Back to Journals » International Journal of General Medicine » Volume 16
Can Patients with Asymptomatic/Mild Illness and Moderate Illness COVID-19 Have White Matter Damage?
Authors Wei C, Yu X, Chen Y, Yang T, Li S, Li J, Chen X
Received 11 August 2023
Accepted for publication 4 October 2023
Published 10 October 2023 Volume 2023:16 Pages 4585—4593
DOI https://doi.org/10.2147/IJGM.S434968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Video abstract of "COVID-19 and white matter hyperintensity" [ID 434968].
Views: 159
Cunsheng Wei,* Xiaorong Yu,* Yuan Chen, Tingting Yang, Shenghua Li, Junrong Li, Xuemei Chen
Department of Neurology, Affiliated Jiangning Hospital with Nanjing Medical University, Nanjing, Jiangsu, 211100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuemei Chen; Junrong Li, Department of Neurology, Affiliated Jiangning Hospital with Nanjing Medical University, 169 Hushan Road, Nanjing, Jiangsu, 211100, People’s Republic of China, Tel +86 13347808579 ; +86 13451908999, Email [email protected]; [email protected]
Background and Purpose: Studies have shown that severe coronavirus pandemic 2019 infection could lead to white matter hyperintensities, but the relationship between asymptomatic/mild illness and moderate illness coronavirus pandemic 2019 and white matter hyperintensities remains largely unknown. This study aimed to investigate the relationship between asymptomatic/mild illness and moderate illness coronavirus pandemic 2019 and the risk of white matter hyperintensities.
Methods: Hospitalized patients who were confirmed to have coronavirus pandemic 2019 for the first time were enrolled. Fazekas scores were used for assessment of the severity of white matter hyperintensities. We also rated the 90-day functional outcome after discharge.
Results: Of the 157 enrolled patients, 124 (78.98%) coronavirus pandemic 2019 patients were classified as having asymptomatic or mild illness, and 33 (21.02%) were classified as having moderate illness. The results showed that the Fazekas scale scores at baseline (periventricular white matter hyperintensities, 1.31± 1.16 vs 2.06± 1.20; Deep white matter hyperintensities, 1.04± 0.97 vs 1.73± 1.13 P < 0.01) and at follow-up (periventricular white matter hyperintensities, 1.38± 1.21 vs 2.09± 1.21; Deep white matter hyperintensities, 1.13± 1.04 vs 1.79± 1.14 P < 0.01) were lower in patients with symptomatic or mild illness than in those with moderate illness. Moreover, no significant difference (7.26% vs 3.03%; P =0.377) was observed between the two divided groups in terms of white matter hyperintensities progression.
Conclusion: Our findings suggest that moderate COVID-19 is related to severe white matter hyperintensities compared with asymptomatic/mild illness but not to the progression of white matter hyperintensities.
Keywords: coronavirus pandemic 2019, white matter hyperintensities, risk, Fazekas scale, progression
Introduction
Coronavirus pandemic 2019 (COVID-19) quickly became a worldwide public health crisis and continues to spread worldwide.1,2 In recent times, the study of long COVID, especially brain complications, has been intensely investigated.3,4 Some studies have shown that severe COVID-19 infection could lead to white matter hyperintensities (WMHs).5,6 The mechanisms may be related to delayed post hypoxic leukoencephalopathy, for which a similar pattern of involvement has been described in patients approximately 10–14 days after a hypoxic insult.7,8 Moreover, postinfectious demyelinating or hemorrhagic encephalitis, toxic and metabolic causes, and posterior reversible encephalopathy syndrome might be underlying causative factors.9
However, the majority of patients with asymptomatic/mild illness and moderate illness COVID-19 do not ultimately develop severe hypoxic, toxic or metabolic abnormalities. Currently, there is a lack of consensus on whether asymptomatic/mild illness and moderate illness COVID-19 increase the risk of WMH; therefore, we designed a short-term follow-up study to investigate the relationship between asymptomatic/mild illness and moderate illness COVID-19 and the risk of WMH.
Methods
Study Population
As China released the control of COVID-19, a large number of infected patients appeared in a short period of time, and non-infected departments including neurology also treated a large number of COVID-19 infected patients. Therefore, in the prospective study, the study group consisted of hospitalized patients in the Department of Neurology, Affiliated Jiangning Hospital of Nanjing Medical University between December 1, 2022, and January 31, 2023, due to fever and cough and who were confirmed to have COVID-19 for the first time by means of a reverse-transcriptase polymerase chain reaction assay (Cobas 6800; Roche Diagnostics, Rotkreuz, Switzerland) of a nasopharyngeal swab specimen. The inclusion criteria were as follows: 1) aged 45 to 85 years and 2) underwent cerebral MRI. The exclusion criteria were as follows: participants with severe head injury; severe cerebral infarction, defined as an NIH Stroke Scale (NIHSS) score ≥ 14; severe cerebral hemorrhage, defined as a baseline intraparenchymal hemorrhage volume ≥ 30 mL or intraventricular hemorrhage; vascular malformation or brain malignancy; or the presence of multiple sclerosis or a lack of complete clinical data. The study flowchart is shown in Figure 1.
 |
Figure 1 Flow diagram of patient selection. |
Data Collection
To assess relevant risk factors at baseline, we examined patient demographic characteristics, history of hypertension, diabetes mellitus, atrial fibrillation and ischemic stroke, presence of coronary heart disease, past or present cigarette or alcohol use, and antiplatelet or statin treatment. Patients underwent a physical examination, systolic and diastolic blood pressure was measured, and laboratory examinations including tests for glucose, homocysteine (HCY), total cholesterol (TC), high-density lipoprotein cholesterol (HDL), triglyceride (TG), low density lipoprotein (LDL), lipoprotein-α (LP-A), glutamic pyruvic transaminase (ALT), glutamic oxaloacetic transaminase (AST), creatinine (Cr), uric acid (UA), troponin I, high C-reactive protein (H-CRP), D-Dime, Pro Calcitonin, and troponin-I were performed.
Chest CT Scan
We performed chest CT scans on each patient. A Siemens SOMATOM Drive 3rd generation dual source CT scanner was used in the study. Patients were placed in a supine position, with their head advanced and their upper limbs raised on both sides. The collected parameters included a 5 mm slice thickness, 0.8 pitch, 120 kV, 220–250 mAs, and 1 mm reconstruction images.
MRI Acquisition
MRI scanning was performed within 3 days of hospitalization on a 3.0 T MRI scanner (Ingenia, Philips Medical Systems, the Netherlands) with an 8-channel receiver array head coil. MRI scanning protocol has been described in detail previously.10,11
Fazekas Scores for Assessment of the Severity of WMH
The scale divides the white matter into periventricular and deep white matter, and each region is given a grade depending on the size and confluence of lesions:12 (1) Periventricular white matter hyperintensities (PVWMH), 0 = absent, 1 = “caps” or pencil-thin lining, 2 = smooth “halo”, 3 = irregular periventricular signal extending into the deep white matter; and (2) Deep white matter hyperintensities (DWMHs), 0 = absent, 1 = punctate foci, 2 = beginning confluence, 3 = large confluent areas. Two experienced neurologists and a neuroradiologist participated in the evaluation of the MRI results, and detailed imaging findings are described in Figure 2.
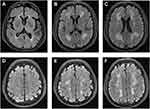 |
Figure 2 Representative T2-FLAIR images illustrating the Fazekas score. Fazekas scores of 1, 2 and 3 on PVWMH are shown in (A–C), and DWMH are shown in (D–F). |
Clinical Spectrum of COVID-19 Infection
(1) Asymptomatic or presymptomatic infection patients were defined as individuals who tested positive for COVID-19 using a virologic test (ie, a nucleic acid amplification test [NAAT] or an antigen test) but had no symptoms consistent with COVID-19. (2) Mild illness patients were defined as individuals who had any of the various signs and symptoms of COVID-19 (eg, fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, loss of taste and smell) but did not have shortness of breath, dyspnea, or abnormal chest imaging. (3) Moderate illness patients were defined as individuals who showed evidence of lower respiratory disease during their clinical assessment or imaging and who had an oxygen saturation measured by pulse oximetry (SpO2) ≥94% on room air at sea level.13
Follow-Up and Progression of WMH
We also rated the 90-day functional outcome after discharge. Any new neurological symptoms (including dizziness, headache, abnormal smell, numbness and weakness in the limbs) at 90 days were recorded and compared with the symptoms at the baseline assessment. All follow-up patients underwent a second MRI scan at 90 days. WMH progression was defined if the Fazekas scale scores were increased by ≥1, regardless of PVWMH or DWMH. These outcome events and MRI imaging were reviewed in detail by 2 experienced neurologists and a neuroradiologist, and a consensus view was achieved.
Statistics
Continuous data are summarized as the mean values with SDs for data with a normal distribution or the median values with interquartile ranges for data with a skewed distribution. Categorical data are presented as frequencies with proportions. A two-sample t-test was used to compare continuous data. Categorical data were analyzed using the chi-square test. All statistical analyses were performed using SPSS 25.0 software (SPSS, Chicago, IL).
Results
Of the 157 enrolled patients, 124 (78.98%) COVID-19 patients were classified as having asymptomatic or mild illness, and 33 (21.02%) were classified as having moderate illness. Patients with asymptomatic or mild illness were younger than those with mild illness (64.44±10.73 vs 74.30±9.12, y, P <0.001). In addition, asymptomatic or mild illness patient groups had a lower ratio of diabetes (25.00% vs 48.48%; P=0.009), coronary artery disease (15.32% vs 33.33%; P=0.021), previous ischemic stroke (65.32% vs 90.91%; P=0.004), atrial fibrillation (4.84% vs 18.18%; P=0.010), bacterial coinfection (0.81% vs 15.15%; P <0.001) and electrolyte disturbances (26.83% vs 69.70%; P <0.001) than the group of patients with moderate illness. Moreover, asymptomatic or mild illness patients presented lower levels of serum CRP (3.92±12.09 vs 18.40±34.86, mg/L, P =0.027), fasting blood glucose (5.94±2.00vs. 7.58±4.01, mmol/L, P =0.032), aspartate aminotransferase (23.08±9.91 vs 30.06±18.19, U/L, P=0.041) and creatinine (69.21±26.61 vs 112.20±143.41, µmmol/L, P=0.002) than patients with moderate illness. However, patients with asymptomatic or mild illness had higher levels of total cholesterol (4.02±1.13 vs 3.40±1.06, mmol/L, P =0.007), LDL cholesterol (2.27±1.03 vs 1.85±0.82, mmol/L, P =0.039), HDL cholesterol (1.04±0.32 vs 0.82±0.30, mmol/L, P =0.001) and uric acid (317.15±81.02 vs 264.58±95.97, µmmol/L, P =0.006) than control subjects. The details are presented in Table 1.
 |
Table 1 Clinical Characteristics of COVID-19 Patients (n=157) |
Follow-up study outcomes were assessed at the end of follow‐up. Of the 124 COVID-19 patients with asymptomatic or mild illness, 10 had dizziness, 5 had headache, 6 had abnormal smell, no subject had numbness and weakness in the limbs, and no one died. Of the 33 COVID-19 patients with moderate illness, 1 patient exhibited deterioration and underwent ventilator-assisted ventilation due to respiratory failure, 3 had dizziness, 4 had headache, 6 had abnormal smell, 1 had weakness in the limbs and was diagnosed with acute stroke, and no one died at the end of follow-up. To further investigate the differences in the Fazekas scale scores in the two groups at baseline and follow-up and for PVWMH and DWMH, the results showed that the Fazekas scale score distributions between the two groups were significantly different not only at the baseline assessment but also at later follow-ups (all P<0.05). Moreover, we also observed a tendency that as the Fazekas scale scores increased, the proportion of asymptomatic or mild illness subjects decreased, but the proportion of moderate illness increased. WMH progression was defined if the Fazekas scale scores were increased by ≥1, and the results indicated that no significant difference (7.26% vs 3.03%; P =0.377) was observed between the two divided groups in terms of WMH progression (Table 2).
 |
Table 2 Fazekas Scale Scores of 157 COVID-19 Patients |
We then compared the Fazekas scale scores between patients with asymptomatic or mild illness and moderate illness by univariate analysis. The means and standard deviations of the Fazekas scale scores in the two groups are also shown in Figure 2. The results showed that the Fazekas scale scores at baseline (PVWMH, 1.31±1.16 vs 2.06±1.20; DWMH, 1.04±0.97 vs 1.73±1.13 P <0.01) and at follow-up (PVWMH, 1.38±1.21 vs 2.09±1.21; DWMH, 1.13±1.04 vs 1.79±1.14 P <0.01) were lower in patients with symptomatic or mild illness than in those with moderate illness (Figure 3).
 |
Figure 3 Fazekas scale scores of the two groups. Asterisks “**” represent P < 0.01 compared with the control group. |
Discussion
This study revealed that moderate COVID-19 is related to severe WMH compared with asymptomatic/mild illness but not to the progression of WMH. This suggests that the severity of brain WMH can be used as an imaging marker for the prognosis of COVID-19 under the current epidemic environment in COVID-19; conversely, asymptomatic/mild illness and moderate illness with COVID-19 will not lead to short-term progression of WMH.
Recently, more studies have shown that COVID-19 infection significantly affects brain function,14–16 and reports indicate that 30–60% of patients with COVID-19 suffer from neurological symptoms,17 including anosmia, agnosia, stroke, paralysis, cranial nerve deficits, encephalopathy, meningitis, delirium, seizures, Guillain‒Barré syndrome, acute necrotizing hemorrhagic encephalopathy, and hemophagocytic lymphohistiocytosis.18,19 A retrospective study of a large sample suggested that the presence of WMH and/or cerebral microbleeds is associated with a critical illness, increased mortality, and worse functional outcome in patients with COVID-19.20 Our study also suggests that the presence of severe WMH is associated with moderate illness with COVID-19 compared with asymptomatic/mild illness; however, we have not observed that progression of WMH is related to asymptomatic/mild illness or moderate illness with COVID-19 infection.
We speculate that this may be caused by the following reasons. First, WMH is a common disease that often occurs in the elderly.21 Research has shown that the incidence of WMH is approximately 50–98% in people over 60 years old, and its incidence is close to 100% in people over 85 years old.22 Therefore, patients with severe white matter damage are often older, these patients often have severe symptoms after being infected with COVID-19, and our data also support this view (Table 1). Age). Second, in addition to age, the severity of WMH is also related to many risk factors for cerebrovascular diseases, such as hypertension and diabetes,11 so these patients are more likely to suffer from severe COVID-19 infection due to more basic diseases. Our data also suggest that asymptomatic/mild illness and moderate illness with COVID-19 will not lead to short-term progression of WMH, thus further indicating that mild to moderate COVID-19 infection will not have a significant impact on white matter damage.
To the best of our knowledge, this is the first study demonstrating the relationship between the severity of WMH and COVID-19. All data were subjected to rigorous quality controls, and rigorous statistical analyses were performed. Meanwhile, to further understand the relationship between COVID-19 infection and the progression of WMH, we performed short-term follow-up and prognostic analysis of patients. This finding will promote scholars to further understand the relationship between WMH and COVID-19 infection. However, there are some limitations in the current study, which are listed as follows. First and most importantly, due to the relatively small overall sample size of the study, and only 33 cases in the severe illness group, we have included as many relevant factors as possible in the univariate analysis (Table 1) as well, so we have not further conducted multivariate analysis by logistic regression or COX regression. However, though we did not conduct multivariate analysis, the data with largely trend supported the relationship between the severity of WMH and COVID-19. Second, the study does not include healthy controls, because of our work mainly focused on the risk of WMH progression in asymptomatic/mild illness and moderate illness with COVID-19 infection, so we did not include healthy controls for comparison, although the risk of WMH in healthy people increases with age. Third, this study is based on clinical research and does not explore the mechanism. Finally, this study is a single-center study involving individuals in the Han population in the same region. Therefore, further large-sample multicenter with multivariate analysis and in-depth mechanistic studies are needed to overcome the above limitations.
Taken together, our findings suggest that moderate COVID-19 is related to severe WMH compared with asymptomatic/mild illness but not to the progression of WMH. Our study further improved the relationship between COVID-19 infection and brain function, and provided evidence for the prevention and treatment of brain complications after long COVID infection. Moreover, a longer follow-up period is needed to evaluate the relationship between COVID-19 and brain complications.
Abbreviations
COVID-19, coronavirus pandemic 2019; WMH, white matter hyperintensities; PVWMH, periventricular white matter hyperintensities DWMHs, Deep white matter hyperintensities; FBG, fasting blood glucose; HbAIc, glycosylated hemoglobin; HCY, homocysteine; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; TG, triglyceride; LDL, low density lipoprotein; LP-A, lipoprotein-α; ALT, glutamic pyruvic transaminase; AST, glutamic oxaloacetic transaminase; Cr, creatinine; UA, uric acid; CRP, C-reactive protein; SD, standard deviation.
Data Sharing Statement
Study data are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
We obtained ethical approval for this study from the ethics committee of the Affiliated Jiangning Hospital of Nanjing Medical University and performed in accordance with the Declaration of Helsinki (reference number, 202203023K01). Written informed consent was obtained from all study participants.
Consent for Publication
All patients gave informed consent for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by Scientific research project of Jiangsu Provincial Health Commission (M2022009); the Nanjing Health Science and Technology Development Special Fund Project (YKK22216); the Clinical Medical Science and Technology Development Fund of Jiangsu University (JLY2021153); Jiangning District Science and Technology Huimin Project (2022092S).
Disclosure
The authors declare no competing interests in this work.
References
1. Fitoussi F, Tonen-Wolyec S, Awaida N, Dupont R, Bélec L. Analytical performance of the point-of-care BIOSYNEX COVID-19 Ag BSS for the detection of SARS-CoV-2 nucleocapsid protein in nasopharyngeal swabs: a prospective field evaluation during the COVID-19 third wave in France. Infection. 2022;50(3):625–633. doi:10.1007/s15010-021-01723-5
2. Zhou HJ, Pan L, Shi H, et al. Willingness to pay for and willingness to vaccinate with the COVID-19 vaccine booster dose in China. Front Pharmacol. 2022;13:1013485. doi:10.3389/fphar.2022.1013485
3. Villaume WA. Marginal BH4 deficiencies, iNOS, and self-perpetuating oxidative stress in post-acute sequelae of Covid-19. Med Hypotheses. 2022;163:110842. doi:10.1016/j.mehy.2022.110842
4. Vollbracht C, Kraft K. Feasibility of vitamin C in the treatment of post viral fatigue with focus on long COVID, based on a systematic review of IV vitamin C on fatigue. Nutrients. 2021;13(4):1154. doi:10.3390/nu13041154
5. Sachs JR, Gibbs KW, Swor DE, et al. COVID-19-associated leukoencephalopathy. Radiology. 2020;296(3):E184–E185. doi:10.1148/radiol.2020201753
6. Rhally A, Griffa A, Kremer S, et al. C-reactive protein and white matter microstructural changes in COVID-19 patients with encephalopathy. J Neural Transm. 2021;128(12):1899–1906. doi:10.1007/s00702-021-02429-6
7. Breit H, Jhaveri M, John S. Concomitant delayed posthypoxic leukoencephalopathy and critical illness microbleeds. Neurol Clin Pract. 2018;8(5):e31–e33. doi:10.1212/CPJ.0000000000000513
8. Lang M, Buch K, Li MD, et al. Leukoencephalopathy associated with severe COVID-19 infection: sequela of hypoxemia? AJNR Am J Neuroradiol. 2020;41(9):1641–1645. doi:10.3174/ajnr.A6671
9. Huang S, Zhou Z, Yang D, et al. Persistent white matter changes in recovered COVID-19 patients at the 1-year follow-up. Brain. 2022;145(5):1830–1838. doi:10.1093/brain/awab435
10. Jiang J, Gao Y, Zhang R, et al. Differential effects of serum lipoprotein-associated phospholipase A2 on periventricular and deep subcortical white matter hyperintensity in brain. Front Neurol. 2021;12:605372. doi:10.3389/fneur.2021.605372
11. Wei C, Yu X, Wang L, et al. Can hyperuricemia predict the progression risk of cerebral small vessel disease? Neurol Res. 2022;44(10):910–917. doi:10.1080/01616412.2022.2067707
12. Ortega G, Espinosa A, Alegret M, et al. Combination of white matter hyperintensities and Aβ burden is related to cognitive composites domain scores in subjective cognitive decline: the FACEHBI cohort. Alzheimer’s Res Ther. 2021;13(1):141. doi:10.1186/s13195-021-00877-6
13. Xu Y, Chen Y, Tang X. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China. Global Health Med. 2020;2(2):66–72. doi:10.35772/ghm.2020.01015
14. Hassett CE, Gedansky A, Migdady I, Bhimraj A, Uchino K, Cho SM. Neurologic complications of COVID-19. Cleve Clin J Med. 2020;87(12):729–734. doi:10.3949/ccjm.87a.ccc058
15. Rai SN, Tiwari N, Singh P, et al. Exploring the paradox of COVID-19 in neurological complications with emphasis on parkinson’s and Alzheimer’s disease. Oxid Med Cell Longev. 2022;2022:3012778. doi:10.1155/2022/3012778
16. Singh N, Rai SN, Singh V, Singh MP. Molecular characterization, pathogen-host interaction pathway and in silico approaches for vaccine design against COVID-19. J Chem Neuroanat. 2020;110:101874. doi:10.1016/j.jchemneu.2020.101874
17. Prasad K, AlOmar SY, Alqahtani SAM, Malik MZ, Kumar V. Brain disease network analysis to elucidate the neurological manifestations of COVID-19. Mol Neurobiol. 2021;58(5):1875–1893. doi:10.1007/s12035-020-02266-w
18. Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38(7):1549.e1543–1549.e1547. doi:10.1016/j.ajem.2020.05.024
19. Hosseini N, Nadjafi S, Ashtary B. Overview of COVID-19 and neurological complications. Rev Neurosci. 2021;32(6):671–691. doi:10.1515/revneuro-2020-0116
20. Agarwal S, Jain R, Dogra S, et al. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. 2020;51(9):2649–2655. doi:10.1161/STROKEAHA.120.030940
21. Chen Y, Wang X, Guan L, Wang Y. Role of white matter hyperintensities and related risk factors in vascular cognitive impairment: a review. Biomolecules. 2021;11(8):1102. doi:10.3390/biom11081102
22. Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain. 2019;142(8):2483–2491. doi:10.1093/brain/awz162
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
