Back to Journals » OncoTargets and Therapy » Volume 16
Cancer and the Vasopressin Gene: Radioimmunoassay Values and Commentary on Copeptin as a Plasma Marker
Authors North WG
Received 21 July 2023
Accepted for publication 3 November 2023
Published 20 November 2023 Volume 2023:16 Pages 973—982
DOI https://doi.org/10.2147/OTT.S425723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Nagashree Seetharamu
William G North
Department of Molecular & Systems Biology, Geisel School of Medicine at Dartmouth College, Hanover, NH, USA
Correspondence: William G North, Email [email protected]
Background: Vasopressin gene expression has been demonstrated to be a common feature of all small-cell lung cancer (SCLC) and breast cancer. Provasopressin (ProVP) is a component of the cancer cell membrane and a likely target for treatment. However, a measurable fraction of this cancer provasopressin is also normally processed and products are released into the circulation. Vasopressin (VP) and vasopressin-associated human neurophysin (VP-HNP), two of three products of processing, were earlier shown to be reliable plasma markers for determining the presence of SCLC and monitoring response to treatment.
Material and Methods: In this study, copeptin, the third product of provasopressin processing, was preliminarily evaluated as a plasma marker for SCLC or breast cancer using radioimmunoassay (RIA). Antibodies directed against the 18 residue C-terminal peptide fragment of copeptin were used to avoid interference from the large-carbohydrate component of this endogenous glycopeptide.
Results: The levels of copeptin in 8 male and 6 female patients with SCLC before treatment ranged from 16 to 319 pmol/L, and these levels were elevated (> 2.5 times) in 10 of 14 cases (70%) when compared with healthy volunteers (normal mean, 18 ± 6 pmol/L). Volunteer values for males were smaller than for females (15± 4 pmol/L and 20± 9 pmol/L), but numbers were small. Patients with breast cancer had plasma levels ranging from 12 to 68 pmol/L, with only three of the six elevated.
Conclusion: While cancer patients displayed a wide range of plasma copeptin levels over 70% with SCLC and 50% with breast cancer had clearly elevated levels. This finding indicates that for such patients, plasma copeptin, like plasma VP and VP-HNP, could be used to detect disease. The control values found for healthy volunteers using our RIA were in a range predictable from established normal plasma levels of both VP and VP-HNP.
Keywords: copeptin, radioimmunoassay, small-cell lung cancer, breast cancer
Introduction
Vasopressin, the antidiuretic hormone in humans, is also an important hormonal component in the regulation of blood pressure.1 The peptide is normally produced by neurons of the hypothalamus under regulation, principally through changes in the osmolality of the blood. The expression of the normal vasopressin gene is also a common feature of all small-cell lung cancers (SCLC) and breast cancers.2–4 This expression uniquely leads to the post-translational glycosylated product provasopressin becoming a surface marker of these tumors. This product may be an effective target for treatment with antibodies.3,5 Alternatively, as can be assessed for the majority of SCLC cases, while most gene product becomes a component of tumor cell membranes, a smaller fraction is enzymatically processed by cancer cells in the same manner as that of central neurons.3,6 Two products of this tumor processing are vasopressin (VP) and vasopressin-associated human neurophysin (VP-HNP)* which are released into the blood, where, for most patients, they are at sufficient levels to become plasma tumor markers and can be used to monitor disease progression.2,7,8 Enzymatic processing of provasopressin also yields a glycosylated peptide of 39 amino acid residues. In all mammals examined, the carbohydrate side chain is attached through an N-glycosidic link to an asparagine residue at position 6 from the N-terminus and is reported to have a molecular size of approximately 2 kDa,6,9,10 or more.3,11,12 This glycosylated product of processing was originally referred to as vasopressin-associated glycopeptide,6 but is now most often referred to as copeptin (Co).10,12 In the current study, we developed a radioimmunoassay (RIA) for copeptin represented by the C-terminal 18 amino acid residues of the copeptin peptide structure (Co22-39) to ascertain whether, like VP and VP-HNP, plasma levels of the glycopeptide might serve to mark the presence of small-cell lung cancer and breast cancer. Such an RIA, like all immunoassays, suffers from the non-availability of intact copeptin as reference standard. However, this RIA might better provide closer approximations of the true values for plasma copeptin than do popular commercial sandwich immunoassays as those by BRAHMS AG (Thermo Scientific, Hennigsdorf, Germany) because such assays are based on a likely faulty logic that antibodies will bind equally well to glycosylated and non-glycosylated versions of the same peptide structure.
*The alternate nomenclature of using Roman numerals for the two major pituitary neurophysins based on electrophoretic mobility is unsuitable because vasopressin-related neurophysin in humans is NP II, whereas that in rats, as the faster of the two, is NP I.
Methods
Antibodies
Rabbit polyclonal antibodies against human copeptin were of the antiserum (Boris Y3) representing a bleed later harvested from the same immunization used to generate antibodies in Boris Y2 antiserum. Boris Y2 antibodies have already been described in previous immunohistochemical studies and have shown specificity for vasopressin-containing neurons of the human hypothalamus.13,14 In short, both antisera were generated in a New Zealand strain rabbit using a synthetic peptide representing the C-terminal 18-amino-acid residues of human copeptin (Co22-39) coupled to thyroglobulin with glutaraldehyde. Boris Y2 antiserum was harvested four weeks following a boost immunization, whereas Boris Y3 antiserum was obtained at exsanguination four weeks following a second boost immunization. Boris Y3 antibodies, without isolation from the serum, were used in RIA at a final serum dilution of 1:15000 (*now available from Woomeratherapeuticsinc.com). The C-terminal region of copeptin was selectively employed for antibody production to avoid interference in binding caused by the large carbohydrate side chain of the asparagine residue at position 6 from the N-terminus of the endogenous glycopeptide. This carbohydrate of copeptin released from the posterior pituitary is of a size (~2000 Kd) almost half that of the entire peptide structure and would be expected to impose a considerable structural distortion of the N-terminal half of the peptide framework.
Immunohistochemistry
Studies demonstrating that the antibodies of the above Boris Y3 antiserum reacted selectively with intact copeptin as a component of the vasopressin precursor, provasopressin, were performed on sections (4 µm) of a formaldehyde-fixed human anterior hypothalamus obtained at autopsy, and formaldehyde-fixed sections of 10 small-cell lung cancers and 10 breast cancers obtained from an archival tissue block library of the Department of Pathology at Dartmouth-Hitchcock Medical Center (Lebanon New Hampshire). Of the SCLC cases, 4 were male and 6 were female, with ages ranging from 50 to 76 years, while 9 of the 10 tumors were primary disease. Nine of the 10 tumors were reported to be positive for the marker synaptophysin, while only one was positive for chromogranin. Of the invasive breast cancer cases, patient ages ranged from 50 to 84 years; 9 of 10 tumors were primary, 9 of 10 were estrogen receptor-positive and one was triple negative disease. Avidin-biotin complex (ABC) staining of the tissue sections was performed as previously described.15 Deparaffinization was carried out by heat exposure at 60°C and washing in xylene. The tissues were then rehydrated by washing (2 × 5 min) with descending concentrations of ethanol (100, 95, and 70%). Antigen retrieval was by incubation with 0.01 M sodium citrate (pH 8.5) for 30 min at 80° C. All subsequent aqueous washings (2x5 min) were with PBS containing 0.4M NaCl, endogenous peroxidase activity was quenched by incubation for 5 min with methanol containing 3% hydrogen peroxide, and tissue sections were blocked with 1.5% normal goat serum (10–20 min). Tissues were incubated with a 1:2000 dilution of Boris Y3 antiserum for one hr at ambient temperature and rinsed with PBS containing 0.4M NaCl. The components of the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame CA) were used in subsequent steps. Final staining was achieved using the ImmunoPure Metal Enhanced DAB Substrate Kit (Pierce, Rockford, IL, USA), with an incubation time of 5 min. No counterstaining of the hypothalamic tissue was performed, but all tumor sections were counterstained with Mayer’s hematoxylin (Sigma-Aldrich, St. Louis, MO, USA). Antibody specificity was assessed in two adjacent sections of invasive cancer tissue by incubating with antigen-blocked diluted BorisY3 (20 µg/mL Co22-39 peptide) as the primary antibody in one case, and with an absence of primary antibody in another. Tissues were dehydrated in ascending concentrations of ethanol, washed in xylene, and coverslipped using Permount mounting medium (Fisher Scientific, Pittsburgh, PA). The staining of tissues was recorded using a Micropublisher camera (QImaging, Burnaby, British Columbia, Canada) connected to a BX51 or BX40 microscope (Olympus, Melville, NY, USA).
High Performance Liquid Chromatography (HPLC)
HPLC of a human neural lobe extract (3%) was performed on a 120 × 5 mm column of Nucleosil (5 µ, ODS-Silica) from Phenomenex (Rancho Palos Verdes, Ca) employing a Beckman model unit 332 plus 100A pump, an Hitachi 155–40 spectrophotometer, a C-RIA Shimadzu Integrated recorder, and the solvent gradient originally described by Swann and coworkers.16 For this gradient, solvent A was an aqueous solution of 0.2M NaH2PO4 adjusted to pH 2.1 with H3PO4, and solvent B was 95% aqueous acetonitrile. In terms of solvent B, the gradient was computed to be 0 to 12% (0–5 min); 12 to 25% (5–20 min), 28–30% (20–50 min); and 30–60% (50–80 min). The flow rate was 1 mL/min and fractions of 1 mL were collected. The human neural lobe obtained at autopsy was extracted for soluble protein with 0.1 M HCl and insoluble material separated by centrifugation at 3000 × g for 10 min at 5°C. Aliquots of fractions from HPLC were spotted on PVDF membrane strips, strips blocked with 5%BSA, and then reacted overnight at 5°C with antibodies to VP, VP-HNP, OT, OT-HNP,2 or the copeptin antiserum (Boris Y3) above. After washing, the strips were incubated with HRPgoat anti-rabbit IgG and then reacted with ECL reagent (Thermo Fisher Scientific).
Radioiodination
Co22-39 peptide was radioiodinated employing a modified lactoperoxidase procedure.17,18 The reaction was performed using 0.5 nmol of Co22-39, 244 mU lactoperoxidase (Calbiochem, 122 U/mg) in 22 µL of 0.05 M sodium phosphate, pH 7.0, 10 µL (1mCi) carrier-free [I125]Na solution (Perkin Elmer, ~17 Ci/mg, 10 mCi/100 µL), and 10 µL of 0.003% H2O2. The reaction was stopped by diluting the mixture with 500 µL phosphate buffer, producing a single site of iodination on the lone tyrosine of the peptide. Radiolabeled Co22-39 was isolated from other reaction components on Sephadex G-25 (0.9x 60 cm) pre-equilibrated with 1.25 mg/mL of BSA. Monoiodination of the isolated labeled peptide was ascertained as previously described by us18 for other products of enzymatic iodination through digestion with protease (Worthington Biochemical Corp. Freehold, N.J.) and comparing the migration of digestion products on Silica Gel TLC with mono-iodotyrosine and di-iodotyrosine. Unlike products generated through oxidation methods, there is no measurable damage to the peptide, and iodinated peptide remains stable for several months at −20°C.
Radioimmunoassay
The assay buffer used was 0.05M sodium phosphate (pH7.5) containing 1.25% BSA, 10−3 M EDTA, and 2 × 10−4 M cystine. Assays were performed with an initial total volume of 300 µL, comprising 150 µL and 200 µL of assay buffer, 50 µL of assay buffer containing Co22-39 reference standard (2.5 to 160 fmol), or 50 µL and 100 µL of plasma sample extract, and 50 µL of diluted antibody preparation. This mixture was incubated at 4°C for 48 h. 125Iodinated Co22-39 peptide (~4000 cpm) was then added with mixing, and incubation continued for a further 24 h at 4°C. Following incubation, the tubes received 50 µL of a 16 mg/mL aqueous solution of bovine gamma-globulin. Antibody-bound peptide/copeptin was separated from free peptide/copeptin by adding 500 µL of 20% polyethylene glycol 5000 with thorough mixing and centrifugation at 10,000 × g for 4 min at 4°C. Pellets from centrifugation were counted for 125Iodo-peptide for 2 min. Included in each assay were incubates with assay buffer controls, and those with no antibody to account for non-specific precipitation of 125Iodo-peptide. Plasma extracts were assayed at two different dilutions and measurements were performed in triplicate. An ln-logit linear transform19 of the standard dose–response displacement of 125Iodo-Co22-39 from the antibodies by the Co22-39 peptide was employed to calculate the amount of copeptin or copeptin product in plasma samples. Cross-reactions in the assay of peptides such as vasopressin and oxytocin were evaluated, as was cross-reaction with the Co22-29 of rat copeptin that has a 50% sequence homology with human Co22-39. The influences of peptide fragments of human Co22-39, namely, human Co27-39, human Co27-37, human Co27-34, and human Co22-31 on the assay were examined, and their binding affinities were used to determine the principal epitope recognized by Boris Y3 antibodies under assay conditions.
The amount of antibody preparation used was designed to bind 25–30% of the total 125Iodo-peptide used in the assay, as this level of binding was consistently found to increase sensitivity over the traditionally recommended binding of 50%. Standard peptide amounts were determined using differential absorbance at 215 and 225 nm.20
Subjects and Plasma Samples
The control subject population comprised 12 healthy adults: five females aged 24–60 years, and seven males aged 23–58 years. The SCLC patient population comprised 10 males and six females, all in the age range 43–63. The breast cancer patients were six females from 40 to 55 years of age with primary ductal (n = 3) and lobular (n = 3) invasive carcinoma. None of the participants showed clinical evidence of congestive heart failure, chronic hypertension, adrenal insufficiency, renal failure, or hypothyroidism. For plasma samples, 10 mL of antecubital venous blood was first withdrawn into heparinized syringes and then spun at 3000x g for 10 mins at 5°C. Orthostatic release of copeptin from the pituitary was minimized by seating subjects for 30 minutes before sampling. Blood was obtained from patients with cancer before the commencement of treatment.
Isolation of Copeptin Products for Assay
For the assay of copeptin in plasma, fresh heparinized samples (10 mL) were extracted for the glycopeptide using a procedure originally developed by us for vasopressin21 but also applicable to oxytocin and other peptides.2 This involves acidifying the plasma with one-tenth the volume of 1.0 M HCl, passage through a small pre-column of octadecasilylsilica (65–100 µ ODS-silica, Sep-Pak, Waters, Milford, MA), washing the column with water (10 mL), and eluting with 2 mL of 80% acetonitrile. The products were dried and reconstituted in 1 mL of assay buffer. Intact glycosylated human copeptin, like rat copeptin,6,11 was demonstrated by us to be eluted through gradient HPLC from ODS-silica columns by approximately 30% aqueous acetonitrile, while Co22-39 appeared at aqueous acetonitrile concentrations of about 25%. The recoveries of the intact glycopeptide itself were not evaluated, but those of Co22-39 were, like other peptides, found to be >80% for doses of 20 and 40 fmol added to peptide-depleted plasma.
Assurance for Animal and Human Studies
Animal approval was provided (1975–2020) by the IACUC of Dartmouth College, an AAALAC-approved facility (animal assurance number A3259-11). Approval for the use of human tissues for these and other studies was provided (1975–2020) by the Institutional Review Board of Dartmouth College under human subject assurance number 00003095, and written informed consent was obtained from all the patients.
Results
Immunohistochemistry
The antibodies of the Boris Y3 antiserum were found to have a specificity similar to that earlier shown for antibodies of the Boris Y2 antiserum. Vasopressin neurons of the human hypothalamus were selectively stained by these antibodies (Figure 1a), as verified by staining the same neurons in an adjacent tissue section with antibodies to VP-HNP (data not shown). Antibodies of the Boris Y3 antiserum were also shown to positively stain all cases of SCLC and all cases of breast cancer examined. This is exemplified by the triple-negative breast cancer and the primary SCLC shown (Figure 1b and d, respectively). In all cases, staining was completely prevented when peptide antigen (Co22-39) was added to the antiserum (Figure 1c and e), and no staining was obtained, in any case, when the primary antibody was omitted and the secondary antibody alone was used. This demonstrated that all staining of tumors with Boris Y3 was specific and there were no false positives.
High Performance Liquid Chromatography
VP, OT, VP-HNP, and OT-HNP subjected to HPLC were eluted at distinctly different points on the gradient at the positions shown in the human pituitary profile of Figure 1f. Antibodies of the BorisY3 antiserum showed copeptin elution as a single peak at an acetonitrile percentage of approximately 30% (~33% solvent B). For the same column and gradient, the Co22-39 peptide was eluted at an acetonitrile percentage of approximately 25% (~28% solvent B).
Radioimmunoassay
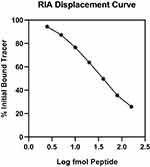
The Co22-39 peptide displaced [125I] Co22-39 in a repeatable dose-dependent manner for a dose range of 2.5 − 160 fmol, with a minimal detectable dose of 5 fmol, defined as displacements >2xSD above binding at zero peptide. Figure 2 shows a typical ln dose–displacement curve with, for a linear logit transform, a slope of −0.92, and an R value >0.98. For such slopes, a 50% displacement was produced in a dose range of 35–40 fmol Co22-39. The intra-assay variability (SD of the mean) of six replicate samples of 25 fmol was 8%, and the inter-assay variability was 14%. Displacements by different volumes of extracted plasma samples had slopes essentially the same as those produced by the reference standard. ODS extracts of plasma stripped of peptides and small proteins prepared by adding 100 mg/mL animal charcoal (Norit, Fisher Scientific Co. Waltham, MA, USA), and centrifuging at 10,000 × g, appeared to have no influence on binding. There was no measurable cross-reaction in the assay from vasopressin, oxytocin, rat Co22-39 (<1:500), nor from any of the fragments of human Co22-39 examined. The binding affinity of Boris Y3 for Co22-39 under assay conditions was found to be 2.6 x !09 M−1, and the binding affinities of the peptide fragments were, in turn, 1.3 × 107 M−1 (Co27-39), 5.7 × 106 M−1 (Co27-37), 4.3 × 106 M−1(Co27-34), and 5.7 × 105 M−1 (Co22-31). While these values demonstrate that all, or most, of Co22-39 is required for high affinity binding to Boris Y3, they also suggest that one principal epitope in this binding is the region Co27-34.
Plasma Values
Using the Co22-39 RIA, estimates of the mean concentration of copeptin in plasma for all normal volunteers were 18 ± 6 (12 to 32) pmol/L (pM), with 15 ± 4 (SEM) pmol/L for seven males and 20 ± 9 pmol/L (pM) for five females. A representation of individual values is shown in Figure 3. The mean value for females was greater than that for males, which could have been due to the greater scatter for the small sampling used. The values for SCLC patients (nine males, six females) ranged from 16 to 319 pmol/L, and 13 of 15 of these values (83%) were elevated as defined by >2.5 times the SD for normal values. This represented eight of nine males and five of six females. For the six females with breast cancer examined, values ranged from 12 to 68 pmol/L with three of these patients having elevated plasma levels compared with healthy females (Figure 3).
Discussion
It is clear from the immunohistochemical and HPLC findings that the antibodies of the Boris Y3 antiserum show a capacity to specifically interact with intact copeptin as a product present in the human neural lobe or as a component of the provasopressin of hypothalamic neurons or tumors. The RIA for copeptin developed here using this antiserum seems to be sensitive and reliable and has a narrow range of values (10–31 pmol/L) for the plasma levels of the healthy volunteers used in the study. However, because of the selective nature of the antibodies employed here or similar antibodies, it could be argued that RIA plasma evaluations represent large C-terminal fragments of the glycopeptide as well as of intact copeptin. Yet such a possibility may not be relevant because, unlike parathyroid hormone measurement by RIA,22 there is no evidence that such fragments represent a significant and non-representative fraction of released copeptin in the plasma. In fact, intact copeptin is reported to be stable in the blood.10 The control values obtained here for plasma copeptin, although only preliminary, seem to be appropriate with respect to established levels of plasma vasopressin and VP-HNP in humans.2,10,18 Assays of plasma copeptin are generally conducted to better evaluate the release of vasopressin from the neural lobe under normal and pathological conditions.10 Copeptin, vasopressin, and VP-HNP are all generated and released from the same precursor, and since they are distributed in a similar volume once released, their relative plasma values should be determined by their half-life in the blood.23,24 However, measured plasma vasopressin levels are predicted to be an underestimate of the expected levels compared to copeptin, and VP-HNP, because a considerable amount of released vasopressin is selectively shared between the blood and a large stable platelet-bound sink.25
Vasopressin plasma levels in normally hydrated humans have been determined to be from 1 to 5 pmol/L.1,2,10,17,23,24 These values were obtained by RIA following peptide extraction with either acetone, acetone-ethanol, or by the ODS-silica extraction first developed by us.17 Plasma values obtained in this manner were shown to also appropriately represent vasopressin levels under abnormal conditions such as hypothalamic and nephrogenic diabetes insipidus, dehydration, and SIADH.
The half-life of vasopressin in human circulation has been reviewed extensively by Lauson24 who tabulated values from many laboratories that show a range of 3–7 minutes, when disappearance is treated as a two-component model. Half-lives in other mammals, including primates, are given to range, in most cases, from 2 to 4 minutes.24,26 More recently, when plotting vasopressin disappearance in volunteers, following a water load from levels produced by hypertonic saline, a much larger apparent half-life of 12 minutes was obtained by Fenske et al.12
Evaluations with our RIA for VP-HNP performed on the plasma of 20 normally hydrated individuals gave values of from <5 to 13.3 pmol/L (mean, 7.3 ±0.5 pmol/L),18 while evaluations by RIA for 14 normally hydrated Long-Evans rats gave vasopressin-associated rat neurophysin (VP-RNP) serum levels higher at 22 ±2 pmol/L.27 These neurophysin values are scattered over a narrow range. Neither VP-HNP nor VP-RNP bind to platelets or to vasopressin at the pH of blood.6 VP-HNP plasma levels, like those for vasopressin, were elevated in all cases of SIADH. For conscious rats, changes in plasma VP-RNP values by RIA are well correlated with changes in plasma osmolality (r = 0.73–0.78), have been used to successfully question the validity of the Verney hypothesis, and to show that vasopressin has a negative feedback influence on its own release.28,29 Moreover, VP-HNP and VP-RNP, as reported for copeptin, are both stable against breakdown in plasma, for 24 h at ambient temperature, >48 hours at 4°C, and for at least 6 months at −20°C.7,30,31 The apparent half-lives of neurophysins were found by us to be approximately one-and-one-half times those of vasopressin, and this is consistent with the findings of others.23,32
The half-life of copeptin in humans has been reported by Fenske et al12 and others10 to be two-to-three times that of vasopressin. This larger half-life reflects the lipophilic nature of copeptin.10–12,33 Given the plasma values and smaller half-lives of vasopressin and likely values of VP-HNP in humans, copeptin levels in normal hydrated individuals might be expected to be in the range of 8–30 pmol/L, which agrees well with the values found here by us for volunteers using the Co22-39 radioimmunoassay (mean,18±6 pmol/L). In apparent agreement, Sailer et al34 found median values for 30 healthy individuals to be 15 pm/L and 10 pm/L using the popular commercial sandwich immunoassays such as Brahms Kryptor and LIA assays (Thermo Scientific, Hennigsdorf, Germany), while much higher values of 48 pm/L were found for the Cloud Clone ELISA assay. However, less comparable low values (expected from vasopressin and VP-HNP levels) ranging from 1.7 to 11.2 pm/L were found with the Kryptor assay by Terzic and coworkers.35 Similar low values were also found by others with the LIA assay. A median value obtained for healthy volunteers with this assay by Morganthaler and coworkers10,36 was 4.2 pmol/L (wide-range of 1–13.8), while Struck and coworkers23 reported a value of 0.88 pmol/L (wide-range 0.3 −18). These lower values are perhaps to be expected because these sandwich assays employ antibodies raised against a non-glycosylated N-terminal peptide region of copeptin (Co7-22) and use a non-glycosylated larger peptide (Co7-39) called PAY33 as reference standard. Conformational differences with copeptin due to glycosylation37 are likely to result in reduced binding of the endogenous peptide to the Co7-22 antibody compared with that of PAY33. Although this would predict lower than actual values for copeptin using these assays, it should not diminish their clinical usefulness in many circumstances.
The results obtained in the current study with cancer patients indicate that plasma copeptin levels, as measured by RIA, are statistically elevated in 83% of patients (>2.5 × SD above normal mean) but may be sufficiently elevated (>2.5 × normal mean) in only 70% of patients to lend them to be useful as markers of SCLC. This is because copeptin levels in future clinical studies could be influenced by unknown conditions for some outpatients. The other two released products generated from provasopressin breakdown, vasopressin and VP-HNP, were both previously shown to be plasma markers of SCLC in patients. Vasopressin is elevated in the plasma of more than half of patients, while VP-HNP, such as copeptin in this study, was clearly elevated in approximately two-thirds of patients.7,8 Plasma levels of both vasopressin and VP-HNP were earlier found to likely reflect tumor burden in such patients and allow treatment to be monitored from changing levels of the two markers.2,3,6–8 It is possible that copeptin levels determined by RIA might also serve such a role.
For the six female patients with breast cancer studied here, plasma copeptin levels by RIA were statistically elevated in half, but only sufficiently elevated (>2.5 × normal mean) in two (33%) to be clearly seen as a possible marker of disease. Similar to SCLC, vasopressin gene expression is common to all types of breast cancer, and all tumors feature surface provasopressin.2,4,5 Release of vasopressin from some breast cancers is evidenced by the ability of breast cancer cells in culture to release copeptin4 and the likely presence of a full complement of processing enzymes in breast cancer cells.38 Copeptin, as determined by RIA, could therefore potentially be a marker in some patients with breast cancer. The findings also suggest that vasopressin and VP-HNP plasma levels, not yet measured in breast cancer patients, could also serve this role, as they do in SCLC patients.
Acknowledgments
These studies were supported in part by the PHS grant NCI CA19603 and the NCI Cancer Center Support Grant P30 CA23108-37.
Disclosure
William G. North, although a faculty member of the Geisel School of Medicine at Dartmouth College and faculty member of the Norris Cotton Cancer Center, also has a commercial interest in Woomera Therapeutics Inc, as President and he holds >30% interest in that company. However, all the studies reported precede that involvement.
References
1. Cowley AW, Liard J-F. Cardiovascular actions of vasopressin. In: Gash DH, Boer GJ, editors. Vasopressin: Principles and Properties. New York: Plenum Press; 1987:389–422.
2. North WG. Neuropeptide production by small cell carcinoma: vasopressin and oxytocin as plasma markers. J Clin Endocrinol Metab. 1991;73(6):1316–1320. doi:10.1210/jcem-73-6-1316
3. North WG. Gene regulation of vasopressin and vasopressin receptors in cancer. Exp Physiol. 2000;85S:27S–40S.
4. Keegan BP, Akerman BL, Pequeux C, North WG. Provasopressin expression by breast cancer cells: implications for growth and novel treatment strategies. Breast Cancer Res Treat. 2006;95(3):265–277. doi:10.1007/s10549-005-9024-8
5. North WG, Pang RHL, Gao G, Memoli VA, Cole BF. Native MAG-1 antibody almost destroys human breast cancer xenografts. Breast Cancer Res Treat. 2010;127(3):631–637. doi:10.1007/s10549-010-1009-6
6. North WG. Biosynthesis of vasopressin and neurophysins. In: Gash DH, Boer GJ, editors. Vasopressin: Principles and Properties. New York: Plenum Press; 1987:175–209.
7. North WG, Maurer LH, Valtin H, O’Donnell J. Human neurophysins as potential tumor markers for small-cell carcinoma of the lung: application of specific radioimmunoassays for vasopressin-associated and oxytocin-associated neurophysins. J Clin Endocrinol Metab. 1980;51:892–896. doi:10.1210/jcem-51-4-892
8. Maurer LH, O’Donnell JF, Kennedy S, Faulkner CS, North WG. Human neurophysins in carcinoma of the lung: relation to histology, disease stage, survival, and the syndrome of inappropriate antidiuretic hormone secretion. Cancer Treat Rep. 1983;67(11):971–976.
9. Holwerda DA. A glycopeptide from the posterior lobe of pig pituitaries. Eur J Biochem. 1972;28(3):340–346. doi:10.1111/j.1432-1033.1972.tb01919.x
10. Morgenthaler NG, Muller B, Struck J, Bergmann A, Redl H, Christ-Crain M. Copeptin, A stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock. 2007;28(2):219–226. doi:10.1097/SHK.0b013e318033e5da
11. North WG, O’Connor EF, Gonzales CB. Single-step isolation and sequencing of vasopressin and oxytocin precursors. Peptides. 1992;13(2):395–400. doi:10.1016/0196-9781(92)90127-O
12. Fenske WK, Schnyder I, Koch G, et al. Release and decay kinetics of copeptin vs AVP in response to osmotic alterations in healthy volunteers. J Clin Endocrinol Metab. 2018;103(2):505–513. doi:10.1210/jc.2017-01891
13. Friedmann A. Vasopressin gene expression by non-neuronal cells: production in the gastrointestinal system and neoplasms of the lung. [PhD Thesis]; Hanover NH: Dartmouth College; 1995.
14. North WG, Pai S, Friedmann A, Yu X, Fay M, Memoli V. Vasopressin gene related products are markers of human breast cancer. Br Cancer Res Treat. 1995;34(3):229–235. doi:10.1007/BF00689714
15. Keegan BP, Memoli VA, Wells W, North WG. Detection of provasopressin in invasive and non-invasive(DCIS) human breast cancer using a monoclonal antibody directed against the C-terminus (MAG1). Breast Cancer. 2001;4:15–22.
16. Swann RW, Gonzalez CB, Birkett SD, Pickering BT. Precursors in the biosynthesis of vasopressin and oxytocin in the rat. Biochem J. 1982;208(2):339–349. doi:10.1042/bj2080339
17. Thorell JI, Johansson BG. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971;251(3):363–369. doi:10.1016/0005-2795(71)90123-1
18. North WG, LaRochelle FT, Melton J, Curtis Mills R. Isolation and partial characterization of two human neurophysins: their use in the development of specific radioimmunoassays. J Clin Endocrinol Metab. 1980;51(4):884–891. doi:10.1210/jcem-51-4-884
19. Rodbard D, Ruder HJ, Vaitukatus J, Jacobs HS. Mathematical analysis of kinetics of radioligand assays: improved sensitivity observed by delayed addition of radiolabelled ligand. J Clin Endocrin Metab. 1971;33(2):343. doi:10.1210/jcem-33-2-343
20. Waddell WJ. A simple ultraviolet spectroscopic method for the determination of protein. J Lab Clin Med. 1956;4:311–314.
21. LaRochelle FT, North WG, Stern P. A new extraction of vasopressin from blood:The use of octadecasilyl-silica. Pflugers Archiv. 1980;387(1):79–81. doi:10.1007/BF00580849
22. Nussbaum SR, Potts JT Jr. Immunoassays for parathyroid hormone 1–84 in the diagnosis of hyperthyroidism. J Bone Mineral Res. 1991;6(Suppl 2):844–850.
23. North WG, Gellai M, Hardy G. Oxytocin and oxytocin-associated neurophysin evaluation by RIA in the Brattleboro rat: turnover. Ann NY Acad Scs. 1982;394(1):167–172. doi:10.1111/j.1749-6632.1982.tb37424.x
24. Lauson HD. Metabolism of neurohypophysial hormones. In:Greep RO, Ashwood, EB, editors. Handbook of Physiology. Washington DC: American Physiological Society; 1974; 4:287–393.
25. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500–2504. doi:10.1016/j.peptides.2005.04.019
26. Leng G, Ludwig M. Neurotransmitters and Peptides: whispered secrets and public announcements. J Physiol. 2008;586(Pt23):5625–5632. doi:10.1113/jphysiol.2008.159103
27. North WG, LaRochelle FT, Hardy GR. Radioimmunoassays for individual rat neurophysins. J Endocr. 1983;96(3):373–386. doi:10.1677/joe.0.0960373
28. Cheng SWT, North WG. Effects of acute increases in plasma osmolality on plasma vasopressin-associated neurophysins in conscious rats: implications for osmoregulation. Neuroendocrine. 1985;40(4):363–368. doi:10.1159/000124099
29. Cheng SWT, North WG. Vasopressin reduces release from vasopressin neurons and oxytocin neurons by acting on V2-like receptors. Brain Res. 1989;479(1):35–39. doi:10.1016/0006-8993(89)91332-2
30. North WG, Ware J, Maurer LH, Chahinian AP, Perry M. Neurophysin as tumor markers for small cell carcinoma of the lung: a cancer and leukemia group B evaluation. Cancer. 1988;62(7):1343–1347.
31. North WG, Maurer LH, O’Donnell JF. The neurophysins and small cell cancer. In: Greco FA, editor. Biology and Management of Lung Cancer. Boston: Martinus Nijhoff Publishers; 1983:143–169.
32. Forsling MJ, Martin MJ, Sturdy JC, Burton AM. Observation of release and clearance of neurophysin and neurohypophysial hormones in the rat. J Endocrinol. 1973;57(2):307–315. doi:10.1677/joe.0.0570307
33. Pitkin DH, Mico BA, Sitrin RD, Lisbet LJ. Charge and lipophilicity govern the pharmacokinetics of glycopeptide antibiotics. Antimicrob Agents Chemother. 1989;29(3):440–444. doi:10.1128/AAC.29.3.440
34. Sailer CO, Refardt J, Blum CA, et al. Validity of different copeptin assays in the diagnosis of the polyuria-polydipsia syndrome. Nat Sci Rep. 2021;11(1):10104. doi:10.1038/s41598-021-89505-9
35. Terzic D, Johansson-Fallgren AS, Ragnarsson O, Goetze JP, Hannarsten O. Evaluation of a sensitive copeptin assay for clinical measurement. The Open Clin Chem J. 2012;5(1):21–26. doi:10.2174/1874241601205010021
36. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112–119. doi:10.1373/clinchem.2005.060038
37. Bosques CJ, Tschampel SM, Woods RJ, Iperioli B. Effects of glycosylation on peptide conformation: a synergistic and computational study. JACS. 2004;126(27):8421–8425. doi:10.1021/ja0496266
38. Du J, Keegan BP, North WG. Key peptide processing enzymes are expressed by breast cancer cells. Cancer Lett. 2001;165(2):211–218. doi:10.1016/S0304-3835(01)00409-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.