Back to Journals » International Journal of Women's Health » Volume 15
Can HPV Test on Random Urine Replace Self-HPV Test on Vaginal Self-Samples or Clinician-Collected Cervical Samples?
Authors Shih YH, Sun L, Hsu ST, Chen MJ, Lu CH
Received 1 May 2023
Accepted for publication 30 August 2023
Published 11 September 2023 Volume 2023:15 Pages 1421—1429
DOI https://doi.org/10.2147/IJWH.S416520
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Yu-Hsiang Shih, Lou Sun, Shih-Tien Hsu, Ming-Jer Chen, Chien-Hsing Lu
Department of Obstetrics and Gynecology, Taichung Veterans General Hospital, Taichung, Taiwan
Correspondence: Chien-Hsing Lu, Department of Obstetrics and Gynecology, Taichung Veterans General Hospital, 1650 Taiwan Boulevard Sect. 4, Taichung, Taiwan, Tel +886-4-23592525, Email [email protected]
Objective: This study investigated whether random urine (RU) samples can be used to accurately identify human papillomavirus (HPV) and whether these samples can replace self-collected vaginal samples in HPV tests.
Methods: A total of 167 patients with abnormal Pap smears were recruited. The patients provided self-collected vaginal and RU samples for HPV testing. Clinicians obtained cervical samples from the patients. Colposcopy examination and cervical biopsy were performed. Hybrid Capture II (HC II) and Cervista tests were used to detect HPV in the RU samples.
Results: The results of tests on clinician-collected cervical samples were used as the benchmark. The sensitivities of the Cervista tests on vaginal samples and the HC II and Cervista tests on RU samples were 75.00%, 49.07%, and 44.44%, respectively. After we adjusted the HPV detection cutoff value for urine samples based on values in the receiver operating characteristic curve, the sensitivities of the HC II and Cervista tests increased to 63.89% and 58.33%, respectively. In 167 patients, 59 had cervix biopsies showing CIN2 or worse (CIN2+). For CIN2+, the sensitivity was 47.5% and 50.8% in the HC II and Cervista tests on RU samples, respectively.
Conclusion: HPV tests on RU samples had approximately 60% sensitivity to HPV tests on clinician-collected cervical samples after the cutoff values were adjusted. For CIN2+, the sensitivity was only approximately 50%. Further studies and improvements in urine-based HPV testing are needed to establish it as a more convenient and accessible method for detecting HPV and cervical dysplasia in patients.
Keywords: HPV, random urine, vaginal HPV test, cervical intraepithelial lesion, Hybrid Capture II, Cervista
Introduction
Multiple studies have consistently demonstrated that human papillomavirus (HPV) testing, either alone or in conjunction with cervical cytology, offers higher sensitivity in detecting high- or low-grade cervical histopathology compared to cervical cytology alone.1 Furthermore, a review article suggests that HPV testing on self-collected vaginal samples presents a promising approach to reaching women who are not actively participating in regular screening programs.2 Despite the potential benefits of self-collected vaginal HPV testing, the uptake remains significantly low. For instance, in Taiwan, the Ministry of Health and Welfare provides self-collected vaginal HPV test kits to women who have not undergone a Pap smear in the past 6 years; however, only a mere 1.6% of women utilized these test kits.3 The underlying reason for this limited adoption could be attributed to various psychosocial factors that warrant further exploration.
An HPV test using a self-collected urine sample, which is non-invasive, would likely be more popular4 and lead to better population coverage in screening programs for individuals who are reluctant to receive a cervical Pap smear or obtain their own vaginal samples. Previous research has consistently demonstrated a significant level of agreement, ranging from 75% to 100%, between paired cervical and urine samples when testing for any type of HPV.5,6 The sensitivity of using urine HPV testing to detect CIN2 or higher lesions varies widely in previous studies, with reported rates ranging from 63% to 89.9%.7–11 To enhance the accuracy of HPV testing, most studies have opted to utilize first-void urine (FVU) samples, considering them to yield the most reliable results.12,13 In certain cases, researchers have even mandated the collection of urine samples as first-void specimens of the day to ensure the highest possible precision.14,15 Additionally, compliance with FVU sampling protocols tends to be lower compared to random urine (RU) sampling, possibly indicating that individuals may be more inclined to provide random urine samples due to the less stringent collection requirements.
Hence, this study aimed to examine the identification of HPV through the application of Hybrid Capture II (HC II) (QIAGEN, Gaithersburg, MD, USA) and Cervista (Hologic, Inc., Bedford, MA) tests on random uterine (RU) samples, extracted from individuals with abnormal Pap smear results. Furthermore, it drew comparisons between these findings and the outcomes of Cervista tests conducted on self-collected vaginal samples, as well as tests conducted on cervical samples collected by clinicians.16,17
Materials and Methods
From May 2014 to December 2015, 252 patients attending a colposcopic clinic due to abnormal Pap smears were invited to participate in this study. The diagnostic criteria for abnormal Pap smears included atypical squamous cells, atypical glandular cells, low-grade squamous intraepithelial lesions, high-grade squamous intraepithelial lesions, adenocarcinoma in situ, adenocarcinoma, or squamous cell carcinoma. Patients who were pregnant, younger than 20 years old, lacking a formal cytology report, or diagnosed with cervical sarcoma or endometrial cancer with cervical involvement were excluded. Informed consent was obtained from the patients after explaining the study, and enrollment and sample collection were conducted. This study was approved by the Taichung Veterans General Hospital institutional review board before enrolling any patient, with approval number 1047904. Out of the 252 patients, 34 were excluded due to meeting the exclusion criteria, and an additional 51 patients declined to participate in the trial. Therefore, a total of 167 patients eventually joined the study. A flowchart describing the selection of the study population is shown in Figure 1.
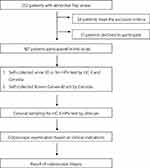 |
Figure 1 A flowchart describing the selection of the study population. |
Specimen Collection
Each patient provided two urine samples, one for the HC II test and the other for the Cervista test, after collecting approximately 30 mL of random urine (RU). Additionally, a vaginal sample was obtained using the Rovers Cervex-Brush by Cervista. During the pelvic examination, the clinician collected a cervical sample using the Digene cervical brush, preserved in medium for the HC II test. Subsequently, patients underwent colposcopy, a visual inspection of the cervix and vaginal walls using a colposcope, to identify abnormalities. Diagnosis involved identifying specific tissue characteristics and performing biopsies to confirm findings.18 The cervical disease status was determined based on histological analysis of the tissue samples.19
Laboratory Analyses
Method of HPV DNA Tests by HC II
HC II is a commercial HPV detection test designed to detect 18 types of HPV. The viral load of HPV DNA was estimated by the ratio of relative light units to positive control values in the samples. A ratio of 1.0 or greater was regarded as positive for HPV DNA.17
Method of HPV DNA Tests by Cervista
The Cervista test is a diagnostic test for the qualitative detection of DNA from 14 high-risk types of HPV. It uses a signal amplification method for detection of specific nucleic acid sequences. A signal to noise value is generated for each of the 3 mixes and is referred to as HPV fold-over-zero (FOZ). If the HPV FOZ ratio is equal to or greater than 1.525, the sample is considered positive for HPV.16
Statistical Analyses
With the cervical HPV test cutoff used as the benchmark, the performance of the tests applied to the vaginal samples and RU samples were evaluated by calculating the sensitivity and specificity for the presence of HPV as determined by the tests on the cervical samples. The study also assessed the sensitivity and specificity of tests on both vaginal and RU samples for detecting cervical intraepithelial neoplasia 2 or worse (CIN2+), a diagnosis dependent on identifying moderate abnormal cell changes in cervical tissue via cervix biopsy.19 The receiver operating characteristic (ROC) curve is a curve representing the performance of a classification model at all classification thresholds. In this study, the ROC curve was utilized to determine the optimal diagnostic cutoff point for the HC II and Cervista tests when applied to RU samples. Following this, the sensitivity and specificity of the tests applied to the RU samples for detecting CIN2+ were recalculated based on the identified optimal cutoff value. To assess the significance of the relative sensitivity and specificity, McNemar’s test was employed with a significance threshold set at 0.05. Additionally, the concordance between RU, vaginal, and cervical samples was measured using Cohen’s Kappa. Statistical analyses were performed using SPSS 13.0 software (SPSS, Chicago, IL).
Results
Ultimately, the study encompassed a total of 167 patients, all exhibiting abnormal Pap smear results. Among them, 108 (64.6%) patients tested positive for HPV using the HC II test on cervical samples, which served as the benchmark. The Cervista tests on vaginal samples showed a sensitivity and specificity of 75.0% and 74.5%, respectively. On the other hand, the HC II tests on RU samples had a sensitivity and specificity of 49.0% and 71.1%, respectively, while the Cervista tests on RU samples had a sensitivity and specificity of 44.4% and 81.3%, respectively (Table 1).
 |
Table 1 Absolute Sensitivity and Specificity for Detecting HPV Using Clinician-Collected Cervical Samples Using Original Cervical HPV Cutoff. Number of Studies: 167 |
In 167 patients, 59 had cervix biopsies showing CIN2 or worse (CIN2+). For detecting biopsy-proven CIN2+, the Cervista tests on vaginal samples had a sensitivity of 67.8% and specificity of 48.1%. However, both the HC II and Cervista tests on RU samples showed low sensitivity, approximately 39%, with specificities of 56.5% and 66.7%, respectively (Table 2).
 |
Table 2 Absolute Sensitivity and Specificity for Detecting CIN2+ Using Original Cervical HPV Cutoff. Number of Studies: 167 |
Due to the unsatisfactory sensitivity of the RU samples, adjustments were made to increase sensitivity. Since the HPV viral load in urine is lower than in the cervix due to fewer exfoliated epithelial cells from voiding, the HPV-positive threshold was reduced to improve sensitivity.
After analyzing the data using statistical software, the ROC curve determined an optimal cutoff of 0.61 for the HC II test on RU samples, resulting in a sensitivity of 63.8% and specificity of 59.3% for detecting HPV infection status. For the Cervista test on RU samples, the adjusted cutoff of 1.23 yielded a sensitivity of 58.3% and specificity of 71.1% (Table 3).
 |
Table 3 Sensitivity and Specificity for Detecting HPV in Clinician-Collected Cervical Samples Using Adjusted (Optimal) HPV Cutoff. Number of Studies: 167 |
Using the adjusted cutoff values, the sensitivity and specificity for detecting CIN2+ in the HC II test on RU samples were 47.5% and 39.8%, respectively, while for the Cervista test on RU samples, the sensitivity and specificity were 50.8% and 53.7%, respectively (Table 4).
 |
Table 4 Absolute Sensitivity and Specificity for Detecting CIN2+ in Random Urine Samples Using Adjusted Cutoff. Number of Studies: 167 |
Comparing the tests on vaginal and RU samples to the benchmark of cervical samples, the relative sensitivity and specificity for CIN2+ were 0.75 and 0.74, respectively, for tests on vaginal samples, with a P value of 0.09, indicating no significant difference. Similarly, there was no significant difference between HC II tests on RU and cervical samples, with a relative sensitivity of 0.63 and relative specificity of 0.59. However, relative to tests on cervical samples, Cervista tests on RU samples had a relative sensitivity of 0.58 with a significant P value of 0.001, indicating a noticeable difference (Table 5).
 |
Table 5 Relative Sensitivity and Specificity for Self-Collected Sample versus Clinician-Collected Sample. Number of Studies: 167 |
The observed concordance between HC II tests on RU and cervical samples, as well as Cervista tests on RU samples, was low at only 38%, with a Kappa value of approximately 0.22 (Table 6).
 |
Table 6 Concordance Between Random Urine Samples and Clinician-Collected Cervical Samples. Number of Studies: 167 |
Discussion
Exploring HPV testing in urine serves as an alternative approach due to the limitations of conventional microscopy-based techniques reliant on identifying morphological markers within cervical cells. Self-collected cervicovaginal samples often lack sufficient cell content and are mixed with vaginal cells, leading to suboptimal morphological quality and reduced sensitivity. These same issues apply to urine samples. Therefore, when utilizing vagina or urine as the sample for HPV testing, the focus shifts from examining cells to detecting the presence of HPV itself.20
In the present study, the sensitivity of the test on self-collected vaginal samples was found to be 75% when the test on clinician-collected cervical samples was used as the benchmark. Additionally, the sensitivity for detecting CIN2+ was 67.8%. For the tests on RU samples, the sensitivity was improved to approximately 60% after adjusting the cutoff values. However, despite the increase in sensitivity, the test results did not show a strong correlation with the pathological findings.
A previous meta-analysis reported that the sensitivity of tests on vaginal samples ranged between 51% and 93% for CIN2+ in primary screenings, and their pooled sensitivity estimates were 76% for CIN2+.21 Another meta-analysis verified the sensitivity of high-risk HPV (hrHPV) assays conducted on vaginal samples for CIN2+; this was based on findings on signal amplification of between 69% and 82% with a pooled absolute sensitivity of hrHPV assays for CIN2+ of 77%,22 which is comparable to our findings.
The rationale behind using urine samples for HPV detection stems from the potential to capture HPV-containing mucus and cellular debris shed from exfoliated cells in the female genital organs, which can be found in the urinary flow. Consequently, the first void urine (FVU) is preferred as it contains a higher concentration of HPV DNA compared to random or midstream urine, due to its ability to collect most of the cellular debris from the urogenital tract. Hence, in most studies, the first void urine is used as the specimen for HPV testing.23,24 In a systematic review and meta-analysis, the individual sensitivities and specificities for detecting any HPV in first void urine ranged from 54% to 99% and 67% to 99%, respectively. Similarly, for the detection of high-risk HPV in first void urine, the individual sensitivities ranged from 51% to 92%, and the specificities varied from 59% to 98%.13 Another study demonstrated a correlation in the incidence of high-risk HPV DNA between FVU and cervical smear samples, ranging from 79% to 80%.25 Furthermore, among patients with a positive urine test for high-risk HPV, 94.3% showed pathologic findings of CIN2/3.26 This indicates that first void urine samples are as reliable as vaginal and cervical samples for HPV detection.23 Sometimes, FVU is defined as the first void urine of the day rather than the initial stream of urine.24 The concordance between HPV detection tests using cervical and first urine of the day samples ranged from 81.2% to 86.1%, which is similar to that obtained using first void urine samples collected later in the day.14 A study by Pattyn et al showed no difference in HPV DNA concentration between FVU collected in the morning and FVU collected later in the day.15
These findings collectively indicate that tests using first void urine (FVU) samples have high sensitivity. However, incorporating FVU sampling into population screening is not practical due to a considerable decrease in compliance once patients leave the clinic. FVU sampling is less convenient, especially for older adults and dependent individuals, as compared to random urine (RU) sampling. Moreover, verifying that a urine sample is truly FVU is challenging, and often midstream or RU samples are collected instead, despite FVU being requested. Unfortunately, data from midstream or RU samples may not provide conclusive results. Senkomago demonstrated that the detection of HPV was similar among tests on first-void, initial, and midstream urine samples.11 However, a meta-analysis reported that tests on FVU samples were significantly more sensitive than those on midstream or RU samples, with a sensitivity of 89.0% (confidence interval (CI): 75.3% to 95.5%) for FVU samples versus 73.9% (CI: 68.3% to 78.8%) for non-FVU samples.27 In the present study, the sensitivity of HPV tests on RU samples was relatively low (49.07% by HC II and 44.44% by Cervista) compared to tests on FVU samples reported in the literature and compared to tests on vaginal samples.
In a previous study, various methods were investigated to enhance the detection rate of HPV DNA in urine. These methods included the addition of a chelating agent to the sample, processing an adequate volume of urine, and ensuring proper storage and extraction to prevent DNA degradation.28 In our study, we aimed to identify additional approaches to increase the sensitivity of HPV tests on urine samples, specifically by adjusting the threshold for HPV positivity. Given that the concentration of HPV in urine is generally lower than in cervical samples, we utilized an ROC curve to determine the optimal cutoff value for HPV-positive urine. While focusing on higher sensitivity for HPV detection in RU samples, we aimed to maintain an acceptable level of specificity without compromising it. The statistical software was utilized to find the optimal cutoff value. Following the adjustment, the sensitivity of the tests using urine samples increased to 60%, with specificities of 59.3% for the HC II test and 71.1% for the Cervista test on RU samples. However, these values were still lower than those obtained from the test on vaginal samples. Furthermore, the concordance between the tests conducted on cervical and RU samples was approximately 37%–38%, which was a disappointing result.
The correlation between HPV test results on urine samples and the presence of cervical intraepithelial lesions remains uncertain. Some studies have reported promising results, while others have shown varying sensitivity and specificity rates. In a cross-sectional study, HPV-positive rates in FVU samples were found to be more than 84% for histology-confirmed CIN1+ and more than 90% for histology-confirmed CIN2+.The sensitivity for detecting CIN2+ in urine samples was reported to be 93%.7 In another cross-sectional study, the sensitivity for detecting CIN2+ using different HPV tests on urine samples was reported to be 83% and 88%, respectively.29 However, not all studies have shown such high sensitivity in urine-based HPV detection for CIN. For example, in a PaVDaG study, the sensitivity for detecting CIN2+ was only 63.1% with HPV detection on urine samples.10 Cho et al also reported lower sensitivities of 66.41% and 73.28% using different HPV commercial kits on FVU samples, with specificities of 32.14% and 46.43%, respectively.30 In our current study, we made adjustments to the cutoff values and observed that the sensitivities of the HC II and Cervista tests on RU samples for detecting CIN2+ were 47.5% and 50.8%, respectively. These results were found to be lower than the sensitivity of 67.8% obtained when using tests on vaginal samples.
The experimental results were deemed unsatisfactory for several reasons. First, for the sake of simplicity, we did not include a chelating agent in the urine samples, and we utilized only 15 mL of urine in each test. This might have contributed to a decreased sensitivity of the tests as demonstrated in previous research.28 Second, not all tests were conducted on all specimens, making interspecimen comparisons challenging, particularly when the tests used were different. If these shortcomings can be improved, it is possible that the experimental results could be enhanced, allowing the use of random urine samples to detect HPV infection and cervical dysplasia in patients. Furthermore, recent studies have shown that patients who have undergone conization due to cervical dysplasia and are HPV positive or persistence have an increased risk of recurrence.31,32 Therefore, the development of a method to detect HPV and cervical dysplasia through urine testing could make patient follow-up more convenient and immediate. This is especially beneficial for monitoring those at higher risk of recurrence, as urine testing offers a non-invasive and easily accessible approach for regular check-ups.
Conclusion
After adjusting the cutoff values, the HPV test on random urine (RU) samples demonstrated an approximate sensitivity of 60% relative to the test on cervical samples. However, for detecting CIN2+, the sensitivity was only around 50%. The experimental results were considered unsatisfactory due to limitations. Further studies and improvements in urine-based HPV testing are needed to establish it as a more convenient and accessible method for detecting HPV and cervical dysplasia in patients.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Taichung Veterans General Hospital institutional review board. The approval number is IRB-1047904. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgements
This research was supported by the Taichung Veterans General Hospital- National Chi Nan University grant 1087903. We thank Tsai-Feng Fu for kind suggestions on the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Banerjee D, Mittal S, Mandal R, Basu P. Screening technologies for cervical cancer: overview. Cytojournal. 2022;19:23. doi:10.25259/CMAS_03_04_2021
2. Tatara T, Wnuk K, Miazga W, et al. The influence of vaginal HPV self-sampling on the efficacy of populational screening for cervical cancer-an umbrella review. Cancers. 2022;14(23):5913. doi:10.3390/cancers14235913
3. Chou HH, Huang HJ, Cheng HH, et al. Self-sampling HPV test in women not undergoing Pap smear for more than 5 years and factors associated with under-screening in Taiwan. J Formos Med Assoc. 2016;115(12):1089–1096. doi:10.1016/j.jfma.2015.10.014
4. Daponte A, Michail G, Daponte AI, Daponte N, Valasoulis G. Urine HPV in the context of genital and cervical cancer screening-an update of current literature. Cancers. 2021;13(7):1640. doi:10.3390/cancers13071640
5. Vorsters A, Micalessi I, Bilcke J, Ieven M, Bogers J, Van Damme P. Detection of human papillomavirus DNA in urine. A review of the literature. Eur J Clin Microbiol Infect Dis. 2012;31(5):627–640. doi:10.1007/s10096-011-1358-z
6. Enerly E, Olofsson N, Nygård O. Monitoring human papillomavirus prevalence in urine samples: a review. Clin Epidemiol. 2013;5:67–79. doi:10.2147/CLEP.S39799
7. Ørnskov D, Jochumsen K, Steiner PH, Grunnet IM, Lykkebo AW, Waldstrøm M. Clinical performance and acceptability of self-collected vaginal and urine samples compared with clinician-taken cervical samples for HPV testing among women referred for colposcopy. A cross-sectional study. BMJ Open. 2021;11(3):e041512. doi:10.1136/bmjopen-2020-041512
8. Cadman L, Reuter C, Jitlal M, et al. A randomized comparison of different vaginal self-sampling devices and urine for human papillomavirus testing-predictors 5.1. Cancer Epidemiol Biomarkers Prev. 2021;30(4):661–668. doi:10.1158/1055-9965.EPI-20-1226
9. Cuzick J, Cadman L, Ahmad AS, et al. Performance and diagnostic accuracy of a urine-based human papillomavirus assay in a referral population. Cancer Epidemiol Biomarkers Prev. 2017;26(7):1053. doi:10.1158/1055-9965.EPI-16-0960
10. Stanczuk G, Baxter G, Currie H, et al. Clinical validation of hrHPV testing on vaginal and urine self-samples in primary cervical screening (cross-sectional results from the Papillomavirus Dumfries and Galloway—PaVDaG study). BMJ Open. 2016;6(4):e010660. doi:10.1136/bmjopen-2015-010660
11. Senkomago V, Des Marais AC, Rahangdale L, Vibat CRT, Erlander MG, Smith JS. Comparison of urine specimen collection times and testing fractions for the detection of high-risk human papillomavirus and high-grade cervical precancer. J Clin Virol. 2016;74:26–31. doi:10.1016/j.jcv.2015.11.005
12. Burroni E, Bonanni P, Sani C, et al. Human papillomavirus prevalence in paired urine and cervical samples in women invited for cervical cancer screening. J Med Virol. 2015;87(3):508–515. doi:10.1002/jmv.24085
13. Bober P, Firment P, Sabo J. Diagnostic test accuracy of first-void urine human papillomaviruses for presence cervical HPV in women: systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:24.
14. Cho HW, Ouh YT, Hong JH, et al. Comparison of urine, self-collected vaginal swab, and cervical swab samples for detecting human papillomavirus (HPV) with Roche Cobas HPV, Anyplex II HPV, and RealTime HR-S HPV assay. J Virol Methods. 2019;269:77–82.
15. Pattyn J, Van Keer S, Biesmans S, et al. Human papillomavirus detection in urine: effect of a first-void urine collection device and timing of collection. J Virol Methods. 2019;264:23–30. doi:10.1016/j.jviromet.2018.11.008
16. Hologic. Cervista™ HPV HR. Secondary Cervista™ HPV HR; 2021. Available from: https://www.hologic.com/package-inserts/diagnostic-products/cervista-hpv-hr-assay.
17. Clavel C, Masure M, Putaud I, et al. Hybrid capture II, a new sensitive test for human papillomavirus detection. Comparison with hybrid capture I and PCR results in cervical lesions. J Clin Pathol. 1998;51(10):737–740.
18. Burness JV, Schroeder JM, Warren JB. Cervical Colposcopy: indications and Risk Assessment. Am Fam Physician. 2020;102(1):39–48.
19. Apgar BS, Kittendorf AL, Bettcher CM, Wong J, Kaufman AJ. Update on ASCCP consensus guidelines for abnormal cervical screening tests and cervical histology. Am Fam Physician. 2009;80(2):147–155.
20. Van Keer S, Pattyn J, Tjalma WAA, et al. First-void urine: a potential biomarker source for triage of high-risk human papillomavirus infected women. Eur J Obstet Gynecol Reprod Biol. 2017;216:1–11. doi:10.1016/j.ejogrb.2017.06.036
21. Arbyn M, Verdoodt F, Snijders PJ, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. doi:10.1016/S1470-2045(13)70570-9
22. Arbyn M, Smith SB, Temin S, Sultana F, Castle P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823. doi:10.1136/bmj.k4823
23. Pattyn J, Van Keer S, Téblick L, Van Damme P, Vorsters A. HPV DNA detection in urine samples of women: ‘an efficacious and accurate alternative to cervical samples?’. Expert Rev Anti Infect Ther. 2019;17(10):755–757. doi:10.1080/14787210.2019.1668776
24. Vorsters A, Van Damme P, Clifford G. Urine testing for HPV: rationale for using first void. BMJ. 2014;349:g6252. doi:10.1136/bmj.g6252
25. Bernal S, Palomares JC, Artura A, et al. Comparison of urine and cervical samples for detecting human papillomavirus (HPV) with the Cobas 4800 HPV test. J Clin Virol. 2014;61(4):548–552. doi:10.1016/j.jcv.2014.10.001
26. Maged AM, Saad H, Salah E, et al. Urine test for HPV genotypes as a predictor of precancerous cervical lesions and for cervical cancer screening. Int J Gynaecol Obstet. 2018;141(3):332–336. doi:10.1002/ijgo.12453
27. Pathak N, Dodds J, Zamora J, Khan K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. Br Med J. 2014;349:g5264. doi:10.1136/bmj.g5264
28. Vorsters A, Van den Bergh J, Micalessi I, et al. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur J Clin Microbiol. 2014;33(11):2005–2014. doi:10.1007/s10096-014-2147-2
29. Sargent A, Fletcher S, Bray K, Kitchener HC, Crosbie EJ. Cross-sectional study of HPV testing in self-sampled urine and comparison with matched vaginal and cervical samples in women attending colposcopy for the management of abnormal cervical screening. BMJ Open. 2019;9(4):e025388. doi:10.1136/bmjopen-2018-025388
30. Cho HW, Hong JH, Min KJ, et al. Performance and diagnostic accuracy of human papillomavirus testing on self-collected urine and vaginal samples in a referral population. Cancer Res Treat. 2020;53:829–836. doi:10.4143/crt.2020.1165
31. Giannini A, Di Donato V, Sopracordevole F, et al. Outcomes of high-grade cervical dysplasia with positive margins and HPV persistence after cervical conization. Vaccines. 2023;11:3.
32. Bogani G, Sopracordevole F, Di Donato V, et al. High-risk HPV-positive and -negative high-grade cervical dysplasia: analysis of 5-year outcomes. Gynecol Oncol. 2021;161(1):173–178. doi:10.1016/j.ygyno.2021.01.020
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
