Back to Journals » Cancer Management and Research » Volume 12
Cancer Patient-Reported Preferences and Knowledge for Liquid Biopsies and Blood Biomarkers at a Comprehensive Cancer Center
Authors Lee MJ , Hueniken K, Kuehne N , Lu L, Jiang SX, Id Said B, McCartney A, Gao Y, Liang M, Obuobi T, Sorotsky H, Eng L , Brown MC , Xu W, Liu G
Received 27 October 2019
Accepted for publication 29 January 2020
Published 13 February 2020 Volume 2020:12 Pages 1163—1173
DOI https://doi.org/10.2147/CMAR.S235777
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Min Joon Lee,1,2 Katrina Hueniken,3 Nathan Kuehne,1 Lin Lu,4 Shirley Xue Jiang,1 Badr Id Said,1 Alex McCartney,1 Yizhuo Gao,1 Mindy Liang,3 Tamara Obuobi,1 Hadas Sorotsky,1 Lawson Eng,1 M Catherine Brown,3 Wei Xu,4 Geoffrey Liu1,3,5
1Department of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; 2Faculty of Medicine, University of Toronto, Toronto, ON, Canada; 3Department of Medical Biophysics, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; 4Department of Biostatistics, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada; 5Department of Epidemiology, Dalla Lana School of Public Health, Toronto, ON, Canada
Correspondence: Geoffrey Liu
Princess Margaret Cancer Centre, 610 University Avenue, Toronto ON M5G 2M9, Canada
Tel +1 416-946-4501 (ext 3479)
Fax +1 416-946-4501 (ext 3067)
Email [email protected]
Background: Blood-based biomarkers (liquid biopsy) are increasingly used in precision oncology. Yet, little is known about cancer patients’ perspectives in clinical practice. We explored patients’ depth of preferences for liquid vs tissue biopsies and knowledge regarding the role of blood biomarkers on their cancer.
Methods: Three interviewer-administered trade-off scenarios and a 54-item self-administered questionnaire were completed by cancer outpatients across all disease sites at the Princess Margaret Cancer Centre.
Results: Of 413 patients, 54% were female; median age was 61 (range 18– 101) years. In trade-off scenario preference testing, 90% (n=372) preferred liquid over tissue biopsy at baseline; when wait times for their preferred test were increased from 2 weeks, patients tolerated an additional mean of 1.8 weeks (SD 2.1) for liquid biopsy before switching to tissue biopsy (with wait time 2 weeks). Patients also tolerated a 6.2% decrease (SD 8.8) in the chance that their preferred test would conclusively determine optimal treatment before switching from the baseline of 80%. 216 patients (58%) preferred liquid biopsy even with no chance of adverse events from tissue biopsy. Patients’ knowledge of blood-based biomarkers related to their cancer was low (mean 23%); however, the majority viewed development of blood biomarkers as important.
Conclusion: Patients had limited understanding of cancer-specific blood-based biomarkers, but 90% preferred liquid over tissue biopsies to assess biomarkers. There was little tolerance to wait longer for results, or for decreased test-conclusiveness. Developing accurate, low-risk tests for cancer diagnosis and management for blood biomarkers is therefore desirable to patients.
Keywords: blood biomarker, liquid biopsy, precision oncology, patient preference, patient knowledge
Introduction
Blood-based biomarkers (liquid biopsy), including cell-free DNA and plasma signatures, are increasingly used in precision oncology.1,2 For many years, blood biomarkers such as PSA or CA19-9 have been used to monitor treatment response and recurrence.3,4 Recent advances in molecular diagnostics have now generated more promising blood biomarkers for targeted therapies, which take into account individuals’ tumor characteristics and potential response and toxicity to specific therapies.1,5 For example, the first epidermal growth factor receptor (EGFR) blood marker was approved by the FDA in 2016 to identity eligible patients with metastatic non-small-cell lung carcinoma (NSCLC) for treatment with erlotinib.2 In 2017, pembrolizumab was approved by the FDA as the first drug for use based on a molecular biomarker rather than a traditional tissue diagnosis.6 More blood biomarkers are anticipated to be approved in coming years across all stages, and broadly across multiple cancer primary sites,2 which may offer safer, less-invasive liquid biopsies to replace diagnostic tests traditionally performed via tissue biopsy.
Transitioning from tissue to liquid biopsy for cancer diagnosis and management has advantages: liquid biopsy results often have high concordance with tissue-based assessments;7–9 patients are accustomed to having blood drawn at routine clinic visits, improving compliance; patients too ill or unable to provide tissue samples are often candidates for liquid biopsies;2,10 and liquid biopsies can easily be used to monitor molecular changes in the tumor in real time.1,10 However, a key knowledge gap is a lack of clinically supported studies correlating some blood biomarkers with traditional tissue markers.10,11 With advancing technology and ongoing research, however, highly specific and sensitive blood biomarker-based tests may eventually replace or work in combination with traditional tissue markers.2,10,11
While much attention has been focused on developing new blood biomarkers and translating this knowledge into clinics, little is known about cancer patients’ perspectives on liquid and tissue biopsies in clinical practice. Several studies have investigated patients’ knowledge and perspectives towards precision oncology, particularly genomic testing, but these studies have not explored patients’ preferences for liquid or tissue biopsy to obtain blood biomarkers or their understanding of how biomarkers are used for their own cancer.12–14
A major focus of research at Princess Margaret Cancer Centre is precision oncology, and much of biomarker-precision oncology research requires patient participation (eg, clinical trials, provision of biological samples). By understanding patient attitudes, knowledge gaps, and resultant perceptual biases that patients have about what it means to develop biomarkers for personalized care, we hope to increase patient engagement in biomarker research as well as overall patient satisfaction in their care. In this study, we seek to understand patients’ preferences and attitudes towards liquid vs tissue biopsies and their current knowledge of the role of blood biomarkers for their specific cancer.
Materials and Methods
Study Population
The study was approved by the University Health Network Institutional Research Ethnics Board (REB#13-6352). A combined interviewer- and self-administered questionnaire was completed by 413 patients with cancer at various sites (ie, thoracic, breast, head/neck, genitourinary, gynecologic, gastrointestinal, hematology clinics) at the Princess Margaret Cancer Centre (PM), Toronto, Canada from May, 2017 to August, 2017. Eligibility criteria included patients with clinically diagnosed malignancy, age over 18 years, and the ability to communicate in English. Patients were approached in the clinic waiting rooms and gave their informed consent.
Study Design
Participants were provided three trade-off scenarios by a research-coordinator and then completed a self-administered 54-item questionnaire.
Trade-off Scenarios
The complete trade-off scenario questionnaire can be found in Supplementary Appendix 1. Briefly, trained research coordinators presented patients with a hypothetical scenario (ie, unrelated to their current cancer) in which preference for biopsy testing using either blood or tissue samples was assessed, and assuming equivalent test characteristics (time to receive biomarker results; accuracy and interpretability of result; safety of test). We then assessed the depth of preference by changing the conditions of the test characteristics (ie, increased wait time, decreased chance of the test conclusively determining treatment options, or chance of adverse side effects) to favour their less-preferred type of biopsy, until the patient switched to their less-preferred, alternative, biopsy type. We performed three separate trade-off scenarios based on wait-time-to-decision-making, test-conclusiveness, and test adverse event rates. Baseline characteristics included a two-week waiting period, 80% chance of test conclusively determining treatment choice (test-conclusiveness), and tissue biopsy chance of hospitalization of 5% (vs 0% for a liquid biopsy/blood biomarker).
Questionnaire
The questionnaire can be found in Supplementary Appendix 2. Briefly, the self-administered questionnaire included questions regarding socio-demographic and clinicopathological information. We also assessed patients’ knowledge on the role of biomarker for their own cancer: level of agreement (Likert scales) with a series of statements designed to elicit patients’ understanding of the role of currently available blood biomarkers for their own cancer.
Twenty-five questions about biomarkers were administered to patients as part of the self-reported questionnaire. The first 16 questions asked whether specific biomarkers (eg, demographic, serum markers, genetic markers, etc.) could be used to diagnose, treat, or manage the patient’s cancer. An additional nine questions asked patients whether biomarkers found in the blood could be used for specific purposes to manage their cancer (eg, for early diagnosis, treatment response, etc.). A biomarker was defined as
… something that is measurable in my body that will help determine how I am managed as a patient. Examples of biomarkers include something measured in the blood or tissue, but can also mean other things such as how much you weigh or other characteristics or you or your cancer.
Patients answered on a five-point scale ranging from “Strongly Disagree” to “Strongly Agree”, including “Don’t Know” as the midpoint for ten of the 16 knowledge questions and “Yes”, “No”, “Unsure” for the remaining six knowledge questions. In addition to these 25 scored knowledge questions, patients were also asked from which information source they had first heard of blood biomarkers, whether they knew if biomarkers were currently being used to determine their treatment and to indicate the importance they placed on future discovery of blood biomarkers for diagnosis and management of their specific cancer.
Answers were scored by medical oncologists, based on current evidence of available, clinically useful biomarkers for patients’ own cancer type. Unique answer keys were generated for 38 cancer sites. Following the survey/interview, a chart review was conducted for each patient to obtain and confirm relevant clinical information, including some information gathered directly from patient-completed questionnaires.
Statistical Analysis
All personally-identifying patient information was removed and replaced by unique study identifiers before running any statistical analysis. Baseline clinico-demographic characteristics were reported for patients who completed the questionnaire.
Frequencies and proportions were used to describe patients’ preferences for liquid or tissue biopsy. Among patients who subsequently decided to switch tests in response to increased wait time, decreased conclusiveness or increased risk of adverse events for their preferred test, we reported the mean and standard deviation of the change in each of these factors that patients were willing to tolerate before they switched to their less-preferred biopsy. The proportion of patients who never switched to their less-preferred biopsy was also reported. Chi-square and t-tests were used to test for associations between clinicodemographic characteristics and initial biopsy preferences, with p-values adjusted by Bonferroni correction to account for multiple comparisons.
Patients’ knowledge of biomarkers for their specific cancer type was reported using frequencies, median, and interquartile range. Strength of relationships between patients’ answers and correct responses was assessed by computing Spearman’s rank-order correlations. Interest in biomarker development was also assessed descriptively and via Spearman correlation.
Results
Questionnaire Characteristics
During pilot feasibility testing, content and face validity was checked by a group of 12 senior (Associate/Full Professor) and junior clinicians (Assistant Professor/Clinical Fellow) in medical, radiation, and surgical oncology in the major sites of gastrointestinal, breast, thoracic, head and neck, genitourinary, sarcoma, melanoma, and hematological malignancy. Detailed qualitative discussions with patients and clinicians during pilot testing (12 patients and 6 clinicians) in three rounds evaluated the readability and interpretability of the questionnaire. Although no formal test-retest reliability testing was performed, nine patients completed at least parts of the survey twice as they had forgotten that they had completed the study already; results were identical 73% of the time, and were off by only one Likert scale category in another 18%. In the case of the three scenarios, results between first and repeated preference testing were identical in 100% of the baseline questions and 88% of the time for depth-of-preference testing when there was ± 1 preference category allowance.
Baseline Patient Characteristics
Of 632 patients approached, 549 were eligible; 413 were ultimately recruited and completed the study questionnaire (effective response rate, 75%; see Figure 1).
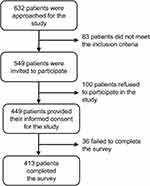 |
Figure 1 CONSORT flow diagram summarizing inclusions and exclusions of participants. |
Baseline patient characteristics are shown in Table 1. Of 413 participants, approximately half were female, with a median age of 61 years. The majority were Caucasian, English-speaking and married, and had completed some post-secondary education. Almost half were currently employed, with the median income of $70,000. Our patient population represented a broad range of tumor subtypes including breast (15%), thoracic (14%), head and neck (18%), genitourinary (15%), gynecologic (15%), and gastrointestinal (16%) cancers, with the median of two years since diagnosis. Compared to the initial stage at diagnosis, the stage at the time of survey represented a substantial increase in the proportion of patients with stage 4 cancers (45% vs 24%). Most patients had received surgery and systemic therapy at the time of recruitment; 8% had received immunotherapy; 14% had prior cancer.
 |
Table 1 Patient Characteristics, N = 413 |
Cancer Patients Preferentially Choose Liquid Biopsy Over Tissue Biopsy
Patients were interviewed by research coordinators in a hypothetical scenario in which either a liquid or tissue biopsy was used to assess a biomarker for an optimal cancer therapy (Table 2). Each scenario presented to patients was made as equivalent as possible at baseline while still maintaining its real-world clinical relevance. In the initial scenario presented to patients at baseline when liquid biopsy and tissue biopsy had equal wait times, equal test-conclusiveness, and low complication rates, 90% (372/413) preferred liquid biopsy over tissue biopsy. There were no significant associations between patients’ clinicodemographic characteristics and their initial choice of biopsy (Supplementary Table S1).
Patients who chose the liquid biopsy were willing to accept a median additional waiting period of 1.8 weeks (ie, 3.8 weeks vs 2 weeks) or a median 6.2% decrease in test-conclusiveness (ie, from 80% to 73.8%) before switching their preference. Nine percent and 6% of these patients were never willing to switch their preference either for any increase in the waiting period or decrease in test-conclusiveness, respectively. Patients who initially chose the tissue biopsy accepted a median additional waiting period of 2 weeks and a median of 3.4% decrease in test-conclusiveness before switching their preference to the liquid biopsy. Five percent and 4% of these patients were never willing to switch their preference for any increase in the waiting period or decrease in the test-conclusiveness, respectively. Among patients with post-secondary education who chose liquid biopsy at baseline, there was a trend towards more quickly changing their preference (depth of preferences) to tissue biopsy when the waiting period was increased (p=0.04), or when the test-conclusiveness of their initial choice was decreased (p=0.02). However, after correcting for multiple comparisons, there were no significant associations between any clinicodemographic characteristics and depth of preferences.
In the third scenario, in which complication rates for patients’ second-choice test were decreased, the majority (N=216, 58%) of patients who initially chose the liquid biopsy were never willing to switch even when complication rates from tissue biopsy (their less-preferred choice) were reduced to zero.
Patients' Knowledge Regarding the Role of Blood Biomarkers for Their Own Cancer
Of the 388/413 patients who completed at least 15 of 25 questions testing their knowledge on the role of biomarkers for their own cancer (Figure 2), general knowledge was low (median 5 correct questions of total 25 questions [IQR 1–9 questions correct]; 23% correct on average). Young (p=0.01), female (p=0.005), those with post-secondary education (p=0.003), those with breast cancer (p=0.05), metastatic disease (p=0.05), or on immunotherapy (p=0.009)/ chemotherapy (p=0.02) had greater number of correct scores (Table 3).
 |
Table 3 Patients' Knowledge Regarding the Role of Blood Biomarkers for Their Own Cancer |
Patients responded “Yes” a median of 10 times per questionnaire (IQR 4–15), or 3.6 times more frequently than a “No” answer [median frequency=1 (IQR 0–4)]. Thus, “Don’t Know” was a very common answer; in fact, 152 (40%) patients checked “Don’t know” for the majority of the 25 questions.
Correctness was weakly correlated with the total number of biomarkers available for each patient’s own cancer type (ρ=0.36, p<0.001). The number of correct “Yes” answers was moderately correlated with the total number of true “Yes”s (ρ=0.54, p<0.001) while the number of correct “No”s was not (ρ =−0.05, p=0.26).
Among all individual biomarker questions, regardless of disease site, patients were most likely to believe that biomarkers did exist for determining the best treatment (N=224, 58%), determining cancer response to treatment (N=215, 55%), and determining how to treat cancer (N=209, 54%). Patients were least likely to believe that there were no useful biomarkers at this time (N=129, 33%) or that age and sex were biomarkers (N=97, 25%) or that behaviours (such as smoking, alcohol use) acted as biomarkers (N=96, 25%). Patients most frequently answered “Don’t know” when asked whether biomarkers for their cancer existed in sputum (N=243, 63%) or whether any useful biomarkers existed currently for their cancer (N=232, 60%).
Patient-Reported Information Source for Blood Biomarkers
Information sources from which patients heard about blood biomarkers are summarized in Table 4. Overall, few patients had heard about blood-based biomarkers, with the most frequently reported information sources being self-learning (43%), internet (38%), and through physicians (45%); 125 (52%) of patients said that they had never heard of blood biomarkers.
 |
Table 4 Information Source from Which Patients Heard About Blood Biomarkers; Counts, Unless Otherwise Specified |
Among the patient population, 344 patients (91%) were being evaluated with blood, tissue, or other clinical biomarkers according to their clinicians’ notes (Table 5). However, only 76 of these patients (22%) were correctly aware of this.
Patient-Reported Values of Future Blood Biomarkers
Patients were asked which future functions of blood biomarkers were most important, including biomarkers that can detect cancer early, can detect cancer recurrence, can predict whether a cancer is or is not responding to treatment, can predict side-effects of treatment, or monitor their treatment compliance (ie, how regularly and appropriately they take the medication); 191 (46%) responded that all five characteristics were “very important” for them. When all five questions were considered together, 85% of answers were “very important” or “important” compared to 3% of answers were “somewhat not important” or “not important” (ie, “important” was answered over 32 times as often as “not important”).
Discussion
Several studies have investigated cancer patients’ knowledge and attitudes towards personalized medicine, specifically in the context of genomic testing.12–14 These studies showed that patients were generally interested in learning more about genomics testing and incorporating it into their care. To our knowledge, our study is the first investigation exploring patients’ attitudes and depth of preference towards liquid biopsies vs tissue biopsies for cancer care, and their current understanding on the role of available blood biomarkers. Our results revealed that patients preferred liquid biopsy over tissue biopsy when each test had relatively equal wait times and conclusiveness and both had low complication rates, but their preferences changed when the test characteristics favored the alternative biopsy type. Overall patients had limited understanding of the role of blood biomarkers, despite their strong interest.
This study had three aims regarding the standardized scenario-based questionnaires. The first aim was to evaluate patients’ initial choice of biopsy, when the test characteristics are equal and complication rates were low for both tests. The second aim was to assess patients’ depth of preference for their initial choice of biopsy. The third aim was to identify patients’ current attitudes toward liquid and tissue biopsy. As expected, most patients chose liquid biopsy as their initial choice of testing, given the equal test characteristics of waiting period and test-conclusiveness. Surprisingly, potential complications of tissue biopsy were not a notable concern for patients who chose liquid biopsy first, as most of these patients would not switch to tissue biopsy, even if there were no potential tissue biopsy-associated complications. Some patients who preferred liquid biopsy described their own experience with tissue biopsy (ie, bleeding, pain) that they wanted to avoid. However, patients’ depth of preferences was relatively shallow, as they were not willing to sacrifice much waiting time or test-conclusiveness, and thus reflect patient desire to maximize treatment efficacy.15
Interestingly, 10% of patients chose tissue biopsy as their initial choice of testing. Some mentioned that their previous experience with tissue biopsies gave them promising results, that tissue biopsies were not too invasive for them, and that liquid biopsies may not provide adequate or meaningful results. Thus, not everyone was willing to rely on liquid biopsies for making decisions related to their cancer care when the traditional option for tissue biopsy was available. Thus, clinicians should consider making clinical decisions together with patients when both options are equally available.
Currently, few blood biomarkers are used in cancer care, and most are limited to specific purposes (ie, predictive biomarkers).2,10 From the self-administered biomarker knowledge questionnaire, we determined that patients generally understood little about these blood biomarkers, across the entire demographic spectrum. Patients tended to answer “don’t know” or overestimate the number of available biomarkers for their cancer. Consistent with a finding by Marron et al, a lack of understanding of direct benefits to patients of blood biomarkers may interfere with research participation and clinical application in the long term.16 As blood biomarkers are not widely available in clinic as diagnostic, prognostic, and predisposition tools, it is imperative to fill in these knowledge gaps to optimize patient care.2,10 However, we observed that patients are optimistic about the development of new biomarkers, which may indicate that increased clinical use of blood biomarkers will be well received.
Our study reveals that few patients have heard about blood biomarkers, and most of them were through self-learning, either through internet or by asking their physicians. Consistent with this finding, only a small fraction of those being evaluated with any biomarkers knew that biomarkers were being used as part of their cancer care. Our results are similar to that of Rogith et al, who found that only a small fraction of cancer patients sought information regarding personalized cancer therapies.17
Some may argue if this knowledge gap really matters in cancer care, especially since each patient’s information needs and seeking behaviour may vary.18 In fact, studies have shown that patients want to be engaged throughout their treatment.19,20 At comprehensive cancer centres with active clinical trials such as PM, patients often come in with questions about specific molecular testing, what test results imply, and how targeted therapies work thereafter. In personalized cancer care, such patient participation play a pivotal role. One way to enhance this care is to educate patients properly. Unfortunately, numerous pre-mature liquid biopsy tests are readily available on social media which can be tempting and giving false ideas to patient. As more blood biomarkers are going to be clinically utilized in near future, it is important to find ways to properly educate patients, so they can correctly understand and use this knowledge for their own care. Only one-third of patients at PM reported hearing about biomarkers from their physicians. This could be due to recall bias, insufficient discussion of biomarkers, or a combination of both. It may be beneficial from a healthcare point of view to develop standardized methods for patient–physician interactions on biomarker education.
When asked which specific functions of future blood biomarkers were important, patients felt it was strongly important to develop those that would improve their cancer treatment (ie, early detection, prediction of treatment response and recurrence) or improve their quality-of-care (ie, predict side-effects of treatment, treatment compliance). Every characteristic was considered important or very important for future development.
A study by Tan et al explored patients’ perspectives on utilizing a urinary biomarker test instead of cystoscopy as part of bladder cancer surveillance.21 Similar to our study, they found that patients preferred a urine biomarker if its sensitivity was close to an existing gold-standard test. In contrast to this study, our scenarios focused on comparing two equivalent hypothetical tests, generalized to multiple disease sites and circumstances.
A limitation of this study was that our questionnaires were not validated with an independent data set. Further, our results (ie, a 9:1 preference for liquid biopsy; and a poor blood biomarker knowledge score across all cancer sites) were striking; clinico-demographic characteristics associated with the minority preference cannot be precisely estimated due to the small number of patients with the minority preference. Although the survey did undergo face and content validity assessment, other forms of validity such as construct and criterion were not fully evaluated; this may not be as large a problem when the results suggest a large majority preference.
There are further limitations to our study. For the scenario-based questions, we could not make complication rates equal between liquid and tissue biopsy as we were trying to measure real-world preferences. These scenarios were also hypothetical, meaning that patient preferences might be different in the real setting. To construct comparison arms, the scenarios had to be artificial in nature; we are aware that morphological, immunohistochemical, and other tissue-based features currently make it infeasible to avoid tissue-testing; however, we are also aware that in some circumstances the tissue obtained may be inadequate for all the testing required, and that a second tissue biopsy may be needed; this second biopsy could be liquid or tissue. We only interviewed patients once and did not follow up with them to explore some of their answers in detail, particularly their attitudes towards liquid biopsies. As this study was conducted at one tertiary, referral cancer centre, our results may not generalize to other institutions. Our study participants were also fluent in English, and disproportionately late-stage, and thus may not represent the general cancer population.
For future studies, detail exploration of which specific areas that patients had interested in further education is warranted. Preferences or knowledge may change over the course of cancer care, especially when more blood biomarkers are approved and utilized in clinical setting. It is therefore desirable to interview patients at multiple time-points, to see their preferences and knowledge change over time. Furthermore, it would be interesting to see how patients’ information needs and information-seeking behaviour may contribute to patients’ preferences for liquid biopsies/blood biomarkers and their interest in learning more about them.
In conclusion, our overall aim was to identify patients’ attitudes towards the emerging field of blood biomarkers and inherent knowledge gaps that must be resolved before translating more markers into clinics. To date, this is among the first large-scale studies that evaluated patients’ preference for liquid biopsies and limited understanding on the role of blood biomarkers for their cancer at a comprehensive cancer center. This evidence supports the idea of continued pursuit of blood biomarker development and patient education in the era of personalized medicine.
Implications for Practice
Patients prefer liquid biopsy over tissue biopsy to assess biomarkers under equivalent conditions. Although patients’ knowledge of blood-based biomarkers for their cancer is limited, discovery and development of new blood-based biomarkers are viewed as important. This supports the idea of continued pursuit of blood-based biomarker development and patient education in the era of precision oncology.
Acknowledgments
We thank all the patients at PM who took time to be interviewed for this study. We also thank all the staff physicians, nurses, coordinators, and volunteers at PM who facilitated with patient recruitment. Presented in part as a poster at the 2018 American Society of Clinical Oncology Annual Meeting; May 31 to June 4, 2018; Chicago, IL, USA and at the 2018 Canadian Centre for Applied Research in Cancer Control Conference; May 27 to May 28, 2018; Montreal, QC, Canada.
Funding
Support was also provided by the Comprehensive Research Experience for Medical Students Research Scholar Program (to M.J.L), Princess Margaret Cancer Centre Alan Brown Chair in Molecular Genomics (to G.L.), Lusi Wong Family Fund (to. G.L.). and the Posluns Family Fund (to G.L).
Disclosure
Geoffrey Liu received honoraria for advisory boards for AstraZeneca, Roche, Novartis, Pfizer, Merck, and Takeda. The authors report no other conflicts of interest in this work.
References
1. Janku F. Tumor heterogeneity in the clinic: is it a real problem? Ther Adv Med Oncol. 2014;6(2):43–51. doi:10.1177/1758834013517414
2. Ilie M, Hofman P. Pros: can tissue biopsy be replaced by liquid biopsy? Transl Lung Cancer Res. 2016;5(4):420–423. doi:10.21037/tlcr
3. Badreddine R, Wang KK. Biomarkers in gastrointestinal cancers. Am J Gastroenterol. 2008;103(8):2106–2110. doi:10.1111/ajg.2008.103.issue-8
4. Adhyam M, Gupta AK. A review on the clinical utility of PSA in cancer prostate. Indian J Surg Oncol. 2012;3(2):120–129. doi:10.1007/s13193-012-0142-6
5. Jackson SE, Chester JD. Personalised cancer medicine. Int J Cancer. 2015;137(2):262–266. doi:10.1002/ijc.v137.2
6. Prasad V, Kaestner V, Mailankody S. Cancer drugs approved based on biomarkers and not tumor type-FDA approval of pembrolizumab for mismatch repair-deficient solid cancers. JAMA Oncol. 2018;4(2):157–158. doi:10.1001/jamaoncol.2017.4182
7. Xu S, Lou F, Wu Y, et al. Circulating tumor DNA identified by targeted sequencing in advanced-stage non-small cell lung cancer patients. Cancer Lett. 2016;370(2):324–331. doi:10.1016/j.canlet.2015.11.005
8. Beije N, Helmijr JC, Weerts MJA, et al. Somatic mutation detection using various targeted detection assays in paired samples of circulating tumor DNA, primary tumor and metastases from patients undergoing resection of colorectal liver metastases. Mol Oncol. 2016;10(10):1575–1584. doi:10.1016/j.molonc.2016.10.001
9. Arriola E, Paredes-Lario A, Garcia-Gomez R, et al. Comparison of plasma ctDNA and tissue/cytology-based techniques for the detection of EGFR mutation status in advanced NSCLC: spanish data subset from ASSESS. Clin Transl Oncol. 2018;20(10):1261–1267. doi:10.1007/s12094-018-1855-y
10. Cheung AH, Chow C, To KF. Latest development of liquid biopsy. J Thorac Dis. 2018;10(Suppl 14):S1645–S1651. doi:10.21037/jtd.2018.04.68
11. Mino-Kenudson M. Rebuttal from Dr. Mino-Kenudson. Transl Lung Cancer Res. 2016;5(4):430–432. doi:10.21037/tlcr
12. Blanchette PS, Spreafico A, Miller FA, et al. Genomic testing in cancer: patient knowledge, attitudes, and expectations. Cancer. 2014;120(19):3066–3073. doi:10.1002/cncr.28807
13. Yusuf RA, Rogith D, Hovick SR, et al. Attitudes toward molecular testing for personalized cancer therapy. Cancer. 2015;121(2):243–250. doi:10.1002/cncr.28966
14. Ciardiello F, Adams R, Tabernero J, et al. Awareness, understanding, and adoption of precision medicine to deliver personalized treatment for patients with cancer: a multinational survey comparison of physicians and patients. Oncologist. 2016;21(3):292–300.
15. Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–115. doi:10.1200/JCO.1997.15.1.110
16. Marron JM, DuBois SG, Glade Bender J, et al. Patient/parent perspectives on genomic tumor profiling of pediatric solid tumors: the Individualized Cancer Therapy (iCat) experience. Pediatr Blood Cancer. 2016;63(11):1974–1982. doi:10.1002/pbc.v63.11
17. Rogith D, Yusuf RA, Hovick SR, et al. Patient knowledge and information-seeking about personalized cancer therapy. Int J Med Inform. 2016;88:52–57. doi:10.1016/j.ijmedinf.2016.01.005
18. Leydon GM, Boulton M, Moynihan C, et al. Cancer patients’ information needs and information seeking behaviour: in depth interview study. BMJ. 2000;320(7239):909–913. doi:10.1136/bmj.320.7239.909
19. Stacey D, Paquet L, Samant R. Exploring cancer treatment decision-making by patients: a descriptive study. Curr Oncol. 2010;17(4):85–93. doi:10.3747/co.v17i4.527
20. Tamirisa NP, Goodwin JS, Kandalam A, et al. Patient and physician views of shared decision making in cancer. Health Expect. 2017;20(6):1248–1253. doi:10.1111/hex.2017.20.issue-6
21. Tan WS, Teo CH, Chan D, et al. Mixed-methods approach to exploring patients’ perspectives on the acceptability of a urinary biomarker test in replacing cystoscopy for bladder cancer surveillance. BJU Int. 2019;124(3):408–417. doi:10.1111/bju.14690
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



