Back to Journals » Cancer Management and Research » Volume 10
Can bone scans guide therapy with radium-223 dichloride for prostate cancer bone metastases?
Authors Gayed I, Salama V, Dawood L , Canfield S, Wan D , Cai C , Joseph U, Amato R
Received 22 February 2018
Accepted for publication 2 May 2018
Published 7 September 2018 Volume 2018:10 Pages 3317—3324
DOI https://doi.org/10.2147/CMAR.S166218
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Isis Gayed,1 Vivian Salama,2 Lydia Dawood,1 Steven Canfield,3 David Wan,1 Chunyan Cai,4 Usha Joseph,1 Robert Amato5
1Nuclear Medicine Section, Department of Diagnostic and Interventional Imaging, University of Texas Health Science Center at Houston, 2Graduate School of Biomedical Sciences, University of Texas MD Anderson Cancer Center, 3Division of Urology, Department of Surgery, 4Division of Clinical and Translational Science, Department of Internal Medicine, 5Division of Oncology, Department of Internal Medicine, University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA
Background: Radium-223 dichloride (Ra-223 Xofigo) has recently been approved as an addition to the host of available therapies in the USA as a treatment option for metastatic castrate-resistant prostate cancer (mCRPC) with bone metastases. This study describes our initial experience in patients treated with Ra-223 dichloride. It attempts to optimize patients’ selection for the best outcome from Ra-223 dichloride therapy.
Methods: Consecutive patients who were referred for treatment with Ra-223 dichloride were prospectively followed. Patients’ demographics, functional status per the Eastern Cooperative Oncology Group (ECOG) performance score, pain level per the numeric rating score (NRS), prostate-specific antigen (PSA), creatinine, and hematological values were compared at baseline and at the end of therapy. Patients also had a bone scan before starting therapy and at the end of therapy. Patients were divided into the favorable response (FR) group if their pain and/or functional status improved and the unfavorable response (UR) group if they did not improve, deteriorated, or deceased. Bone scan findings before and after Ra-223 dichloride therapy were compared in both the FR and UR groups.
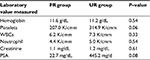
Results: Twenty patients were treated with Ra-223 dichloride. Twelve patients had innumerable bone metastases, three patients had super scans, and three patients had two to seven bone lesions. Two patients were lost to follow-up after the first injection. There were eight patients in the FR group and 10 patients in the UR group. Patients with UR had mean ECOG and NRS pain scores of 1.3 and 5.0 versus 0.8 and 4.4 in the FR group. The mean PSA and creatinine levels in the UR group were 445.2 ng/mL and 1.2 mg/dL versus 22.7 ng/mL and 1.1 mg/dL in the FR group. The mean hemoglobin, platelets, and absolute neutrophil values were 11.2 g/dL, 314.9 K/cmm, and 7.3 K/cmm in the UR group versus 11.6 g/dL, 207.0 K/cmm, and 6.2 K/cmm in the FR group. Seven of the eight patients with FR had a bone scan at the end of therapy showing improvement in five patients, a mixed response in one patient, and progression in another patient. Five patients in the UR group completed five or six injections and had bone scans showing flare of bone metastases in three patients, progression in one patient, and improvement in the fifth patient. Three patients in the UR group died after the first or second injections. Two of these patients had baseline super scans and the third one had widespread bone metastases.
Conclusion: mCRPC patients with lower PSA levels at baseline and fewer bone lesions are more likely to respond favorably to Ra-223 dichloride therapy.
Keywords: Radium-223 dichloride, castrate-resistant bone metastases, prostate cancer, radioactive therapy
Introduction
Prostate cancer is the most commonly diagnosed noncutaneous cancer in men with a higher risk in Blacks than in non-Hispanic Whites. Globally, >1.1 million men are diagnosed with prostate cancer yearly.1,2 It is estimated that ~161,360 men in the USA will be diagnosed with prostate cancer in 2017 according to the new cancer statistics.3 Additionally, prostate cancer is reported as the second leading cause of cancer death in men in the USA, after lung cancer.
The primary treatment goal in prostate cancer patients who fail local therapy or who are not candidates for local therapy is androgen suppression.4 However, the disease eventually relapses and progresses, despite maximum androgen blockage, to metastatic castration-resistant prostate cancer (mCRPC) with a median time-to-progression of 18 months (range 16–24 months).5–7 Bone metastases occur in >90% of patients with mCRPC.2 These are mainly osteoblastic bone metastases, which significantly impact the patient’s quality of life (QoL) due to severe pain and limitation of mobility. These patients may also suffer from the risk of skeletal-related events (SREs), including spinal cord compression, pathologic bone fracture, the need for bone surgery or radiation, and hypercalcemia and pancytopenia, which all may negatively impact the physical activity of the patients. Moreover, it has been proven that the mortality rate is increased in patients with bone mCRPC with an overall survival that approaches 3 years.8
Recently, there has been increasing interest in using bone-targeted agents to reduce the rate of SREs and improving overall survival. Radium-223 dichloride (Ra-223 Xofigo) is one of the bone-targeted radioisotope agents that has recently been approved in the USA and is reported to have a high safety and efficacy for the treatment of bone metastases in mCRPC patients and prolonging survival at an average of 3.6 months with improved QoL.9,10
Ra-223 dichloride is a bone-seeking α-emitter agent that is, similar to calcium, absorbed into bone matrix at the site of high bone turnover caused by bone metastases. It is excreted from the body mainly through the gastrointestinal tract and, to a lesser extent, through the urine with a half-life of ~11.4 days.8 Being an α-emitter, Ra-223 is characterized by a short range track of <0.1 nm (compared with other β-emitters), and high localized energy radiation causing strong nonrepairable dsDNA breaks in the metastatic cancer cells and subsequently cell death, with minimal radiation and less damage to the surrounding tissues, including the bone marrow, thus causing minimal myelosuppression.10
Treatment with Ra-223 dichloride is well tolerated by the patients. Nevertheless, adverse effects have been reported during therapy, including nausea, diarrhea, fatigue, weight loss, thrombocytopenia, and neutropenia.9–12
After USA Food and Drug Administration approval of Ra-223 dichloride therapy for mCRPC patients, we thought to prospectively collect patients’ information to identify those who respond favorably to the treatment. We attempted to enroll all patients referred for Ra-223 dichloride injections in order to optimize future patient referral for such therapy for best clinical outcomes.
Methods
After obtaining the University of Texas Health Science Center at Houston’s Institutional Review Board (IRB) approval and signed written and informed consent forms, we prospectively included all patients referred to our facility for Ra-223 dichloride therapy. All patients had prostate cancer metastases to bones and had failed treatment with multiple chemotherapies and hormonal therapy. The study was ethically conducted and adhered to the study protocol.
All patients’ data were collected including demographics and blood laboratory results including hemoglobin (Hgb) levels, white blood cell (WBC) count, neutrophil count, platelets, creatinine, alkaline phosphatase (ALP), and total prostate-specific antigen (PSA) levels. All patients had a bone scan to prove the presence of bone metastases prior to the initiation of therapy except for one patient whose bone scan was performed after the second dose of therapy; he was initially treated based on the appearance of multiple metastases on CT and MRI. The number of bone lesions seen on bone scan and their locations was recorded up to 12 lesions. When bone metastases were innumerable due to their widespread nature in all the axial and most of the appendicular bones, the number was recorded as multiple bone metastases (m). Bone scans were repeated at the end of the treatment regardless of the number of injections received except in patients who died while receiving therapy. Diffuse bone metastases throughout the axial and appendicular skeleton were considered as a super scan. Bone lesions were compared in number and change in osteoblastic activity between the pretherapy and post-therapy scans. The change in osteoblastic activity was graded on a semiquantitative scale as mild, moderate, or marked increase in tracer uptake. If the number of lesions had decreased or remained the same with evidence of slight increase in size (flare) and/or if decreased in osteoblastic activity on the semiquantitative scale, the patient was considered improved. When there is no change in number, size or osteoblastic activity of the lesions, the patient was considered stable. If the number of lesions had increased >2 lesions and/or increased in osteoblastic activity per the semiquantitative scale in ≥2 lesions on the post-therapy scan, the patient was considered as having progressive disease.
Prior to every injection, the patients were also interviewed to evaluate their pain level, pain medications received, and their functional status. The patients’ pain level was graded using the numeric rating score (NRS) from 1 to 10. Their functional status was graded per the Eastern Cooperative Oncology Group (ECOG) classification from 0 to 5. This information was re-evaluated prior to each of the six injections. Patients whose blood results did not meet the criteria for re-administration of injections were managed by their referring physician with supportive measures or were delayed for a few weeks to allow the recovery of their blood results. We also collected information about adverse effects experienced after every injection and prior to re-administration of the subsequent dose. Patients whose pain level decreased as per the NRS score and/or their mobility and functionality improved per the ECOG classification were included into the favorable response (FR) group.
Statistical analysis
Descriptive statistics were summarized for demographic, laboratory variables, and clinical characteristics. Mean ± standard deviation (SD) was reported for continuous variables. Frequency (percentage) was reported for categorical variables. Laboratory values and NRS pain score were compared between patients in the FR and unfavorable response (UR) groups by two sample t-test or Wilcoxon rank sum test as appropriate. For the skewed data, the logarithm transformation was applied before the comparison. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA), and a P-value of <0.05 was considered significant.
Results
Twenty-one patients were referred for Ra-223 dichloride therapy in our institution; 20 patients were included. One patient refused to be included in our prospective data collection and follow-up. The average age and standard deviation of the patients was 72.3±7.4 years. The demographics of the patients in both the FR and UR groups are described in Table 1.
  | Table 1 Patients’ characteristics and history of prior therapies in both the FR and UR groups Abbreviations: FR, favorable response; SD, standard deviation; UR, unfavorable response. |
The median interval between baseline bone scan and the first dose of treatment was 27 days and between the last dose and follow-up bone scan was 24 days. Two patients discontinued Ra-223 dichloride injections after the fourth dose, two patients after the fifth dose, and one patient after the first dose due to significant decrease in Hgb levels.
Twelve patients had innumerable bone metastases, three patients had super scans, and five patients had two to seven bone lesions. Two patients were lost to follow-up after the first injection. There were eight patients in the FR group and 10 patients in the UR group. The findings of the bone scan in both the FR and UR groups are summarized in Figure 1. Patients with UR had the mean pretherapy ECOG and NRS pain scores of 1.3 and 5.0 versus 0.8 and 4.4 in the FR group. The mean baseline PSA level was 445.2 mg/dL in the UR group, which was higher than the FR group at 22.7 mg/dL. However, the difference did not reach a statistically significant level (P=0.08) likely due to the small size of this study. The mean hematological values and creatinine levels for the two groups were comparable with no statistical significant difference as outlined in Table 2.
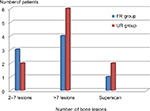  | Figure 1 Distribution of the number of bone lesions seen on baseline bone scan in the FR and UR groups. Abbreviations: FR, favorable response; UR, unfavorable response. |
Seven of the eight patients in the FR group had both baseline and follow-up scans at the end of therapy. Five of these patients demonstrated improvement in bone metastases (71.4%). One patient in the FR group experienced progression of bone metastases despite clinical improvement and decrease in pain level, and one patient had a mixed response on bone scan. The eighth patient refused to repeat post-therapy bone scan. Only five of the 10 patients in the UR group had follow-up repeat bone scans at the completion of therapy or discontinuation of therapy. Three patients died during the therapy, and two patients were extremely sick and were unable to undergo repeat imaging. Repeat bone scan in the UR group showed flare of bone lesions in three patients, progression of bone metastases in one patient, and improvement of bone metastases in one patient despite clinical deterioration and worsening of pain.
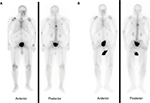
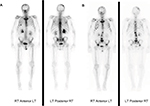
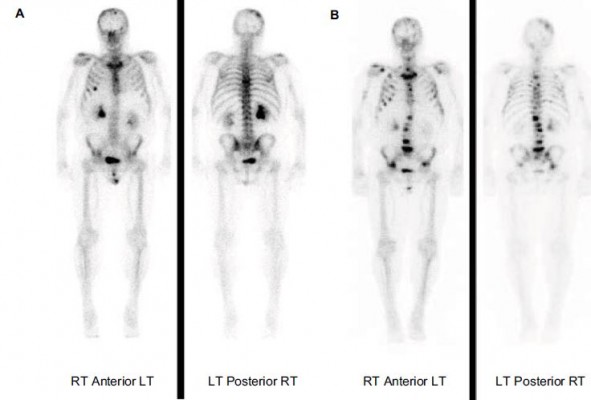
Thus, the findings on the post-therapy bone scan correlated with the pain level and functional status of the patients in five of the seven patients (71.4%) with FR and in one of the five patients with UR. In contrast, three of the five (60%) patients in the UR group showed evidence of flare, which is an indication of healing on the bone scan, but the patients’ pain and functional status had deteriorated (Figure 2). Additionally, one patient showed improvement on repeat bone scan while his pain increased. Thus, disparity between the patients’ clinical status and post-therapy bone scan findings was noted in a total of four of the five (80%) patients with repeat bone scans in the UR group. Examples of disparity between the clinical outcome and the bone scan finding in one patient in each of the FR (Figure 3) and UR (Figure 4) groups are demonstrated.
The three patients who died during the therapy had baseline bone scans demonstrating innumerable bone metastases in one patient and super scan in the remaining two patients. One died of pulmonary infection, and the other two patients died as a result of disease progression and bone marrow failure. A total of eight patients experienced further decrease in their Hgb levels during the therapy to anemic levels (four patients in each of the FR and UR groups), six patients had neutropenia (two in the FR group and four in the UR group), and three patients developed thrombocytopenia (three in the FR group and none in UR group). One patient complained of swelling of his legs, one patient had urosepsis, and another patient had small thrombosis of a vein at the site of injection, which subsequently resolved without intervention.
Discussion
Our study aimed to optimize the selection of patients for treatment with Ra-223 dichloride and evaluate the effects of the therapy on bone scan. Our study results indicated that PSA level before the initiation of the therapy can distinguish between patients who responded favorably and those who responded unfavorably to the treatment. The mean PSA level at baseline before starting Ra-223 dichloride injections was much lower in the FR group than in the UR group. Our results complement the results of further analysis of the percentage decline of laboratory values of total alkaline phosphatase (tAP), lactate dehydrogenase (LDH), and PSA levels after the Ra-223 dichloride as prognostic indicators of overall survival in ALSYMPCA trial patients.13 The authors found that declines in tAP and LDH at 12 weeks correlated with longer overall survival compared to placebo group.
We also found that patients with fewer bone lesions on baseline bone scan are more likely to have FR compared to patients with >7 lesions and less bone marrow reserve. Thus, FR is excepted with less tumor burden as seen on bone scan. Patients with innumerable lesions or super scans are more likely to exhibit a UR. Numerous bone metastases also tend to increase patients’ pain when they flare after Ra-223 dichloride injections. These findings favor early use of Ra-223 dichloride therapy in patients developing bone metastases. The ALSYMCA trial, which was used by the FDA to approve the use of Ra-223 dichloride therapy, included 85% of patients with >6 lesions and 40% of the patients had >20 lesions or a super scan. Thus, all patients were grouped together in the Ra-223 dichloride treatment arm with the impression that all patients with mCRPC responded well to Ra-223 dichloride therapy with overall 3.6 months prolongation of life.14 Our study adds further characterization and sub-selection insights into the group of patients who would benefit most with this new treatment with Ra-223 dichloride. Our results are supported by previous study from Etchebehere et al15 who demonstrated that tumor burden indices reflected in total volume of fluoride avid bone metastases and total fluoride metastases uptake correlated well and are significant independent predictors of overall survival.
Both Bräuer et al16 and Ahmadzadehfar et al17 demonstrated the superior role of Gallium 68 prostate-specific membrane antigen (Ga 68 PSMA) positron electron tomography (PET) in the evaluation of patient suitability for Ra-223 therapy over bone scan. Many of the patients in their studies demonstrated more bone metastases with Ga 68 PSMA PET than with bone scans. Additionally, Ga 68 PSMA PET shows disease in lymph nodes and soft tissue in addition to bone metastases, which helps better triage the patient for the appropriate therapy. Yet, till Ga-68 PSMA receives FDA approval for routine clinical use in the USA, bone scans and F-18 sodium fluoride PET scans will remain the imaging modalities to evaluate patient suitability for Ra-223 therapy.
Sequencing of treatments with chemotherapy is an area of much debate and many clinical trials.2 There is emerging evidence of the benefit of docetaxel in hormone-sensitive prostate cancer patients.18–21 Thus, similar evaluation of Ra-223 dichloride therapy in hormone-sensitive cancer patients should be considered. Also, Sarotor et al14 found that the likelihood of having to undergo external beam radiation therapy for pain and spinal cord compression was reduced in 614 patients who received Ra-223 therapy versus 307 patients who received placebo. Oncologists also need to change prior perception of using radio-isotopes (Strontium 89, Renium-186 hydroxyethylidene diphosphonate, and Samarium 137) as palliative pain control agents at a late stage of the disease, with limited therapeutic effect, to the new role of Ra-223 dichloride as a front-line therapeutic agent for bone metastases in prostate cancer patients.22–24 Our results suggest that earlier treatment with Ra-223 dichloride in mCRPC patients with less tumor burden, as reflected in lower PSA levels and less bone metastases, have FR and improved outcomes. These patients are also more likely to complete all six injections of the therapy without significant side effects.
The three patients who died during the therapy had widespread bone metastases, and more patients in the UR group (four patients) had neutropenia than in the FR group (two patients). These findings indicate the importance of starting the therapy with higher bone marrow reserve to achieve the most benefit from the total of six injections of Ra-223 dichloride. Patients with limited bone marrow reserve tend to do poorly with such treatments.22,25,26 Additionally, Du et al27 do not recommend therapy with Ra-223 dichloride in patients with <3 months life expectancy, that is, patients with widespread bone metastases and on the verge of bone marrow failure.
The limitation of our study is the small number of patients included. However, these therapies are costly, and it is difficult to obtain insurance coverage in many patients. Yet, we have gained good insight and experience from these patients for better future selection and referral to this therapy and with better outcome to the patients. Our study provides preliminary results for future larger studies to further optimize patient selection for treatment with Ra-223 dichloride. We also share in this study our experience of the three patients who died from infections and bone marrow failure shortly after Ra-223 dichloride therapy.
Conclusion
Ra-223 dichloride therapy is more beneficial in patients with less bone metastatic tumor burden, as reflected by lower PSA levels and lower number of bone lesions on pretherapy evaluations. Treatment of mCRPC patients should be considered early on, in the course of the disease, for better patient outcomes. Bone scan findings on the post-therapy scan may not correlate with the patients’ clinical outcome in many instances.
Acknowledgments
We would like to thank Ms Karen Swaby, Ms Grace Rizk and Ms Monica Atta for their support of this research protocol. An abstract for a poster presentation was submitted and accepted for the Society of Nuclear Medicine Meeting, May 2016.
Disclosure
The authors report no conflicts of interest in this work.
References
Florimonte L, Dellavedova L, Maffioli LS. Radium-223 dichloride in clinical practice: a review. Eur J Nucl Med Mol Imaging. 2016;43(10):1896–1909. | ||
Wilson JM, Parker C. The safety and efficacy of radium-223 dichloride for the treatment of advanced prostate cancer. Expert Rev Anticancer Ther. 2016;16(9):911–918. | ||
The American Cancer Society [webpage on the Internet]. The American Cancer Society: Key Statistics for Prostate Cancer Facts; [Updated January 5, 2017]. Available from: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed August 18, 2017. | ||
El-Amm J, Aragon-Ching JB. Radium-223 for the treatment of castration-resistant prostate cancer. Onco Targets Ther. 2015;18:1103–1109. | ||
Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85. | ||
Tilki D, Schaeffer EM, Evans CP. Understanding mechanisms of resistance in metastatic castration-resistant prostate cancer: the role of the androgen receptor. Eur Urol Focus. 2016;2(5):499–505. | ||
Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer. 2008;16(8):879–889. | ||
Mottet N, Bellmunt J, Bolla M, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Actas Urol Esp. 2011;35:565–579. | ||
Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;350(16):1655–1664. | ||
Saad F, Charles J, Gillessen S, et al; Radium-223 International Early Access Program Investigators. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016;17(9):1306–1316. | ||
Anido Herranz U, Fernández Calvo O, Afonso Afonso FJ, et al. Radium-223 dichloride: a new paradigm in the treatment of prostate cancer. Expert Rev Anticancer Ther. 2015;15:339–348. | ||
Mulford D, Scheinberg D, Jurcic J. The promise of targeted alpha-particle therapy. J Nucl Med. 2005;46(suppl 1):199S–204S. | ||
Nilsson S, Franzen L, Parker C, et al. Two-year survival follow-up of the randomized double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013;11(1):20–26. | ||
Sarotor O, Coleman R, Nilsson S, et al. Effect of radium 223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomized trial. Lancet Oncol. 2014;15(7):738–746. | ||
Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223 Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56(8):1177–1184. | ||
Bräuer A, Rahbar K, Konnert J, Bögemann M, Stegger L. Diagnostic value of additional 68Ga-PSMA-PET before 223 Ra-dichloride therapy in patients with metastatic prostate carcinoma. Nuklearmedizin. 2017;56(1):14–22. | ||
Ahmadzadehfar H, Azgomi K, Hauser S, et al. Ga-PSMA-11 PET as a gatekeeper for the treatment of metastatic prostate cancer with 223Ra: proof of concept. J Nucl Med. 2017;58(3):438–444. | ||
Sartor O, Coleman R, Nillson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol. 2017;28(5):1090–1097. | ||
Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. | ||
Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815–825. | ||
Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol. 2015;194(6):1537–1547. | ||
Liepe K, Shinto A. From palliative therapy to prolongation of survival: (223) RaCl2 in the treatment of bone metastases. Ther Adv Med Oncol. 2016;8(4):294–304. | ||
James ND, Bloomfield D, Luscombe C. The changing pattern of management for hormone-refractory, metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2006;9(3):221–229. | ||
Piffanelli A, Dafermou A, Giganti M, et al; Italian Association of Nuclear Medicine (AINM). Radionuclide therapy for painful bone metastases. An Italian multicentre observational study. Writing Committee of an Ad Hoc Study Group. Q J Nucl Med. 2001;45(1):100–107. | ||
Sartor O. Isotope therapy for castrate-resistant prostate cancer: unique sequencing and combinations. Cancer J. 2016;22(5):342–346. | ||
Song YP, Ellis T, Walshaw R, et al. Comparing clinical outcomes for radium-223: do older patients do worse? Int J Radiat Oncol Biol Phys. 2017;98(4):955–957. | ||
Du Y, De Vincentis G, Fanti S, et al. Practical recommendations for radium-223 treatment of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44(10):1671–1678. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.