Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Can Blood Biomarkers Be Used to Assess Oxidative Stress in COPD Patients After Pulmonary Rehabilitation
Authors Beykumül A , Ersoy Y, Gülbaş G, Neselioglu S
Received 14 December 2022
Accepted for publication 21 August 2023
Published 5 October 2023 Volume 2023:18 Pages 2179—2186
DOI https://doi.org/10.2147/COPD.S400415
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Ayşegül Beykumül,1 Yuksel Ersoy,2 Gazi Gülbaş,3 Salim Neselioglu4
1Pulmonary Rehabilitation Unit, Turgut Ozal Medical Center, Inonu University, Malatya, Turkey; 2Department of Physical Medicine and Rehabilitation, Faculty of Medicine, Inonu University, Malatya, Turkey; 3Department of Chest Diseases, Faculty of Medicine, Inonu University, Malatya, Turkey; 4Department of Medical Biochemistry, Faculty of Medicine, Yildirim Beyazit University, Ankara, Turkey
Correspondence: Ayşegül Beykumül, Pulmonary Rehabilitation Unit, Turgut Ozal Medical Center, Inonu University, Elazig Road 11th. Km. Battalgazi, Malatya, 44280, Turkey, Tel +905355581415, Fax +904223411220, Email [email protected]
Purpose: To determine the level of oxidative stress in the body due to pulmonary rehabilitation (PR) with thiols and disulfide and to investigate their relationship with indirect markers such as creatine kinase (CK), creatine kinase – MB (CK-MB), and lactate dehydrogenase (LDH), which show cell destruction.
Patients and Methods: Patients with chronic obstructive pulmonary disease (COPD) are included in inpatient and outpatient care. They were evaluated before and after for PR, and an exercise program was prescribed. In addition, native thiol (NT), total thiol (TT), disulfide (DS), LDH, CK, and CK-MB values were tested.
Results: The mean age of 21 patients was 63± 7.31 years. Eleven of them were outpatients and 10 of them were inpatients. Most of the patients were male (M/F=20/1, 95.2/4.8%). There was a significant difference in pulmonary function tests (PFT), St. George Respiratory Questionnaire (SGRQ), and 1 repetition maximum (1RM) before and after the treatment (p< 0.001). There was a correlation between PFT and 1RM upper extremity. While there was no significant difference between thiols and disulfide, according to GOLD scores, there was a significant difference in patients with level 3-MMRC. No correlation was found between LDH, CK, CK-MB, and thiols, DS. ΔCK was found to be associated with ΔDS, and ΔCK-MB with ΔNT, and ΔTT.
Conclusion: PR contributes to the antioxidant process by improving respiration and reducing oxidative stress. The decrease in LDH, CK with PR, increase in CK-MB, and correlation of CK with thiols and DS gave a different interpretation. In this case, it should be considered that oxidative stress may also be increased in people with high CK values.
Keywords: CK, CK-MB, COPD, LDH, oxidative stress, pulmonary rehabilitation
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease that is not reversible, characterized by progressive airflow limitation and respiratory symptoms. COPD commonly results from an inflammatory process due to exposure to harmful gases and particles, especially cigarette smoke, that is preventable and treatable.1 Oxidative stress is key in driving COPD-related inflammation, even in people who have quit smoking. Reactive oxygen species (ROS) formation occurs continuously during normal metabolic processes in every cell. Activated phagocytic cells such as neutrophils and macrophages produce large amounts of ROS.2 PR has recently been recognized as a standard and effective treatment.1 PR reduces oxidative stress and helps to maintain the oxidant-antioxidant balance.3 Thiols are an organic compound containing a sulfhydryl (-SH) group, which is critical in preventing the formation of any oxidative stress state in cells.4 Studies in COPD have shown that thiols decrease due to inflammation and Disulfide (DS) increases, creating a picture favoring oxidative stress.5,6
Creatine kinase (CK) is an important enzyme regulator of high-energy phosphate production and utilization in contractile tissues. Serum CK activity is a sensitive indicator of skeletal muscle and myocardium injuries. It is one of the indices of cellular necrosis and is widely used in diagnosing skeletal muscle diseases in clinical situations such as tissue damage in skeletal muscles.7 It is used in the clinic to obtain important information about the situation in these tissues by evaluating the CK in the skeletal muscle and CK–MB in the heart tissue.8 CK-MB, used to diagnose myocardial infarction, is also a good predictor of inflammatory skeletal muscle diseases. High CK is also an indicator of inflammation and myopathy in COPD.9 Lactate dehydrogenase (LDH) is released from cells into the bloodstream when cells are damaged or destroyed. Therefore, the LDH test can be used as a general marker of cell injury.10,11
Although there are many studies on the relationship between pathophysiology and oxidative stress in COPD, few studies have been studied with thiols. However, no studies are showing the effect of PR on thiols. Therefore, we aimed to determine the thiols and disulfide levels in COPD patients receiving PR and to investigate whether CK, CK-MB, and LDH could be secondary to exercise and be used in follow-up in COPD patients receiving PR.
Methods
Data Collection and Ethical Considerations
The study was carried out between May 2019-January 2020 in the COPD outpatient clinic of the Department of Chest Diseases. Approval was obtained from Malatya Clinical Research Ethics Committee, (Inonu University, Malatya, Turkey) for this prospective, randomized, interventional study (approval number: 2019/107). Detailed information was given to the patients before the procedure, and all signed a consent form. This study was conducted following the principles of the Helsinki Declarations revised in 2013.
Patient Population
Patients over 40 years of age, diagnosed with COPD and followed up in our clinic, who did not have any cardiological, neurological, musculoskeletal or psychiatric problems that would prevent them from exercising (using a treadmill, bicycle ergometer, arm ergometer) were included in the study. Patients who did not have COPD, had lung cancer and other malignancies, neurological and/or musculoskeletal, psychiatric, and cardiac problems that would prevent them from exercising, received PR treatment in the last 6 months, and acute problems such as recent myocardial infarction, and pulmonary embolism were excluded.
Study Design
In the evaluation, haemograms, biochemistry (including CK, CK-MB, and LDH) and c-reactive protein (CRP) as a routine diagnosis, pulmonary function test (PFT),12 and body mass index (BMI) of COPD patients (patients with previous and/or newly COPD diagnosis) and without exacerbation were measured.
A 6-minute walking test (6MWT)13 was applied for physical capacity, a hand grip test14 for hand grip strength (HGS) evaluation, and 1 repetition maximum test (1RM)15 was applied to determine the ideal weight for upper and lower extremity training. Quality of life was assessed with St. George Respiratory Questionnaire (SGRQ).16 All evaluations except oxidative stress values were performed in our hospital.
Blood was taken from all patients in 4 cc biochemistry tubes to send to Yildirim Beyazit University Ankara Training and Research Hospital Medical Biochemistry Laboratory, and centrifuged at 4000 rpm for 10 minutes (Hettich Zentrifugen, Rotina 380, Germany). The samples whose plasma was separated were kept at −80C° and when they reached the planned number, they were packaged with dry ice and sent. Dynamic thiol/Disulfide balance was evaluated with an automated chemical analyzer (an automated clinical chemistry analyzer -Roche-Cobas 501, Mannheim, Germany). According to the pre-treatment value, the increase in the native thiol, total thiol values, and the decrease in the disulfide value was evaluated as a decrease in the oxidative stress level.5,6
The exercise program was applied 5 days a week, a total of 16 sessions. Stretching exercises for warming up and cooling down 5 days a week, 20 minutes of treadmill, 20 minutes of bicycle ergometer, 20 minutes of arm ergometer, a total of 60 minutes of aerobics, 3 days a week, starting with 40% of 1 RM, weight exercises were performed on the upper and lower extremities. Pulse and oxygen saturation (SPO2) were measured before and after training and after two minutes of recovery.
Statistical Analysis
Statistical analysis of the data was done with IBM SPSS 22.0. In defining the data regarding our variables in the study, quantitative data were presented as mean, standard deviation (SD), and qualitative data were presented as number (n) and percentage (%). Shapiro Wilk normality test was used to test whether the quantitative data regarding our variables showed normal distribution. The paired-sample t-test and The Pearson correlation test were used to analyze the data conforming to the normal distribution. Paired sample t-test was used to evaluate the data before and after the study. The results were evaluated at the 95% confidence interval at the p<0.05 significance level. In general, the interpretation of the “Cohen d” value was accepted as: d≥1 very large effect, 0.8=large effect, 0.5=moderate effect, 0.2=small effect.
Results
The mean age of 21 patients included in our study was 63±7.31. Demographic data of the patients are given in Table 1. A significant difference was found in the pre-and post-treatment findings in 6MWD, PFT, SpO2, and all pulse onset scores, pulse recovery, SGRQ, and 1RM. The effect size is given in Table 2.
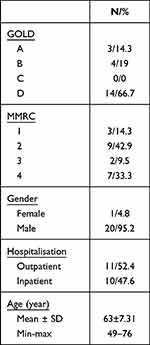 |
Table 1 Demographic Features |
 |
Table 2 Pre- and Post-Treatment Findings |
When the correlations between the LDH, CK, and CK-MB differences of patients’ pre and post-treatment with the differences of NT, TT, DS, and indices were examined (Table 3), a moderately strong negative correlation was found between CK and NT, TT, a positive correlation between DS and LDH, and a negative weak correlation between CK.
 |
Table 3 Correlations of Differences in LDH, CK, CK-MB Values with Differences in NT, TT, DS, and Indices in Pre and Post-Treatment |
In the estimated linear regression model (Table 4), the relationship between ΔDS value and ΔCK was found to be statistically significant. It was observed that there was a 4-unit increase in ΔDS value for a 1-unit increase in ΔCK value.
 |
Table 4 Estimation of ΔNT, ΔTT, ΔDS by ΔCK Value – Linear Regression Model |
Discussion
In our study, we aimed to investigate the relationship between LDH, CK, and CK-MB values, and thiols and DS, which indirectly provide information about cell and muscle destruction in the body, to show the possible effect of PR on oxidative stress. Similar to the literature, we observed that the exercise capacity, respiratory functions, quality of life, and muscle strength of patients with PR increased compared to pre-treatment.1,17 Increasing muscle strength with PR was associated with improved PFT values.18 It was observed that thiols, which are oxidative stress markers, increased as respiratory functions improved. According to the results of this study, determining that thiol and disulfide values are associated with CK and CK-MB, and especially following the CK value, may help to closely monitor the oxidative balance, anti-inflammatory effect, and muscle damage-repair process in PR training.
In our study, when the exercise capacity and respiratory functions of outpatients and inpatients were compared before and after treatment, it was determined that outpatients benefited more clinically and statistically. While inpatients did not exert much physical effort outside the treatment period, outpatients have better outcomes due to their daily commute from home, increased effort capacity while continuing their routines, and increased participation in daily life.
Our study is the first to evaluate thiol-disulfide homeostasis in COPD patients receiving PR therapy. Therefore, no study in the literature includes reference values of thiol, disulfide, and indices in COPD patients. The study of Erden et al, conducted with 50 patients with COPD and 33 healthy controls, showed that patients with COPD had lower thiol levels than healthy volunteers.19 Babaoglu et al. Thiol levels were no different in patients with COPD, Asthma, and ACOS (Asthma-COPD overlap syndrome).5 In our study, although the values of thiols and DS before and after treatment changed, they were not statistically significant.
When the measurements of our patients before and after the treatment program were compared, we recognized that the 1RM values in the upper and lower extremities increased by 2–5 kg. When we evaluated all patients, there was a statistically and clinically significant difference in the lower extremity 1RM value and a statistically significant difference in the upper extremity 1RM values. In the study of Dourado et al, in which they compared three different exercise programs in patients with COPD, there was no difference between the groups before and after treatment, similar to our study. However, they found a statistically significant difference in 1RM values.20 In some PR studies, there was no increase in HG.21–24 While there was a difference in the 1RM value in our patients, there was no significant difference in grip strength. We thought that it might be a result of the intensity-related fatigue during patient treatment because all tests of the evaluated patients were performed on the same day, with rest periods in between, but sequentially.
6 MWD is one of the indicators of functional capacity.25 In the literature, it has been shown that a significant increase in 6 MWD leads to an improvement in the perception of ADL and dyspnea and improves patients’ quality of life.10,26–28 Before and after PR treatment in both inpatients and outpatients compared, statistically and clinically significant improvements were observed in 6 MWDs. As muscle strength and effort capacity increased, patients with dyspnea had a decrease in dyspnea and a decrease in anxiety, and significant increases in activities of daily living. Patients became more active, social, and hopeful that they could achieve their pre-treatment well-being. The progress they noticed in themselves daily with exercise motivated them even more. All these developments are factors that increase the quality of life of patients. The changes in all SGRQ scores of the patients were evaluated as clinically and statistically significant. These results were similar to the guidelines.17,29,30
It is thought that there is an increase in CK value in COPD due to the deterioration of the balance between muscle building and destruction in favor of destruction. Barreiro et al. The total CK activity in the muscles of individuals with COPD was found to be higher than in healthy individuals. It also found a positive correlation between CK activity, protein expiration, and FEV1 and a negative correlation with CK carbonylation in patients with COPD. They stated that oxygen radicals contribute to the deterioration of CK function in the extremity muscles by selectively targeting CK in the extremity muscles.31 It has been determined that CK elevation at rest is a marker of asymptomatic early myopathy. CK is also an indicator of inflammation and myopathy in COPD.11
Our study observed that LMM and CK values were negatively correlated, and CK values increased as LMM decreased. It was determined that thiol values were associated with SFT values, and NT and TT values increased as FVC, FEV1, FEV1/FVC, and FEF25–75 values increased. A study conducted with workers working in the cement industry found that SFT values were negatively associated with oxidative stress.32 The increase in antioxidants is thought to improve PFT values, especially in FVC.
Since the outcome is due to sarcomeric damage, strenuous exercise that damages skeletal muscle cells causes an increase in total serum CK.11 A statistically significant correlation was found between CK and thiols and DS values between pre-and post-treatment differences. It is thought that there is a negative relationship between oxidative stress parameters and CK. As the difference between NT, TT, and DS values increases, the difference in CK value decreases. By looking at the changes in the CK value, an idea about the oxidative stress level of the patient can be obtained. Marin-Corral et al, in severe-moderate COPD and healthy individuals, when the control group and severe COPD patients were compared, respiratory muscle functions were found to be impaired, CK activities decreased, and superoxide anion increased. Patients with severe COPD have had an increase in the diaphragm muscle. That indicates that the main target of ROS in muscles is to reduce CK activity.33 In the literature, LDH is expected to increase with CK and CK-MB, especially in cellular damage, inflammation, and muscle destruction.7,11 Cepelak et al found that resting serum LDH activity was increased in COPD patients compared to healthy smokers and non-smokers.34 Kanda et al showed that CK rises earlier (24 hours) after exercise than LDH.10 LDH elevation is also observed in the acute phase after exercise.35 At the same time, higher LDH values have been associated with higher perceived shortness of breath.36 CK-MB only not only myocardial damage but also a marker of exercise-induced skeletal muscle damage in marathon runners. It has been shown that CK-MB increases in athletes after chronic intense exercise as well as acute vigorous exercise.37
When LDH, CK, and CK-MB values were analyzed in our study, it was observed that LDH and CK decreased contrary to expectations, and CK-MB increased after treatment. We think that our patients’ low CK and LDH levels after exercise are due to the antioxidant anti-inflammatory effect of the exercise and the light exercise program we applied.
Limitations of this study were that the patient population mainly consisted of male patients and the unequal distribution of treatment groups according to the combined GOLD assessment.
Conclusion
Our study observed that the respiratory functions, effort capacities, peripheral muscle strength, and ADL of the patients with COPD who received PR treatment improved compared to the pre-treatment level. When the effect of PR on oxidative stress was examined, it was thought that it contributed to the antioxidant process by improving respiratory functions and reducing oxidative stress. The fact that the CK value was associated with thiols and DS gave a different interpretation, as the patients showed a decrease in LDH and CK values and an increase in CK-MB values. Oxidative stress may be increased in people with high CK values. With CK monitoring, it may be possible to follow the oxidation process in patients with COPD. In fact, we accept that it will be important to follow the oxidative stress status of the patient with CK as a step, and we think that it may be useful in terms of monitoring the effectiveness of the antioxidant therapy used especially in COPD. In future studies, specific studies on LDH and CK subgroups may provide important information demonstrating mitochondrial function and oxidative stress.
Acknowledgments
We would like to thank Dr. Ozcan Erel for providing us with laboratory facilities. We thank Dr. H. Gozde Gozukara Bag for her support in the statistical analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global Strategy for the Diagnosis, Management, and Prevention of COPD. The global initiative for chronic obstructive lung diseases (GOLD) 2022 report. Available from: https://goldcopd.org/2022-gold-reports.
2. Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71–86. doi:10.1016/j.ccm.2013.10.004
3. Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017;47(8):
4. Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. WAO J. 2012;5(1):9–19. doi:10.1097/WOX.0b013e3182439613
5. Babaoglu E, Kilic H, Hezer H, et al. Comparison of thiol/disulfide homeostasis parameters in patients with COPD, asthma, and ACOS. Eur Rev Med Pharmacol lSci. 2016;20(8):1537–1543.
6. Erel O, Neselioglu S. A novel and automated assay for thiol/disulfide homeostasis. Clin Biochem. 2014;47(18):326–332. doi:10.1016/j.clinbiochem.2014.09.026
7. Brancaccio P, Limongelli FM, Maffulli N. Monitoring of serum enzymes in sport. Br J Sports Med. 2006;40(2):96–97. doi:10.1136/bjsm.2005.020719
8. Skitek M, Kranjec I, Jerin A. Glycogen phosphorylase isoenzyme BB, creatine kinase isoenzyme MB and troponin I for monitoring patients with percutaneous coronary intervention a pilot study. Med Glas. 2014;11:13–18.
9. Stomme JH, Rustad P, Steensland H, et al. Reference intervals foresight enzymes in blood of adult females and males measured by the international federation of clinical chemistry reference system at 378C: part of the Nordic reference interval project. Scand J Clin Lab Invest. 2004;64(4):371–384. doi:10.1080/00365510410002742
10. Kanda K, Sugama K, Sakuma J, Kawakami Y, Suzuki K. Evaluation of serum leaking enzymes and investigation into new biomarkers for exercise-induced muscle damage. Exerc Immunol Rev. 2014;20:39–54.
11. Brancaccio P, Maffulli N, Buonauro R, Limongelli FM. Serum enzyme monitoring in sports medicine. Clin Sports Med. 2008;27(1):1–18. doi:10.1016/j.csm.2007.09.005
12. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardization of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
13. Brown CD, Wise RA. Field tests of exercise in COPD: the six-minute walk test and the shuttle walk test. COPD. 2007;4(3):217–223. doi:10.1080/15412550701480125
14. Higgins SC, Adams J, Hughes R. Measuring hand grip strength in rheumatoid arthritis. Rheumatol Int. 2018;38(5):707–714. doi:10.1007/s00296-018-4024-2
15. Onat A, Aksu H, Uslu N, et al. Smoking among Turkish adults: addiction is on the way to increase in our women. Turkish Cardiol Soc Res. 1999;27:697–700.
16. Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi:10.1164/ajrccm/145.6.1321
17. Ates I, Ozkayar N, Inan B, et al. Dynamic thiol/disulfide homeostasis in patients with newly diagnosed primary hypertension. J Am Soc Hypertens. 2016;10(2):159–166. doi:10.1016/j.jash.2015.12.008
18. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;23(2):CD003793.
19. Erden E, Motor S, Ustun I, et al. Investigation of Bisphenol A as an endocrine disruptor total thiol, malondialdehyde and C-reactive protein levels in chronic obstructive pulmonary disease. Eur Rev Med Pharmacol Sci. 2014;18(22):3477–3483.
20. Dourado VZ, Tanni SE, Antunes LCO, et al. Effect of three exercise programs on patients with chronic obstructive pulmonary disease. Brazil J Med Biol Res. 2009;42(3):263–271. doi:10.1590/S0100-879X2009000300007
21. Steiner MC, Barton RL, Singh SJ, et al. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2003;58(9):745–751. doi:10.1136/thorax.58.9.745
22. Faager G, Soderlund K, Skold CM, et al. Creatine supplementation and physical training in patients with COPD: a double-blind, placebo-controlled study. Int J Chron Obstruct Pulmon Dis. 2006;1(4):445–453. doi:10.2147/copd.2006.1.4.445
23. Fuld JP, Kilduff LP, Neder JA, et al. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax. 2005;60(7):531–537. doi:10.1136/thx.2004.030452
24. Aldhahir AM, Al Rajeh AM, Aldabayan YS, et al. Nutritional supplementation during pulmonary rehabilitation in COPD: a systematic review. Chron Respir Dis. 2020;17:1–21. doi:10.1177/1479973120904953
25. Ortega F, Toral J, Cejudo P, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(5):669–674. doi:10.1164/rccm.2107081
26. Agarwala P, Salzman SH. Six-Minute walk test: clinical role, technique, coding, and reimbursement. Chest. 2020;157(3):603–611. doi:10.1016/j.chest.2019.10.014
27. De Blasio F, Polverino M. Current best practice in pulmonary rehabilitation for chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2012;6(4):221–237. doi:10.1177/1753465812444712
28. Bernard S, Whittom F, Leblanc P, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(3):896–901. doi:10.1164/ajrccm.159.3.9807034
29. Troosters T, Demeyer H, Hornikx M, et al. Pulmonary rehabilitation. Clin Chest Med. 2014;35(1):241–249. doi:10.1016/j.ccm.2013.10.006
30. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4:CD003793.
31. Barreiro E, Gea J, Matar G, Hussain SNA. Expression and carbonylation of creatine kinase in the quadriceps femoris muscles of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2005;33(6):636–642. doi:10.1165/rcmb.2005-0114OC
32. Shanshal SA, Al-Qazaz HK. Spirometric outcomes and oxidative stress among cement factory workers: results from a cross-sectional study. J Occup Environ Med. 2020;62(10):e581–85. doi:10.1097/JOM.0000000000001991
33. Marin-Corral J, Minguella J, Ramirez-Sarmiento AL, Hussain SNA, Gea J, Barreiro E. Oxidised proteins and superoxide anion production in the diaphragm of severe COPD patients. Eur Respir J. 2009;33(6):1309–1319. doi:10.1183/09031936.00072008
34. Cepelak I, Dodig S, Romic D, Ruljancic N, Popovic-Gale S, Malic A. Enzyme catalytic activities in chronic obstructive pulmonary disease. Arch Med Res. 2006;37(5):624–629. doi:10.1016/j.arcmed.2006.01.004
35. Saey D, Michaud A, Couillard A, et al. Contractile fatigue, muscle morphometry and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(10):1109–1115. doi:10.1164/rccm.200408-1005OC
36. Spruit MA, Pennings HJ, Does JD, Möller GM, Janssen PP, Wouters EFM. Serum LDH and exercise capacity in COPD. Thorax. 2008;63(5):472. doi:10.1136/thx.2007.086363
37. Miller TD, Rogers PJ, Bauer BA, O’Brien SRW, Bailey KR, Bove AA. Does exercise training alter myocardial creatine kinase MB isoenzyme content? Med Sci Sports Exerc. 1989;21(4):437–440. doi:10.1249/00005768-198908000-00016
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
