Back to Journals » Cancer Management and Research » Volume 9
Can 18F-FDG PET predict the grade of malignancy in thymic epithelial tumors? An evaluation of only resected tumors
Authors Nakagawa K , Takahashi S, Endo M, Ohde Y, Kurihara H, Terauchi T
Received 15 July 2017
Accepted for publication 3 October 2017
Published 5 December 2017 Volume 2017:9 Pages 761—768
DOI https://doi.org/10.2147/CMAR.S146522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Kazuo Nakagawa,1 Shoji Takahashi,2 Masahiro Endo,3 Yasuhisa Ohde,2 Hiroaki Kurihara,4 Takashi Terauchi5
1Department of Thoracic Surgery, National Cancer Center Hospital, Tokyo, 2Division of Thoracic Surgery, 3Division of Diagnostic Radiology, Shizuoka Cancer Center, Shizuoka, 4Department of Diagnostic Radiology, National Cancer Center Hospital, 5Department of Nuclear Medicine, Cancer Institute Hospital, Tokyo, Japan
Objective: Although 18-fluorine fluorodeoxyglucose positron emission tomography (18F-FDG PET) is thought to be useful for predicting the histological grade of thymic epithelial tumors (TETs), it remains controversial. To date, just a few of many previous studies have included only resected cases. Therefore, we investigated 18F-FDG PET findings only in patients with resected TETs.
Patients and methods: A total of 112 patients with TETs consisting of 92 thymomas and 20 thymic carcinomas (TCs), resected at two institutes (Shizuoka Cancer Center [Shizuoka] and National Cancer Center Hospital [Tokyo]) between October 2002 and December 2015, were evaluated. Spearman rank correlation coefficient was used to assess the association between the maximum standardized uptake value (SUVmax) in the tumor and both the histological subtype and tumor stage. The cutoff value of SUVmax for differentiating thymoma from TC was calculated.
Results: The SUVmax was strongly related to both the World Health Organization (WHO) histological subtype and tumor stage based on the eighth edition of the tumor-node-metastasis (TNM) classification (Spearman rank correlation coefficient =0.485 and 0.432; p = 0.000 and 0.000, respectively). There was a significant difference between thymoma and TC. The optimal SUVmax cutoff value for differentiating thymoma from TC was 4.58 (sensitivity: 80% and specificity: 78.3%). In contrast, there was no significant difference between low-risk (type A, AB, and B1) and high-risk (type B2 and B3) thymoma, or between type B3 thymoma and the other subtypes.
Conclusion: Our results suggest that 18F-FDG PET is useful for differentiating thymoma from TC, but not for predicting the histologic grade of thymoma.
Keywords: 18F-FDG PET, thymic epithelial tumors, thymoma, thymic carcinoma, WHO histological subtype, TNM classification
Introduction
Although many studies have examined the clinical value of 18-fluorine fluorodeoxyglucose positron emission tomography (18F-FDG PET) for thymic epithelial tumors (TETs),1–16 its clinical usefulness and significance are still unclear. Hence, the National Comprehensive Cancer Network (NCCN) guidelines state that 18F-FDG PET in the initial evaluation for anterior mediastinal lesions is “as indicated”.17 To date, most previous studies have demonstrated that 18F-FDG PET is useful for predicting the grade of malignancy in TETs, and in particular that there is a significant difference in 18F-FDG uptake in the tumor between thymoma and thymic carcinoma (TC). Although some studies have reported the optimal cutoff value of maximum standardized uptake value (SUVmax) for differentiating thymoma from TC,2,6,7,15,16 the most appropriate threshold remains controversial. In terms of the grade of malignancy of thymoma, most studies on 18F-FDG PET for TETs were conducted based on a simplified World Health Organization (WHO) classification:18 low-risk group (type A, AB, and B1) and high-risk group (type B2 and B3),3,4,8,10,12,13,15,16 or low-risk group (type A, AB, and B1) and high-risk group (type B2, B3, and TC),5,7,9,11,14 because patients with type B2 or B3 thymoma or TC have a worse prognosis than those with type A, AB, or B1. In contrast, Marchevsky et al suggested that the WHO classification could be simplified into three categories (type A-B1, type B2, and type B3), excluding TC.19 More recently, Weis et al evaluated whether or not the WHO histological type had an independent impact on prognosis using a worldwide retrospective database of the International Thymic Malignancy Interest Group, and demonstrated in a multivariate analysis that the histological subtype did not have an independent prognostic impact on survival.20 Thus, the validity of these simplified classifications is still controversial and should be evaluated further.
In addition to the histological classification, tumor stage has also been thought to correlate with the degree of 18F-FDG uptake in the tumor. Recently, a new tumor-node-metastasis (TNM) staging system for TETs has been established.21 However, no previous study has evaluated the relationship between the degree of 18F-FDG uptake in the tumor and this new TNM staging system.
Furthermore, previous studies involved a relatively small number of patients (less than 50) and most included not only patients with resected tumors but also those with biopsy alone. In this study, we investigated the relationships between 18F-FDG uptake in the tumor and both histologic subtype and tumor stage in the largest number of patients with only resected TETs.
Patients and methods
Patients
A total of 112 consecutive patients with TETs (92 with thymoma and 20 with TC) who underwent surgical resection with curative intent at Shizuoka Cancer Center (Shizuoka) and National Cancer Center Hospital (Tokyo) between October 2002 and December 2015 were enrolled in this study. All 112 patients underwent preoperative 18F-FDG PET examination at each institution. In all these patients, the final histological diagnoses were made using the resected specimens. All patients underwent chest radiography and contrast-enhanced computed tomography (CT) as well as 18F-FDG PET before surgery. Several patients also underwent magnetic resonance imaging (MRI). Before surgery, if the tumor was strongly suspected to be a thymoma radiologically, we usually performed resection without preoperative pathological diagnosis, because the clinical thoracic oncological conference agreed to omit a preoperative diagnosis. Otherwise, we performed CT-guided percutaneous core-needle biopsy to achieve a preoperative diagnosis after the radiologic evaluation. We reviewed the medical records of these patients to obtain demographic data, as well as their radiological, operative, and pathological findings. The institutional review board at the National Cancer Center Hospital, Tokyo (No 2014-082) approved the study. The requirement to obtain informed consent from patients was waived because of the retrospective nature of the study, and no specific patient-identifiable information was utilized.
18F-FDG-PET imaging
Patients fasted for at least 4 hours before 18F-FDG PET examinations. Patients received an intravenous injection of 200–250 MBq of [18] fluoro-2-deoxy-D-glucose and then rested for ~1 hour before undergoing imaging. Image acquisition was performed with an Advanced NXi PET and Discovery PET/CT scanners (GE Medical Systems, Milwaukee, WI, USA). Two-dimensional emission scanning was performed from the groin to the top of the skull. Acquired data were reconstructed by iterative ordered subset expectation maximization. To evaluate 18F-FDG uptake, the tumor was first examined visually, and then 18F-FDG PET data were evaluated semiquantitatively based on the SUVmax. To measure the SUVmax, a region of interest (ROI) was placed over the tumor after correcting for radioactive decay. The maximum activity in the tumor ROI was then calculated as tumor activity/injected dose/body weight of each patient.
Histological subtype and tumor stage
The histological subtype of TETs was determined according to the WHO classification18 as follows: type A, AB, B1, B2, B3, or TC. For the staging of patients with TET, the eighth edition of the TNM classification21 was used.
Statistical analysis
The mean and standard deviation of SUVmax in each tumor were calculated. We used a Spearman correlation coefficient analysis to identify correlations between SUVmax of each tumor and either the histological subtype or tumor stage. We also used Wilcoxon ranked sum tests to evaluate the difference in SUVmax in the tumor between thymoma and TC, as well as between two groups in a simplified WHO histological classification. The cutoff value of SUVmax for differentiating thymoma from TC was calculated using a receiver operating characteristic (ROC) curve analysis. The optimal cutoff values were calculated by means of the Youden Index. A p-value of less than 0.05 was considered significant. SPSS Statistics version 22.0 for Windows (IBM Corporation, Armonk, NY, USA) was used to perform all statistical analyses.
Results
Patient characteristics
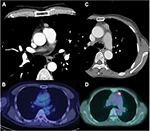
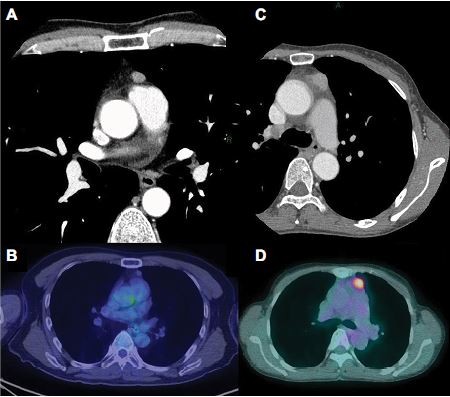
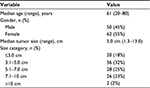
The baseline characteristics of the patients are shown in Table 1. There were 50 men and 62 women with a median age of 61 years (range: 40–79 years). Two patients had myasthenia gravis before surgery. With regard to preoperative therapy, one patient with type AB thymoma received preoperative steroid therapy and one TC patient received induction chemoradiotherapy. All patients underwent macroscopically complete resection. Regarding the type of operation, 67 patients underwent resection of tumor with total thymectomy (64 through median sternotomy and 3 through video-assisted thoracic surgery [VATS]), while 45 underwent resection of tumor with partial thymectomy (4 through median sternotomy, 6 through anterolateral thoracotomy, and 35 through VATS). The median tumor size was 5.0 cm (range: 1.5–13.0 cm). Twenty (18%) patients had a tumor of 3 cm or less in diameter. Figure 1 shows CT and PET-CT findings of two tumors (type AB thymoma and TC) that measured 3 cm or less.
  | Table 1 Baseline characteristics of the patients |
Histological subtypes based on WHO classification
Regarding the histological subtypes determined by resected specimens, type A was observed in 12 patients, AB in 45, B1 in 19, B2 in 10, B3 in 6, and TC in 20. Thirty-three (29.5%) of the 112 patients underwent CT-guided biopsy. Of these, six were diagnosed with thymoma, but the WHO histological subtype could not be determined. In addition, of these six patients, the diagnosis was changed from thymoma to TC after resection in one patient. The histological subtype determined using biopsy specimen was equivalent to that determined by resected specimen in 20 patients (1 exhibited type A, 1 type AB, 2 type B1, 1 type B2, 2 type B3, and 13 TC). In the remaining seven patients, the WHO histological subtype determined using biopsy specimen was not equivalent to that determined by resected specimen. After resection, the histological subtype was changed from B1 to B2 in two patients, from type B2 to AB in two patients, from B3 to B1 in one patient, and from TC to B3 in two patients.
Tumor stage
Among the 112 patients, 89 (79.5%) were in stage I, 3 (2.7%) in stage II, 11 (9.8%) in stage III, and 9 (8.0%) in stage IV. Of the 11 patients in stage III, 5 had a tumor with invasion to the lung, 5 had a tumor with invasion to both the lung and pericardium, and 1 had a tumor with invasion to the left brachiocephalic vein. Of the nine patients in stage IV, two patients had a tumor with perithymic lymph node metastasis (N1 disease), six had a tumor with deep thoracic lymph node metastasis (N2 disease), and one had a tumor with pleural dissemination.
Correlation between SUVmax and either WHO histological subtype or tumor stage
Upon visual inspection, all tumors showed 18F-FDG uptake to various degrees. Table 2 shows the relationship between SUVmax in the tumor and the histological subtype based on WHO classification. The degree of 18F-FDG uptake in the tumor tended to gradually increase from type A to TC. The correlation between SUVmax in the tumor and the histological subtype was statistically significant (Spearman correlation coefficient =0.485, p = 0.000). Table 3 shows the relationship between SUVmax in the tumor and the tumor stage. The degree of 18F-FDG uptake in the tumor also tended to gradually increase from stage I to IV. The correlation between SUVmax in the tumor and tumor stage was also statistically significant (Spearman correlation coefficient =0.432, p = 0.000).
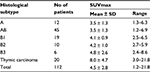  | Table 2 SUVmax in the tumor for each histological subtype Abbreviation: SUVmax, maximum standardized uptake value. |
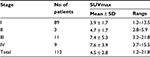  | Table 3 SUVmax in the tumor for each stage Abbreviation: SUVmax, maximum standardized uptake value. |
Distinction between thymoma and TC or two groups of a simplified WHO classification for thymoma
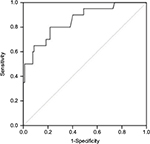
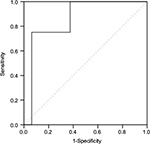
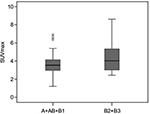
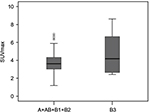
There was a significant difference in SUVmax in the tumor between thymoma and TC (p = 0.000). The usefulness of the SUVmax value for differentiating thymoma from TC was evaluated. When an SUVmax of 4.58 was used as a cutoff value with a sensitivity of 80.0% and a specificity of 78.3%, the area under the curve was measured to be 0.855 (95% CI, 0.760–0.950) using a ROC analysis (Figure 2). For tumors that measured 3 cm or less, when an SUVmax of 4.57 was used as a cutoff value with a sensitivity of 75.0% and a specificity of 93.8%, the area under the curve was 0.859 (95% CI, 0.672–1.000) using a ROC analysis (Figure 3). In terms of differences in SUVmax between two groups according to a simplified WHO classification, there was no significant difference between low-risk (type A, AB, and B1) and high-risk (type B2 and B3) thymoma (p = 0.101; Figure 4). Furthermore, no significant difference was observed between type B3 and the other subtypes of thymoma (p = 0.607; Figure 5).
Discussion
Although there have been many studies on the usefulness of 18F-FDG PET in TETs,1–16 they involved relatively few patients, and most included not only patients with surgical resection but also those with biopsy alone. With regard to the histological diagnosis of TETs, it can sometimes be difficult to determine the histological subtype based on the WHO classification with the use of small biopsy specimens.22 Indeed, in 33 (29.5%) of the112 patients who underwent CT-guided biopsy in this study, the histological subtype determined using biopsy specimens was consistent with that determined by analysis of resected specimens in 20 (60.6%) patients. In the remaining 13 (39.4%) patients, the histologic subtype could not be determined using biopsy specimens or was inconsistent with that determined by the use of resected specimens. Lococo et al stated that pathological specimens obtained by percutaneous biopsy were deemed to be unreliable in comparison to 18F-FDG PET findings.12 Furthermore, not only histological subtype but also tumor stage can be determined more accurately in resected specimens.23 Therefore, in this study we enrolled only patients with surgical resections.
In routine clinical practice, it is important to be able to accurately predict whether a mediastinal mass is a thymoma or another malignant tumor including TC. In the initial management of anterior mediastinal lesions, the NCCN guidelines state that biopsy should be avoided if a resectable thymoma is strongly suspected based on clinical and radiological findings.17 Conversely, a biopsy should be performed for an anterior mediastinal lesion that is suspected to be TC based on radiological evaluation unless the procedure needs a transpleural approach. Furthermore, when an anterior mediastinal mass is suspected to be TC, special treatment strategies such as the surgical approach24 should be taken into consideration. Currently, a minimally invasive approach for TETs should be performed only at specialized centers with surgeons who have expertise in these techniques. This perspective is based on the results of retrospective studies on minimally invasive resection for only early-stage thymomas.17,24 In addition, TC is sometimes found to be a more advanced disease at the pathological evaluation after surgical resection than during clinical evaluation.25 Hence, at present, such a minimally invasive approach is not appropriate for a thymic lesion that is suspected to be TC.
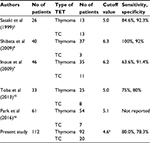
In previous studies,2,6,7,15,16 the optimal cutoff value of SUVmax in the tumor for differentiating thymoma from TC was from 5 to 6 (Table 4). This study shows a cutoff value of 4.58, which is slightly lower than those reported in previous studies. Our result may be attributed to the percentage of patients with small-sized TC included in the study. The threshold of SUVmax that separates thymoma and TC needs to be further evaluated. The incidence of small-sized mediastinal tumor is expected to increase in the near future due to the worldwide spread of CT screening. Yano et al investigated clinicopathological findings and prognosis of small-sized anterior mediastinal tumors (SSAMTs) of 3 cm or less and stated that SSAMTs were good candidates for VATS; however, conversion to sternotomy should be considered if there are intraoperative findings of pericardial invasion or a rapid pathological diagnosis of TC.26 Our cutoff value of 4.57 in tumors measuring 3 cm or less showed a high specificity of 93.8%. Thus, 18F-FDG PET might be useful for such small anterior mediastinal lesions when deciding among surgical strategies.
In contrast to some studies, no significant difference in SUVmax was observed between low-risk (type A, AB, and B1) and high-risk (type B2 and B3) thymoma in this study. While several studies have shown that there is a significant difference between the two groups,4,12 others have not.3,8,10,13,15 A recent study demonstrated that type B3 thymoma had worse disease-free and overall survival than the other histological subtypes,27 and Benveniste et al demonstrated a significant difference in SUVmax between type B3 and the other subtypes.13 Although we conducted the same analysis in this study, we did not observe a significant difference in SUVmax between type B3 and the other subtypes. The usefulness of 18F-FDG PET for predicting the malignancy grade of thymomas remains controversial and further evaluation will be needed to clarify this point.
In this study, the tumor stage was determined according to the eighth edition of the TNM classification.21 We evaluated the relationship between SUVmax in the tumor and tumor stage based on the TNM staging system for the first time, and demonstrated that the correlation between them was statistically significant. The proportion of patients with stage I disease was remarkably high (79.5%), as noted by Fukui et al in their study.28 Further studies will be needed on the association between the findings of 18F-FDG PET and the new TNM staging system.
It is becoming clear that 18F-FDG PET may play an important role as a surrogate end point for assessing the clinical efficacy of novel oncologic therapies.29 However, the value of 18F-FDG PET in the assessment of a therapeutic response in patients with TETs has not been fully investigated. Recently, Thomas et al reported for the first time the usefulness of 18F-FDG PET for monitoring treatment efficacy in patients with unresectable stage III or IV TETs, and demonstrated that there was a close correlation between early metabolic response and the subsequent best response using Response Evaluation Criteria In Solid Tumors.30 Further investigations will also be needed to determine whether or not 18F-FDG PET is suitable for therapy monitoring in patients with TETs.
Limitations
This study has several limitations. First, we included only patients with TETs. However, in routine clinical practice, several diseases such as malignant lymphomas and germ cell tumors, as well as benign lesions such as thymic cyst and hyperplasia should be considered for the differential diagnosis of thymic lesions. Accordingly, 18F-FDG PET findings of all thymic lesions, not only benign lesions but also malignant tumors, should be investigated simultaneously in future studies. Second, while 18F-FDG PET can be useful as a staging procedure in malignant tumors, we could not investigate its usefulness for staging patients with TETs. In addition, while 18F-FDG PET can play an important role in predicting the prognosis of patients with malignant tumors, prognostic information was not available in this study. From a clinicopathological viewpoint, 18F-FDG PET in TETs may be able to provide useful information regarding the prognosis of patients with TETs. The value of 18F-FDG PET for staging and predicting prognosis in patients with TETs should also be evaluated further. Finally, although our study included more patients with TETs than previous studies, there were still not enough patients for each histological subtype. In particular, we included a large number of patients with type AB, but only a small number of patients with type B3, which has a more aggressive nature among the five histological subtypes of thymoma. Additional studies with sufficient number of patients will be needed to clarify the usefulness of 18F-FDG PET in TETs. Due to the rarity of these tumors, it is imperative that such studies are conducted in a multi-institutional setting.
Conclusion
In conclusion, the degree of 18F-FDG uptake in TETs was significantly correlated with both the histological subtype based on WHO classification and tumor stage. 18F-FDG PET is useful for differentiating thymoma from TC, but not for predicting the grade of malignancy of thymoma.
Acknowledgments
We wish to thank Ace Statistics Support Co, Ltd. for their invaluable help with the statistical analysis of the data. This work was supported in part by Grant-in-Aid for Cancer Research (25-A-13) from the Ministry of Health, Labour and Welfare of Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
Liu RS, Yeh SH, Huang MH, et al. Use of fluorine-18 fluorodeoxyglucose positron emission tomography in the detection of thymoma: a preliminary report. Eur J Nucl Med. 1995;22(12):142–147. | ||
Sasaki M, Kuwabara Y, Ichiya Y, et al. Differential diagnosis of thymic tumors using a combination of 11C-methionine PET and FDG PET. J Nucl Med. 1999;40(10):1595–1601. | ||
Sung YM, Lee KS, Kim BT, Choi JY, Shim YM, Yi CA. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med. 2006;47(10):1628–1634. | ||
Endo M, Nakagawa K, Ohde Y, et al. Utility of 18FDG-PET for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer. 2008;61(3):350–355. | ||
Luzzi L, Campione A, Gorla A, et al. Role of fluorine-fluorodeoxyglucose positron emission tomography/computed tomography in preoperative assessment of anterior mediastinal masses. Eur J Cardiothorac Surg. 2009;36(3):475–479. | ||
Shibata H, Nomori H, Uno K, et al. 18F-fluorodeoxyglucose and 11C-acetate positron emission tomography are useful modalities for diagnosing the histologic type of thymoma. Cancer. 2009;115(11):2531–2538. | ||
Inoue A, Tomiyama N, Tatsumi M, et al. 18F-FDG PET for the evaluation of thymic epithelial tumors: correlation with the World Health Organization classification in addition to dual-time-point imaging. Eur J Nucl Med Mol Imaging. 2009;36(8):1219–1225. | ||
Kumar A, Regmi SK, Dutta R, et al. Characterization of thymic masses using 18F-FDG PET-CT. Ann Nucl Med. 2009;23(6):569–577. | ||
Terzi A, Bertolaccini L, Rizzardi G, et al. Usefulness of 18-F FDG PET/CT in the pre-treatment evaluation of thymic epithelial neoplasms. Lung Cancer. 2011;74(2):239–243. | ||
Fukumoto K, Taniguchi T, Ishikawa Y, et al. The utility of [18F]-fluorodeoxyglucose positron emission tomography-computed tomography in thymic epithelial tumours. Eur J Cardiothorac Surg. 2012;42(6):e152–156. | ||
Eguchi T, Yoshida K, Hamanaka K, et al. Utility of 18F-fluorodeoxyglucose positron emission tomography for distinguishing between the histological types of early stage thymic epithelial tumours. Eur J Cardiothorac Surg. 2012;41(5):1059–1062. | ||
Lococo F, Cesario A, Okami J, et al. Role of combined 18F-FDG-PET/CT for predicting the WHO malignancy grade of thymic epithelial tumors: a multicenter analysis. Lung Cancer. 2013;82(2): 245–251. | ||
Benveniste MF, Moran CA, Mawlawi O, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol. 2013;8(4):502–510. | ||
Matsumoto I, Oda M, Takizawa M, et al. Usefulness of fluorine-18 fluorodeoxyglucose-positron emission tomography in management strategy for thymic epithelial tumors. Ann Thorac Surg. 2013;95(1):305–310. | ||
Toba H, Kondo K, Sadohara Y, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography and the relationship between fluorodeoxyglucose uptake and the expression of hypoxia-inducible factor-1α, glucose transporter-1 and vascular endothelial growth factor in thymic epithelial tumours. Eur J Cardiothorac Surg. 2013;44(2):e105–112. | ||
Park SY, Cho A, Bae MK, Lee CY, Kim DJ, Chung KY. Value of 18F-FDG PET/CT for predicting the World Health Organization malignant grade of thymic epithelial tumors: focused in volume-dependent parameters. Clin Nucl Med. 2016;41(1):15–20. | ||
NCCN Clinical Practice Guidelines in Oncology. Thymomas and Thymic Carcinomas. Available from: http://www.nccn.org/professionals/physician_gls/PDF/thymic.pdf. Accessed September 1, 2017. | ||
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th edition. Lyon: IARC; 2015. | ||
Marchevsky AM, Gupta R, McKenna RJ, et al. Evidence-based pathology and the pathologic evaluation of thymomas: the World Health Organization classification can be simplified into only 3 categories other than thymic carcinoma. Cancer. 2008 15;112(12):2780–2788. | ||
Weis CA, Yao X, Deng Y, et al; Contributors to the ITMIG Retrospective Database. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol. 2015;10(2):367–372. | ||
Brierley JD, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 8th ed. New York: Wiley-Blackwell; 2017. | ||
den Bakker MA, Roden AC, Marx A, Marino M. Histologic classification of thymoma: a practical guide for routine cases. J Thorac Oncol. 2014;9(9 Suppl 2):S125–S130. | ||
Tomiyama N, Müller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr. 2001;25:388–393. | ||
Toker A, Sonett J, Zielinski M, Rea F, Tomulescu V, Detterbeck FC. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol. 2011;6(7 Suppl 3):S1739–1742. | ||
Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149(1):95–100. | ||
Yano M, Sasaki H, Moriyama S, et al. Clinicopathological analysis of small-sized anterior mediastinal tumors. Surg Today. 2014;44(10):1817–1822. | ||
Roden AC, Yi ES, Jenkins SM, et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol. 2015;10(4):691–700. | ||
Fukui T, Fukumoto K, Okasaka T, et al. Clinical evaluation of a new tumour-node-metastasis staging system for thymic malignancies proposed by the International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee and the International Thymic Malignancy Interest Group. Eur J Cardiothorac Surg. 2016;49(2):574–579. | ||
Shankar LK, Hoffman JM, Bacharach S, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006; 47(6):1059–1066. | ||
Thomas A, Mena E, Kurdziel K, et al. 18F-fluorodeoxyglucose positron emission tomography in the management of patients with thymic epithelial tumors. Clin Cancer Res. 2013;19(6):1487–1493. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.