=0.215, Waldχ2=11.347, p=0.001) showed significant correlations with CRF among colorectal cancer patients; While anxiety symptoms (B=0.162, Waldχ2=1.840, p=0.175), body image perception (B=0.013, Waldχ2=0.048, p=0.826) and other factors had no correlations with CRF.
 |
Table 4 Generalized Linear Model Analysis on the Factors Affecting CRF (n=363) |
Positive coping and depressive symptoms predominantly influenced the physical, affective and cognitive aspects of fatigue; Negative coping predominantly influenced the physical and affective aspects of fatigue; Per capita monthly income predominantly influenced the physical and cognitive aspects of fatigue (see Table 5). The results suggest that we should develop targeted interventions based on the specific characteristics of fatigue.
 |
Table 5 Influencing Factors of Physical, Affective, and Cognitive Fatigue of CRF (n=363) |
Influencing Factors Model of CRF Among Colorectal Cancer Patients
Based on the findings of this study, we preliminarily constructed a model on the influencing factors of CRF in colorectal cancer patients (see Figure 4). When managing fatigue among colorectal cancer patients, health professionals are advised to be alert to these influencing factors in this model. Whether this model is suitable for other cancer populations, further validation is needed through future research.
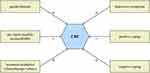 |
Figure 4 Influencing factors model of CRF among colorectal cancer patients. Abbreviation: CRF, cancer-related fatigue. Notes: +: positive correlation; -: negative correlation. |
Discussion
CRF is a common side effect of cancer and its treatments among colorectal cancer patients, with reported prevalence rates ranging from 57% to 100%;42,43 however, it has not been effectively managed and treated in clinical practice partially due to its complicated and multiple influencing factors. A comprehensive understanding and evaluation regarding the related factors of CRF is particularly necessary to promote fatigue management and improve clinical outcomes for this population.44 Being diagnosed with a cancer and receiving related treatments can lead to the change of coping patterns (eg, coping with avoidance, passiveness, and dependence), psychological distress (eg, anxiety, depression, fear, and anger), and body image concerns (eg, stoma, scarring, skin color change, and loss of hair or body parts) in patients with colorectal cancer. Therefore, this study selected the following common variables, coping styles, anxiety, depression, and body image as major psychosocial factors, and tried to determine their effects on CRF. The findings of final generalized linear model showed that: the psychosocial factors of depressive symptoms, positive coping, and negative coping were independent influencing factors of CRF; whereas, anxiety symptoms, body image perception were not. Depressive symptoms and positive coping mainly influenced the physical, affective and cognitive dimensions of fatigue; Negative coping mainly influenced the physical and affective dimensions of fatigue.
The present survey demonstrated that CRF was also pervasive among colorectal cancer patients receiving postoperative adjuvant therapy, the mean score of CFS was 21.612 (at moderate fatigue level), with an incidence of 69.4% for clinical fatigue. As for the scores of the three subscales of CFS, the physical fatigue had the highest score and the cognitive fatigue yielded the lowest score, indicating that CRF had the greatest impact on the physical function of colorectal cancer patients. These findings were similar to those reported by Deng in postoperative patients with colorectal cancer.45 However, the incidence and mean score of CRF in the present sample were lower than those reported by Wei’s study of colorectal cancer patients after fast-track surgery, in their report, the mean score of CRF was 43.70 (at severe fatigue level) with an incidence of 100%.43 A longitudinal study using Fatigue Assessment Scale (FAS) to evaluate the course of fatigue among colorectal cancer patients and their partners found that: Fatigue scores for patients at preoperative measurement, postoperative 3, 6, 12 months follow-up were 19.2±5.56, 22.3±6.7, 21.7±7.0, 20.6±6.5, respectively.46 The incidences of fatigue for patients at preoperative measurement, postoperative 3, 6, 12 months follow-up were 23%, 46%, 43%, 32%, respectively.46 A meta-analysis revealed that fatigue was the most severe symptom (mean=50.14) with a pooled frequency of 38.1% among colorectal cancer survivors after cancer treatments.47 The above findings were different from ours. These inconsistencies may be related to the patients’ conditions, time since surgery, course of disease, treatment methods, fatigue assessment tools, and study design. In short, the relatively high incidence and levels of CRF suggested the necessity for medical staff to strengthen the assessment and management of CRF for colorectal cancer patients.
Coping styles refer to the methods and strategies adopted by individuals to reduce or avoid stress and adapt to the new situation, which usually include positive coping styles and negative coping styles. Adequate and good coping strategies are essential for patients to deal with cancer- and/or treatment-related symptoms,48 such as fatigue. The results of descriptive statistics showed that the scores of positive coping and negative coping subscales were 16.653 and 8.289, respectively, which were relatively low compared with the total score of each subscale. The mean scores of items regarding the positive coping and negative coping subscales were 1.388 (16.653/12) and 1.036 (8.289/8), respectively. Despite the relatively low scores of positive coping and negative coping, thankfully the majority of patients with colorectal cancer tended to adopt positive, problem-solving centered, or adaptive coping strategies in our study, such as trying to discover the beneficial side of things, diverting attention through some meaningful activities, disclosing distress or troubles to others, seeking support, changing the value system, etc. Generalized linear model revealed that positive coping was a negative predictor of CRF (B=−0.319, p<0.001), while negative coping was a positive predictor of CRF (B=0.215, p=0.001), which were basically consistent with Jiang’s findings in breast cancer patients.49 Previous study demonstrated that positive coping could reduce patients’ psychological distress such as anxiety and depression to a certain extent, make them face the disease bravely, accept the reality calmly, cooperate with treatment positively, seek social support actively, and develop healthy behaviors, thereby alleviate CRF.50 Good coping strategies can also enhance self-efficacy and quality of life. Healthcare professionals can promote patients’ positive coping by providing solution-focused nursing intervention, stress management methods (eg, muscle relaxation exercises, mindfulness meditation), education and training on coping strategies, self-efficacy enhancing intervention, social support resources, etc, and guide them to adopt more positive and effective coping strategies, and avoid negative or maladaptive coping strategies, so as to enhance psychological adaptability and reduce CRF. Coping styles can be strongly influenced by cultural factors, such as cultural norms and values, educational levels, etc. For example, our study found that colorectal cancer patients with a college education or above scored significantly higher in positive coping than those with other educational levels. It should be noted that positive coping and negative coping are relative; positive coping does not necessarily have positive effects, or negative coping necessarily results in negative consequences. Different coping styles may yield different outcomes for different individuals at different times and in different scenarios.32 Further research is warranted to identify the most effective coping mechanisms in this cancer population.
Depression is one of the common psychological distressing symptoms, which may seriously affect the psychological adjustment and quality of life of colorectal cancer patients. In our study, the prevalence of depression in patients with colorectal cancer undergoing postoperative adjuvant therapy was 28.4%, indicating that depression was also relatively common in this population, corresponded with prior research.51 Based on the results of generalized linear model, depressive symptoms exerted significant effects on CRF (B=1.223, p<0.001), suggesting that the higher the levels of depression, the higher the scores of CRF. Similarly, Deng52 and Ma9 also found this relationship in their research; however, this relationship was not found in Li’s study,53 which may be related to the different study designs, cancer types, measurement tools of CRF, and so on. Depression itself, or by resulting in other symptoms, affects the patients’ health outcomes, including increased mortality and decreased quality of life.54 Therefore, healthcare providers should take some tailored psychosocial interventions (eg, cognitive behavioral therapy, mindfulness-based stress reduction therapy, acceptance and commitment therapy, art therapy, etc) to help cancer patients regulate their emotions, alleviate depression and CRF.
Due to cancer and its related treatments, colorectal cancer patients often experience a variety of physical and psychosocial concerns, such as stoma, hair or body parts loss, lower sexual function, stigma, social alienation. These problems may affect their body image, self-identity, and cause body image distress or disorder, which is more common in colorectal cancer patients during the postoperation and treatment stages. Body image was defined as an individual’s subjective thoughts and perceptions of their physical appearance.23 The levels of body image were assessed using BIS, a brief instrument for measuring body image changes in cancer patients. In the present study, the mean score of BIS was 5.419, with a prevalence rate of 11.8% for body image disorder. The levels of body image were consistent with the findings of previous study, but the incidence of body image disorder was lower than that reported in Song’s study,55 which may be associated with the different cut-off values of body image disorder, in their study, a BIS score of ≥5 was defined as having body image distress. In Han’s systematic review, the pooled frequency of body image distress was reported to be 78.5% among colorectal cancer survivors,47 which was much higher than our research findings. As far as we know, this study was the first to test the relationship between body image and CRF among colorectal cancer patients. Although univariate analysis exhibited a correlation between body image and CRF (r=0.30, p<0.001), surprisingly, the results of generalized linear model showed that body image was not an independent predictor of CRF among colorectal cancer patients. Regarding the relationship between body image and CRF in other cancer populations, further exploration is needed.
Body image disorder is related to anxiety, depression, and poorer quality of life of patients with colorectal cancer, but is often overlooked.55,56 Body image disorder can cause social alienation, which leads to higher rates of morbidity and mortality, having a satisfactory perceived body image contributes to better treatment and health outcomes and a better ability to deal with the disease.23 Compared to other symptoms, body image disorder was rarely discussed between clinicians and survivors with colorectal cancer.57 Hence, medical workers should also pay specific attention to those patients with body image concerns, tailor communication and take appropriate psychological interventions (eg, psychoeducation about the cognitive model of body image, cognitive-behavioral therapy on building a renewed image after cancer treatment)58 accordingly, so as to help them correct wrong cognition, promote psychological adjustment, and contribute to good clinical outcomes.
Limitations and Strengths
There are several limitations that should be mentioned in the present study. Firstly, the study was performed in a single geographic region in Anhui Province, China. Therefore, our sample may not entirely represent all colorectal cancer patients around the world. Secondly, in view of the potential biases in questionnaire surveys, such as recall bias, could not be avoided. Lastly, this kind of cross-sectional study may be insufficient to establish a definitive causal relationship between CRF and its psychosocial factors, the conclusions are exploratory and should be treated with caution. Future large-scale prospective studies are needed to further confirm these causal relationships.
Despite the above mentioned shortcomings, this study extended and enriched the existing findings by analyzing the influencing factors of CRF from the perspectives of supportive care. The results of the study provided fundamental data for the development and implementation of effective interventions to improve the CRF of patients with colorectal cancer.
Conclusion
The preliminary findings of this study indicated that depressive symptoms, positive coping, and negative coping were significant modifiable influencing factors of CRF among colorectal cancer patients. Active coping strategies exhibited significant negative correlations with CRF, depressive symptoms and passive coping strategies showed significant positive correlations with CRF, depression levels and CRF yielded the highest correlation. In order to improve the CRF of patients with colorectal cancer, it would be necessary to develop tailored interventions for promoting positive coping behavior, diminishing negative coping behavior and reducing depressive symptoms. Additionally, appropriate consideration of the patients’ gender (female), income condition (low level) and the type of anticancer treatment is also needed. Coping strategies learning could mitigate depression and anxiety, improve quality of life of colorectal cancer patients. Healthcare providers working with these patients should receive corresponding education and training in these complementary approaches, so as to better improve the patients’ health outcomes.
Data Sharing Statement
The data sets used and analyzed for the study were available from the corresponding author and the first author on reasonable request.
Ethical Approval and Consent to Participate
This study was performed in accordance with the principles of the Helsinki Declaration. Approval was granted by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University and The First Affiliated Hospital of Bengbu Medical University & Affiliated Cancer Hospital of Bengbu Medical University (Approval number: 2020#482). Informed consent was obtained from all individual participants included in the study.
Acknowledgments
We would like to express our gratitude to all the participants. We also thank Jing Zhang for her assistance in communication with the managers of relevant hospitals. FLDFH
Funding
This work was supported by Science and Technology Plan Project of Sichuan Province (2020YFS0157, PI: Xiaolian Jiang).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Liu Z, Li Z, Zhang Y, et al. Interpretation on the report of global cancer statistics 2020. J Multidiscip Cancer Manag. 2021;7(2):1–13. doi:10.12151/JMCM.2021.02-01
3. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–732. doi:10.1038/s41575-019-0189-8
4. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174. doi:10.1016/j.tranon.2021.101174
5. Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi:10.1136/gutjnl-2014-309086
6. Rabeneck L, Chiu HM, Senore C. International perspective on the burden of colorectal cancer and public health effects. Gastroenterology. 2020;158(2):447–452. doi:10.1053/j.gastro.2019.10.007
7. Al Maqbali M. Cancer-related fatigue: an overview. Br J Nurs. 2021;30(4):S36–S43. doi:10.12968/bjon.2021.30.4.S36
8. Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(1):64–84. doi:10.6004/jnccn.2019.0001
9. Ma Y, He B, Jiang M, et al. Prevalence and risk factors of cancer-related fatigue: a systematic review and meta-analysis. Int J Nurs Stud. 2020;111:103707. doi:10.1016/j.ijnurstu.2020.103707
10. Husson O, Mols F, van de Poll-Franse LV, Thong MS. The course of fatigue and its correlates in colorectal cancer survivors: a prospective cohort study of the profiles registry. Support Care Cancer. 2015;23(11):3361–3371. doi:10.1007/s00520-015-2802-x
11. Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist. 2000;5(5):353–360. doi:10.1634/theoncologist.5-5-353
12. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3(7):961–968. doi:10.1001/jamaoncol.2016.6914
13. Bower JE, Asher A, Garet D, et al. Testing a biobehavioral model of fatigue before adjuvant therapy in women with breast cancer. Cancer. 2019;125(4):633–641. doi:10.1002/cncr.31827
14. Adam S, van de Poll-Franse LV, Mols F, et al. The association of cancer-related fatigue with all-cause mortality of colorectal and endometrial cancer survivors: results from the population-based profiles registry. Cancer Med. 2019;8(6):3227–3236. doi:10.1002/cam4.2166
15. Schmidt ME, Bergbold S, Hermann S, Steindorf K. Knowledge, perceptions, and management of cancer-related fatigue: the patients’ perspective. Support Care Cancer. 2021;29(4):2063–2071. doi:10.1007/s00520-020-05686-5
16. Pearson EJ, Morris ME, Mckinstry CE. Cancer-related fatigue: a survey of health practitioner knowledge and practice. Support Care Cancer. 2015;23(12):3521–3529. doi:10.1007/s00520-015-2723-8
17. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi:10.1038/nrclinonc.2014.127
18. Mitchell SA. Cancer-related fatigue: state of the science. PM R. 2010;2(5):364–383. doi:10.1016/j.pmrj.2010.03.024
19. Koornstra RH, Peters M, Donofrio S, van den Borne B, de Jong FA. Management of fatigue in patients with cancer--a practical overview. Cancer Treat Rev. 2014;40(6):791–799. doi:10.1016/j.ctrv.2014.01.004
20. Susanne K, Michael F, Thomas S, Peter E, Andreas H. Predictors of fatigue in cancer patients: a longitudinal study. Support Care Cancer. 2019;27(9):3463–3471. doi:10.1007/s00520-019-4660-4
21. Peoples AR, Roscoe JA, Block RC, et al. Nausea and disturbed sleep as predictors of cancer-related fatigue in breast cancer patients: a multicenter NCORP study. Support Care Cancer. 2017;25(4):1271–1278. doi:10.1007/s00520-016-3520-8
22. Tabrizi FM, Alizadeh S. Cancer related fatigue in breast cancer survivors: in correlation to demographic factors. Maedica. 2017;12(2):106–111.
23. Albert JG, Lo C, Rosberger Z, et al. Biopsychosocial markers of body image concerns in patients with head and neck cancer: a prospective longitudinal study. Curr Oncol. 2022;29(7):4438–4454. doi:10.3390/curroncol29070353
24. Davis C, Tami P, Ramsay D, et al. Body image in older breast cancer survivors: a systematic review. Psychooncology. 2020;29(5):823–832. doi:10.1002/pon.5359
25. Paterson CL, Lengacher CA, Donovan KA, Kip KE, Tofthagen CS. Body image in younger breast cancer survivors: a systematic review. Cancer Nurs. 2016;39(1):E39–E58. doi:10.1097/NCC.0000000000000251
26. Faul F, Erdfelder E, Lang AG, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi:10.3758/bf03193146
27. Choi J, Kim S, Choi M, Hyung WJ. Factors affecting the quality of life of gastric cancer survivors. Support Care Cancer. 2022;30(4):3215–3224. doi:10.1007/s00520-021-06683-y
28. Okuyama T, Akechi T, Kugaya A, et al. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19(1):5–14. doi:10.1016/s0885-3924(99)00138-4
29. Okuyama T, Tanaka K, Akechi T, et al. Fatigue in ambulatory patients with advanced lung cancer: prevalence, correlated factors, and screening. J Pain Symptom Manage. 2001;22(1):554–564. doi:10.1016/s0885-3924(01)00305-0
30. Shun S, Beck SL, Pett MA, Berry PH. Psychometric testing of three Chinese fatigue instruments in Taiwan. J Pain Symptom Manage. 2006;32(2):155–167. doi:10.1016/j.jpainsymman.2006.02.011
31. Zhang FL, Ding Y, Han LS. Reliability and validity of the Chinese version of cancer fatigue scale. Chin J Ment Health. 2011;25(11):810–813.
32. Xie YN. A preliminary study on reliability and validity of simplified coping style questionnaire. Chin J Clin Psychol. 1998;6(2):53–54.
33. Nie A, Su X, Zhang S, Guan W, Li J. Psychological impact of covid-19 outbreak on frontline nurses: a cross-sectional survey study. J Clin Nurs. 2020;29(21–22):4217–4226. doi:10.1111/jocn.15454
34. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092
35. Wang Y, Chen R, Zhang L. Evaluation of the reliability and validity of the generalized anxiety disorder 7-item scale among inpatients in general hospital. J Clin Psychiatry. 2018;28(3):168–171.
36. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
37. Negeri ZF, Levis B, Sun Y, et al. Accuracy of the patient health questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis. BMJ. 2021:
38. Hopwood P, Fletcher I, Lee A, Al GS. A body image scale for use with cancer patients. Eur J Cancer. 2001;37(2):189–197. doi:10.1016/s0959-8049(00)00353-1
39. Zhang Y. Study on Self-Image and Quality of Life of Patients with Prostate Cancer. Shanghai: Fudan University; 2010.
40. Khang D, Rim HD, Woo J. The Korean version of the body image scale-reliability and validity in a sample of breast cancer patients. Psychiatry Invest. 2013;10(1):26–33. doi:10.4306/pi.2013.10.1.26
41. Li X. Medical Statistics. Beijing: Higher Education Press; 2016.
42. Vardy JL, Dhillon HM, Pond GR, et al. Fatigue in people with localized colorectal cancer who do and do not receive chemotherapy: a longitudinal prospective study. Ann Oncol. 2016;27(9):1761–1767. doi:10.1093/annonc/mdw252
43. Wei JN, Li SX. The relationship between nutritional risks and cancer-related fatigue in patients with colorectal cancer fast-track surgery. Cancer Nurs. 2018;41(6):E41–E47. doi:10.1097/NCC.0000000000000541
44. Wang S, Jiang N, Song Y, et al. Correlates of cancer-related fatigue among colorectal cancer patients undergoing postoperative adjuvant therapy based on the theory of unpleasant symptoms. Curr Oncol. 2022;29(12):9199–9214. doi:10.3390/curroncol29120720
45. Deng SH, Chen M, Xu F, Jiang XX. Study on the cancer-related fatigue and influencing factors of colorectal cancer patients after surgery. J Yangtze Univ. 2016;13(30):48–51. doi:10.16772/j.cnki.1673-1409.2016.30.021
46. Traa MJ, De Vries J, Roukema JA, Den Oudsten BL. The association between patient’s and partner’s fatigue in couples coping with colorectal cancer: a longitudinal study. Support Care Cancer. 2016;24(10):4113–4121. doi:10.1007/s00520-016-3226-y
47. Han CJ, Yang GS, Syrjala K. Symptom experiences in colorectal cancer survivors after cancer treatments: a systematic review and meta-analysis. Cancer Nurs. 2020;43(3):E132–E158. doi:10.1097/NCC.0000000000000785
48. Dahal A, Meheta RK. Fatigue experience and coping strategies among cancer patients receiving chemotherapy. J Nepal Health Res Counc. 2018;16(3):285–290.
49. Jiang PL, Wang SH, Jiang DM, Yu LL. Cancer related fatigue in patients with breast cancer after chemotherapy and coping style. J Cent South Univ. 2011;36(4):323–328. doi:10.3969/j.issn.1672-7347.2011.04.008
50. Luo WZ. Analysis of the Influencing Factors of Cancer-Related Fatigue in Patients with Thyroid Cancer Treated by 131I. Tangshan: North China University of Science and Technology; 2017.
51. Peng YN, Huang ML, Kao CH. Prevalence of depression and anxiety in colorectal cancer patients: a literature review. Int J Environ Res Public Health. 2019;16(3):411. doi:10.3390/ijerph16030411
52. Deng YQ, Wang QL. Relationship of anxiety, depression, sleep quality and medical coping style with cancer related fatigue in tumour patients. Mil Med J S Chin. 2018;32(6):410–412.
53. Li Z, Mi D, Wen Z, Yu X, Shao P, Wang Y. Multidimensional independent predictors of cancer-related fatigue in lung cancer patients in gansu. Clinical Focus. 2018;33(4):323–328.
54. Cheng V, Oveisi N, Mctaggart-Cowan H, Loree JM, Murphy RA, De Vera MA. Colorectal cancer and onset of anxiety and depression: a systematic review and meta-analysis. Curr Oncol. 2022;29(11):8751–8766. doi:10.3390/curroncol29110689
55. Song L, Pang Y, Zhang J, Tang L. Body image in colorectal cancer patients: a longitudinal study. Psychooncology. 2021;30(8):1339–1346. doi:10.1002/pon.5688
56. Song L, Han X, Zhang J, Tang L. Body image mediates the effect of stoma status on psychological distress and quality of life in patients with colorectal cancer. Psychooncology. 2020;29(4):796–802. doi:10.1002/pon.5352
57. Stuhlfauth S, Melby L, Hellesø R. Everyday life after colon cancer: the visible and invisible challenges. Cancer Nurs. 2018;41(6):E48–E57. doi:10.1097/NCC.0000000000000506
58. Graboyes EM, Kistner-Griffin E, Hill EG, et al. Efficacy of a brief cognitive behavioral therapy for head and neck cancer survivors with body image distress: secondary outcomes from the BRIGHT pilot randomized clinical trial. J Cancer Surviv. 2023. doi:10.1007/s11764-023-01454-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


