Back to Journals » Infection and Drug Resistance » Volume 16
Calcium-Sensitive Receptors Alters Intestinal Microbiota Metabolites Especially SCFAs and Ameliorates Intestinal Barrier Damage in Neonatal Rat Endotoxemia
Authors Sun Y, Song J, Lan X, Ma F, Jiang M, Jiang C
Received 10 May 2023
Accepted for publication 17 August 2023
Published 30 August 2023 Volume 2023:16 Pages 5707—5717
DOI https://doi.org/10.2147/IDR.S420689
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yan Sun,1,* Jiayu Song,2,* Xue Lan,1 Fei Ma,2 Mingyu Jiang,3 Chunming Jiang1,2
1Department of Neonatology, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, 150001, People’s Republic of China; 2Department of Neonatology, Zhuhai Women and Children’s Hospital, Zhuhai, Guangdong, 519060, People’s Republic of China; 3Department of Pediatrics, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, 150001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunming Jiang, Department of Neonatology, The First Affiliated Hospital of Harbin Medical University, 23 Youzheng Street, Nangang District, Harbin, Heilongjiang, 150001, People’s Republic of China, Tel +86-13303601657, Fax +86 0451-53670428, Email [email protected]
Purpose: The calcium-sensing receptor (CaSR) acts as a major modulator of tissue responses related to calcium homeostasis and expresses highly in the mammalian intestine. Endotoxemia tends to impair intestinal barrier function and poses significant obstacles in clinical treatment. This work is designed to decipher whether CaSR can protect lipopolysaccharide (LPS)-induced intestinal barrier dysfunction in neonatal rats by targeting intestinal metabolites.
Patient and Methods: In this study, we utilized gas chromatography (GC) combined with liquid chromatography-mass spectrometry (LC-MS) to quantitatively analyze SCFAs and metabolites in fecal samples of 24 neonatal rats with LPS induced endotoxemia.
Results: Our results showed that CaSR alleviated endotoxin damage to the intestinal tight junction structure and upregulated the levels of butyric acid, propionic acid, valeric acid, and isovaleric acid in short-chain fatty acids (SCFAs). Non-targeted metabolomics analysis indicated that CaSR improved intestinal metabolic disorders by regulating glycerophospholipid metabolism, α-linolenic acid metabolism, as well as sphingolipids metabolism.
Conclusion: CaSR can alter intestinal microbiota metabolites, especially SCFAs, and improve intestinal barrier damage in neonatal rat endotoxemia.
Keywords: calcium-sensing receptor, lipopolysaccharide, endotoxemia, short chain fatty acids, metabolomics
Introduction
Infection remains a chief reason for neonatal mortality around the world. From 2000 to 2010, 64% of deaths of under 5-year-old children were due to infections, of which 40.3% died in the neonatal period. Despite the decreased percentage of infectious deaths (51.8%) by 2013, the percentage of infectious deaths in the neonatal period increased to 44%.1,2 Most infections are driven by Gram-negative bacteria. Particularly, lipopolysaccharide (LPS) is a major ingredient of the Gram-negative bacteria’s outer membrane, which enters the bloodstream to activate nuclear transcription factors, induce the release of inflammatory factors, activate inflammation-related cell signaling pathways, and trigger cascade reactions, eventually leading to a series of pathological and physiological changes in the body, namely endotoxemia.3 Endotoxemia can manifest as neonatal sepsis in neonates, especially in premature infants, and is the most severe condition of neonatal infection that accounts for 11–30% of all neonatal deaths, often accompanied by shock, necrotizing enterocolitis, and neurological infections.4,5 Recent research has found that increased intestinal permeability and barrier dysfunction are implicated in endotoxemia development.6
The calcium-sensing receptor (CaSR), belongs to the G protein-coupled receptor superfamily, is widely distributed in the gastrointestinal tract, nervous system, cardiovascular system, breast, and bone tissues, and its expression is regulated by calcium ions, inflammatory factors, and various bacterial metabolites.7 CaSR is expressed in the apical and basolateral membranes of villus cells of the small intestine, as well as in surface and crypt epithelial cells of the colon in rodents and humans.8,9 CaSR plays an important role in the gut. In mice with intestinal epithelial specific CaSR knockout, intestinal barrier integrity was reduced, intestinal microbiome composition was altered, intestinal pattern recognition receptor expression was altered, and local and systemic innate responses were skewed from regulatory to irritant, thus aggravating colitis induced by DSS.10,11 CaSR is considered a potential immunotherapeutic target for intestinal homeostasis in a manner. CaSR agonist (R-568) can reverse the secretion of the intestinal fluid triggered by cholera toxin and heat-stable Escherichia coli enterotoxin by stimulating phosphodiesterases to degrade cyclic nucleotides. The allosteric activator of CaSR may provide a unique therapy for secretory diarrheas.12 However, the function of CaSR in neonatal sepsis has not been investigated, and the purpose of this study is to explore the role of CaSR in neonatal rats.
The intestinal microbiota is pivotal in the production and absorption of nutrients, metabolism, and immune system maintenance in the host.13 Accumulating studies have indicated intestinal dysbacteriosis as one of the etiological factors in a variety of diseases, including irritable bowel syndrome, diverticulosis, celiac disease, and type 2 diabetes.14–17 The critical role of intestinal microbiota in many gastrointestinal and extra-gastrointestinal diseases has also been confirmed.18 Gastrointestinal microbiota includes over 1500 species and 50 phyla, with over 99% of intestinal microbiota belonging to Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. Of these, Bacteroidetes and Firmicutes are the major components of healthy intestinal microbiota.19,20 The intestinal microbiota status was evaluated by the ratio of Firmicutes to Bacteroidetes.21
Short-chain fatty acids (SCFAs), consisting of acetic, propionic, and butyric acid, are common metabolites in the intestinal microbiota. These SCFAs are the cardinal energy sources of intestinal epithelial cells, which contribute to maintaining the intestinal mucosal mechanical barrier.22 SCFAs promote the production of antimicrobial peptides including lysozyme, defensin, and mucin genes, increase the antimicrobial peptide secretion, and intensify the intestinal immune function.23 Also, SCFAs are closely related to host physiological functions.24 Emerging studies25,26 report that SCFAs, as ligands of GPCRs, are pivotal modulators of host metabolism and intestinal microorganisms. Moreover, SCFAs exert anti-inflammatory functions in the colon epithelium and advance the intestinal barrier via promoting the assembly of the tight junction.27,28 Consequently, SCFAs are accepted as pivotal parameters for evaluating intestinal homeostasis, in particular intestinal barrier integrity.
This work quantitatively analyzed metabolites, especially SCFAs, in fecal samples using gas chromatography (GC) coupled with liquid chromatography-mass spectrometry (LC-MS) to further explore the protective mechanism of CaSR on endotoxemia-induced intestinal injury.
Materials and Methods
Animals and Design of Experiments
Twenty-four Sprague-Dawley (SD) neonatal rats (5-day old, 12.5–15 g) supplied by Beijing Vital River Laboratory Animal Technology Co., Ltd. [SCXK-2021-0011] were raised in standard conditions (21°C-23°C, relative humidity 40%-70%, a cycle of 12 h light and 12 h darkness), with standard laboratory diet and drinking water at liberty. The operations were ratified by the Ethics Committee of First Affiliated Hospital of Harbin Medical University [IACUC No. 2022114] and followed the Guide for the Care and Use of Laboratory Animals. Animals need to be executed for tissue sampling and analysis. According to the regulations of animal welfare and animal protection, we performed cervical dislocation on animals under deep anesthesia to minimize the pain of experimental animals. The successful establishment of the animal model was confirmed by observing the drowsiness, activity, and trembling of animals. Twenty-four neonatal rats were randomized into the control, LPS, and CaSR agonist (R-568) groups. As reported in previous studies, neonatal rats experienced intraperitoneal injections of LPS (10 mg/kg) for establishing endotoxemia models.29–31 Rats in the control group were subjected to injection with an equal amount of physiological saline. In the CaSR agonist group, rats were given R-568 solution (10 mg/kg) by intraperitoneal injections 1 h before LPS injection. After 24 h of LPS intervention, the rats were intraperitoneally injected with 10% chloral hydrate solution and euthanized by cervical dislocation. The intestinal tissue was removed in a sterile environment and fixed with 2.5% glutaraldehyde or stored at −80°C. All tissue samples were retained at −80°C for reserve.
Ultrastructure of Intestinal Epithelial Intercellular Tight Junctions
The ileum tissue was subjected to 2-h fixation in 2.5% glutaraldehyde at 4°C, embedding in an Epon mixture with a 1:1 ratio of Epon 812 and acetone before cutting into 60–80 nm sections and dying with uranyl acetate together with lead citrate, followed by observation with a transmission electronic microscope (TEM) (HT7700, available from Hitachi, Tokyo, Japan).
Non-Targeted Metabolomics Analysis
Fecal samples were collected from 8 rats in each group of control group, LPS group and CaSR agonist group. The fecal samples (25 mg) and the solution of methanol: water (4:1, v/v) were blended at 50 Hz for 6 min in a Wonbio-96c high-throughput tissue crusher (supplied by Wanbo Biotechnology Co., Ltd., Shanghai, China), which was placed for 30 min at −20°C to precipitate proteins. Next, the mixture was subjected to 15-min centrifugation (13,000 g, 4°C) to collect the supernatant for LC-MS/MS.
UHPLC-Q Exactive system (Thermo Fisher Scientific) was utilized as an instrument platform for LC-MS assay. In detail, the HSS T3 column (100 mm × 2.1 mm i.d., 1.8 μm) was employed for separating 2 μL of samples, which was then entered into mass spectrometry measurement. The mobile phases consisted of 0.1% formic acid each in acetonitrile: isopropanol (1:1, v/v). Subsequently, the harvest of the mass spectrometric data was achieved with Thermo UHPLC-Q Exactive Mass Spectrometer that was equipped with an electrospray ionization (ESI) source under the positive or negative ion mode. The raw data of metabolites were analyzed by uploading to the Majorbio cloud platform. For accurate quality, the MS fragment spectra were appraised in the Human Metabolome Database (HMDB), followed by quantification of the isotope ratio of differential metabolites. The control group, LPS group and CaSR agonist group were compared pairwise. The mapping of the differential metabolites into their biochemical pathways was achieved via the KEGG (Fisher’s exact test, P adjust < 0.05) for metabolic enrichment pathway analysis. Afterward, multivariate statistical analyses were executed with the ropls R package, including partial least-squares discriminant analysis (PLS-DA) and orthogonal PLS-DA (OPLS-DA). The significantly differential metabolites were determined with the screening criteria of variable importance in projection (VIP) ≥ 1 and P < 0.05.
Detection of SCFAs
The fecal samples of rats were collected, weighed (25 mg), and transferred into a 2 mL test tube. The samples were added with 500 µL water comprising 0.5% phosphoric acid and then ground at 50 Hz for 3 min, repeated twice. After 15-min centrifugation at 4°C and 13,000 g, the supernatant was transferred to a centrifuge tube (1.5 mL) and added with N-butanol solvent (0.2 mL) comprising internal standard 2-ethyl butyric acid (10 mg/mL). Then, the sample was subjected to 10-s vortex and 5-min centrifugation at 4°C and 13,000 g. Subsequently, the sample was passed via a filter (0.22 mm) and analyzed by Agilent 8890 B gas chromatography (supplied by Agilent Technologies, CA, USA) coupled with a mass selective detector (MSD, Agilent 5977B). The analyte compounds were isolated with an HP-FFAP capillary column ((30 m long × 0.25 mm diameter × 0.25 µm film thickness). The GC parameters included a constant flow rate (1 mL/ min), injection volume (1 uL), inlet temperature (260°C), splitting mode (10:1), as well as a solvent delay (2.5 min). The temperature of the GC column was initially maintained at 80°C and increased to 120°C (40°C/min) and then 200°C (10°C/min). Besides, the temperatures of the ion source and transmission line were kept at 230°C. The scanning mode was selected ion monitor (SIM). In the sample, the SCFA compounds’ absolute content was quantified with the application of Masshunter software (v10.0.707.0, Agilent, USA).
Statistic Methods
IBM SPSS Statistics 26.0 was implemented for statistical analysis based on Student’s t-test (two-group comparison) or one-way analysis of variance (ANOVA) coupled with Tukey’s multiple comparison tests (multi-group comparison). Data were indicated as mean ± standard deviation (SD). A notable difference was noted in a p-value below 0.05.
Results
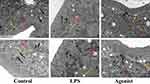
CaSR Agonist Protects the Integrity of Intestinal Epithelial Tight Junctions in LPS-Induced Neonatal Rat Endotoxemia
Twenty-four neonatal rats were randomized into the control, LPS, and CaSR agonist (R-568) groups. Neonatal rats experienced intraperitoneal injections of LPS (10 mg/kg) for establishing endotoxemia models. In the CaSR agonist group, rats were given R-568 solution (10 mg/kg) by intraperitoneal injections 1 h before LPS injection. The rats were euthanized 24 h after LPS intervention. The intestinal epithelial tight junctions’ integrity prevents microbial toxins along with other pernicious intracellular contents from crossing the intestinal epithelium.32 Tight junctions are crucial parameters of the intestinal barrier’s integrity and impermeability. To assess the effect of CaSR agonists on the integrity of tight junction of intestinal epithelium, We compared the tight junction structure and other organelles between the control group, LPS group and CaSR agonist-treated under transmission electron microscopy (TEM). In the control group, tight connective structures and organelles were observed in the intestinal epithelial cells, which were normal with many large lipid droplets. In LPS group, the microvilli of intestinal epithelial cells were sparse, the compact connective structure was cracked, the desmosomes were reduced, the intracellular lipid droplets were significantly reduced, and the mitochondria were slightly swollen. In the agonist group, the tight junction structure returned to normal, the intracellular lipid droplets were smaller than those in the control group, the number of lipid droplets was significantly increased than that in the LPS group, the nucleus was not swollen, and some mitochondria were slightly swollen (Figure 1). These partial results suggest that administration of CaSR agonists mitigated LPS-induced intestinal epithelial tight junction breakdown and lipid droplet reduction.
CaSR Agonists Alters the Fecal Metabolites in LPS-Induced Neonatal Rat Endotoxemia
The intestinal microbiota’s potential mechanism participating in host metabolism has relevance to the metabolites produced by intestinal microbiota. To investigate the impact of changes in intestinal metabolic pathways, we used LC-MS non-targeted metabolomics to analyze fecal metabolites. After normalization, missing value encoding, and filtering low-quality peaks, 10,644 peaks were detected in the positive ion mode while 9059 peaks, were in the negative ion mode. We performed PCA analysis of the control group, LPS group and CaSR agonist group in two groups, as shown in Figure 2A, and found that LPS significantly changed intestinal metabolite profiles compared with the control group. At the same time, as shown in Figure 2B, the resolved metabolite profile of the CaSR agonist group was also significantly changed compared with that of the LPS group. The same results were also shown using OPLS-DA score analysis (Figures 2C and D).
According to the screening criteria of VIP > 1.0, fold > 1.0 or < 1.0, and P < 0.05, 1801 definite metabolites (1101: positive; 700: negative) were noted in three groups. As shown in the volcanic plot (Figure 3A), the Agonist group presented significant changes in 695 metabolites compared to the LPS group, of which 357 were upregulated and 338 were downregulated. The heat map analysis showed notable alternations in the top 50 metabolites of the LPS and Agonist groups, which contributed to distinguishing the metabolic differences (Figure 3B). Lipids, benzene, lipid molecules, carboxylic acids, as well as organic oxygen compounds, were the chief differential metabolites. We subsequently made an analysis of potential metabolic pathways following the matched differential metabolites. Given KEGG classification, the differential metabolites between the LPS and Agonist groups included 7 types of metabolic pathways, namely metabolism, cellular processes, drug development, human diseases, biological systems, and environmental and genetic information processing (Figure 3C). Intriguingly, enriched lipid metabolism was noted in the metabolic category, and pathogenic E. coli infection was markedly enriched in the human disease category (Figure 3C).
The topology method was utilized for pathway enrichment analysis following the differential metabolites between LPS and Agonist groups (Figure 4). KEGG topology analysis disclosed 10 enriched metabolic pathways. Of these, the degradation of furfural, biosynthesis of cutin, cork base, and wax, α-linolenic acid metabolism, glycerol phospholipid metabolism, sphingolipid metabolism, biosynthesis of tyrosine, phenylalanine, and tryptophan, metabolism of serine, glycine, and threonine, phenazine biosynthesis, arginine biosynthesis, sesquiterpene biosynthesis, and triterpene biosynthesis were the main metabolic pathways of CaSR intervention, which may be related to alleviating intestinal mucosal barrier damage. Glycerol phospholipid, sphingolipid, and α-linolenic acid metabolism were filtered out to be the major metabolic pathways in the LPS and Agonist groups (P < 0.05).
 |
Figure 4 Pathway impact in topology analysis. The size and color of each circle were based on the pathway impact value and P-value, respectively. |
CaSR Agonists Affects the Levels of SCFAs in LPS-Induced Neonatal Rat Endotoxemia
The preliminary results observed an elevation in the SCFAs-related bacteria abundance in the intestinal microbiota after CaSR intervention. Considering the non-targeted metabolomics conducted by LC/MS, we adopted GC/MS to estimate the concentration of SCFAs because of the strong volatility of SCFAs. The concentration and total content of 7 types of SCFAs were shown in Figure 5. Elevated total content of SCFAs was observed in the LPS group in contrast to the control group (P < 0.001), with distinct decreases in acetic acid and isobutyric acid (respectively P < 0.001 and P < 0.05). Relative to the LPS group, the CaSR group exhibited an enhancement in 4 SCFAs, namely propionic, butyric, isovaleric, and valeric acid (Figure 5).
 |
Figure 5 SCFAs contents in different groups. **P < 0.01, ***P < 0.001, Control vs LPS; #P < 0.05, Agonist vs LPS. |
Discussion
Neonatal sepsis is a refractory and fatal disease among neonates with substantial morbidity and mortality, which poses great challenges for pediatric management worldwide.33 Further improving sepsis diagnosis and therapy has far-reaching significance for global health.34 The intestine is widely deemed as the organ dysfunction “engine”.35 As important components of the intestine, intestinal microflora mediates diverse physiological functions including metabolism and biosynthesis. Nevertheless, critical diseases result in various alterations in microbial diversity, such as a decrease in diversity and excessive growth of pathogenic bacteria.36,37 Prescott et al38 found a significant dose-effect relationship between intestinal ecological disorders and sepsis progression through clinical observation of 43,095 hospitalized patients. Damage to the intestinal barrier and changes in intestinal microflora can be considered both as a result of sepsis and a cause of its progression. The mechanism and therapeutic potential of intestinal microflora in the occurrence and development of sepsis are increasingly highlighted, which may effectively contribute to improving the prognosis of sepsis.39
SCFAs possess notable anti-inflammatory activities and maintain glycolipid metabolism homeostasis.40 Emerging evidence has suggested the critical role of SCFAs in intestinal homeostasis, barrier function, inflammatory responses, and epithelial cell integrity.41 As a research hotspot in recent years, the role of SCFAs in sepsis has also been gradually explored. Consistently, our results confirmed that the total level of SCFAs in the LPS-induced sepsis model was significantly reduced. Nastasi et al found that SCFAs, especially propionic acid, and butyric acid, can inhibit the expressions of cytokines IL-6 and IL-12 induced by LPS in human mature dendritic cells.42 SCFAs trigger the generation of prostaglandin E2 and the IL-10 levels by stimulating GPCRs, and can also activate inflammasomes, promoting the production and secretion of IL-18.43 In clinical studies using SCFAs to treat inflammatory diseases, the improvement of clinical and histological indicators in inflammatory bowel diseases supports the direct anti-inflammatory effect of butyric acid at the inflammatory site.44,45 In type 2 diabetes, a metabolic disorder with low-grade inflammation, the reduction of butyric acid-producing organisms can be observed.46 Multiple studies47,48 have shown that SCFAs suppress histone acetylation, thereby inhibiting gene transcription, whiOKUMURA and FU et al49,50 indicated that intravenous injection of sodium butyrate (NaB) inhibits NF-κB activation and restores the production of tight junction proteins ZO-1 and claudin-1 to block the vicious cycle of cytokine storms, improve intestinal barrier function, and increase the survival rate. A negative correlation was noted between systemic butyric acid levels and sepsis, and an increase in butyric acid levels may improve the condition of sepsis. This study found that Relative to the LPS group, the Agonist group exhibited an enhancement in propionic, butyric, isovaleric, and valeric acid, suggesting that the reduction of intestinal inflammation may have relevance with elevated butyric acid levels. Accumulating evidence supports the critical role of SCFAs in shaping host metabolism through inflammatory pathways in the local and peripheral immune systems. Increasing attention also concerns the potential of SCFAs as critical molecular signals between host and microbiota, or as metabolic substrates for controlling host cell metabolism.
Various junction proteins in intestinal epithelial cells, such as claudin, occludin, ZO-1, ZO-2, etc., form a mechanical barrier for epithelial cells. The integrity of tight junctions is crucial for maintaining intestinal balance.51 SCFAs can upregulate tight junction proteins in septic intestinal tissues, thereby improving intestinal function. The dietary approach based on SCFAs has gradually become a focus of researchers. SCFAs, especially butyrates, are prominent substrates for retaining colonic epithelium. Butyrate is a favorable fuel for colon cells, and intestinal epithelial cells are the main sites of butyrate isolation in vivo.52–54 SCFAs also act in the epithelial barrier integrity by coordinating the tight junction protein modulation. Between the lumen and the hepatic portal vein system, tight junction proteins also modulate the intracellular molecular pathways. The increased permeability is related to the bacteria translocation and/or the bacterial cell wall components, thus triggering related inflammatory cascade reactions.55 Butyric acid seems to be the most important regulatory factor for tight junction proteins and enhances intestinal barrier function via enhancing the claudin-1 and ZO-1 levels and the occludin redistribution.56 As reported, butyrate reverses the abnormal level of ZO-1, reduces LPS translocation, and inhibits neutrophil infiltration, pro-inflammatory cytokine secretion, as well as macrophage activation thereby relieving liver injury in rats.57 Meanwhile, butyric acid can activate the function of PPAR-γ, elevate the expressions of in vivo and in vitro transporters such as MCT-1 and MCT-4, and promote the absorption of SCFAs by intestinal epithelial cells to provide energy for intestinal epithelial cells.58 SCFAs upregulate the expressions of ZO-1, occludin, claudin-3, and claudin-4 by activating AMPK, inhibiting the MLCK/MLC2 pathway, and phosphorylating PKCβ2.59 Mitochondria are known to maintain the energy homeostasis of intestinal epithelial cells.60 SCFAs can significantly promote the genes encoding mitochondrial adenosine triphosphate (ATP) synthase subunits and mitochondrial uncoupling proteins to maintain mitochondrial function.61 Moreover, energy balance disorder is a typical characteristic of inflammatory intestinal tissue. Observation under TEM found that the tight junction structure of the intestinal epithelium in neonatal rats with endotoxemia was damaged, and mitochondria were slightly swollen. CaSR intervention alleviated the damage to the tight junction structure of the intestinal epithelium. These results may be attributed to alternations in the intestinal metabolite composition, contributing to reduced production of SCFAs and damage to tight junction structures. CaSR may regulate this process and participate in the protective effect of the intestinal barrier. This paper adopted LC/MS for revealing the intestinal metabolic profile in neonatal rats with endotoxemia. The metabolic spectrum results showed significant differences in the metabolites between each group, with the differential metabolites mainly related to lipid metabolism including carboxylic acids, phospholipids, and lipid molecules. Through the KEGG topology analysis of the LPS and Agonist groups, we found that the significantly differential metabolites were mainly implicated in sphingolipids metabolism, α-linolenic acid metabolism, and glycerol phospholipid metabolism. CaSR notably augmented the α-linolenic acid abundance. As a polyunsaturated fatty acid with an anti-inflammatory effect, α-linolenic acid plays an anti-inflammatory and antioxidant role in a variety of ways, including acting as a substrate for cyclooxygenase/oxygenase to regulate the production of lipid mediators such as eicosanoid.62,63 CaSR may alleviate inflammatory responses by regulating a variety of unsaturated fatty acid metabolic pathways.
To summarize, Targeted and non-targeted metabolomics analysis indicates that CaSR may be involved in regulating the production of SCFAs and a wide range of metabolites. Specific alternations in the microbiota-metabolism axis may contribute to the protective influence of CaSR on the intestinal barrier in endotoxemia.
Conclusion
In conclusion, our results demonstrate that CaSR agonists alter intestinal metabolites and ameliorate intestinal damage caused by endotoxemia. However, it is unclear how CaSR acts on endotoxemia through metabolite changes, possibly by affecting the synthesis of SCFAs and fecal metabolites to reduce intestinal damage, and further evidence is needed.
Data Sharing Statement
Data supporting the outcomes of this work can be acquired from the corresponding author following reasonable requirements.
Ethics Approval and Informed Consent
The authors are responsible for the work to ensure that questions relating to the accuracy or completeness of the work are properly examined and resolved. The operations were ratified by the Ethics Committee of First Affiliated Hospital of Harbin Medical University [IACUC No. 2022114] and followed the Guide for the Care and Use of Laboratory Animals.
Acknowledgments
We are thankful to the reviewers for their instructive comments on this work.
Author Contributions
C.J. finished study design, Y.S., X.L., M.J. finished experiments on animals, J.S., F.M. finished data analysis, Y.S., J.S. finished manuscript editing. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the Innovation Fund of the First Hospital of Harbin Medical University [grant numbers 2021M21], and the Zhuhai Science and Technology project in the field of social development [grant numbers 2220004000335 and 2320004000185].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi:10.1016/S0140-6736(14)61698-6
2. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi:10.1016/S0140-6736(12)60560-1
3. Kayagaki N, Wong MT, Stowe IB, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi:10.1126/science.1240248
4. Santos RP, Tristram D. A practical guide to the diagnosis, treatment, and prevention of neonatal infections. Pediatr Clin North Am. 2015;62(2):491–508. doi:10.1016/j.pcl.2014.11.010
5. Bersani I, Speer CP. Nosocomial sepsis in neonatal intensive care: inevitable or preventable? Z Geburtshilfe Neonatol. 2012;216(4):186–190. doi:10.1055/s-0032-1321837
6. Yoseph BP, Klingensmith NJ, Liang Z, et al. Mechanisms of intestinal barrier dysfunction in sepsis. Shock. 2016;46(1):52–59. doi:10.1097/SHK.0000000000000565
7. Chavez-Abiega S, Mos I, Centeno PP, et al. Sensing extracellular calcium - an insight into the structure and function of the calcium-sensing receptor (CaSR). Adv Exp Med Biol. 2020;1131:1031–1063.
8. Owen JL, Cheng SX, Ge Y, et al. The role of the calcium-sensing receptor in gastrointestinal inflammation. Semin Cell Dev Biol. 2016;49:44–51. doi:10.1016/j.semcdb.2015.10.040
9. Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu Rev Physiol. 2009;71:205–217. doi:10.1146/annurev.physiol.010908.163128
10. Elajnaf T, Iamartino L, Mesteri I, et al. Nutritional and pharmacological targeting of the calcium-sensing receptor influences chemically induced colitis in mice. Nutrients. 2019;11(12):3072. doi:10.3390/nu11123072
11. Cheng SX, Lightfoot YL, Yang T, et al. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014;588(22):4158–4166. doi:10.1016/j.febslet.2014.05.007
12. Geibel J, Sritharan K, Geibel R, et al. Calcium-sensing receptor abrogates secretagogue- induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci U S A. 2006;103(25):9390–9397. doi:10.1073/pnas.0602996103
13. Wang Z, Xu CM, Liu YX, et al. Characteristic dysbiosis of gut microbiota of Chinese patients with diarrhea-predominant irritable bowel syndrome by an insight into the pan-microbiome. Chin Med J. 2019;132(8):889–904. doi:10.1097/CM9.0000000000000192
14. Agahi A, Hamidi GA, Daneshvar R, et al. Does severity of alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front Neurol. 2018;9:662. doi:10.3389/fneur.2018.00662
15. Xiao K, Sun Y, Song J, et al. Gut microbiota involved in myocardial dysfunction induced by sepsis. Microb Pathog. 2023;175:105984. doi:10.1016/j.micpath.2023.105984
16. Liu Z, Wang N, Ma Y, et al. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front Microbiol. 2019;10:390. doi:10.3389/fmicb.2019.00390
17. Zhang B, Yue R, Chen Y, et al. Gut microbiota, a potential new target for Chinese herbal medicines in treating diabetes mellitus. Evid Based Complement Alternat Med. 2019;2019:2634898. doi:10.1155/2019/2634898
18. Abhari K, Shekarforoush SS, Hosseinzadeh S, et al. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr Res. 2016;60:30876. doi:10.3402/fnr.v60.30876
19. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi:10.1038/nature08821
20. Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi:10.1146/annurev-immunol-020711-074937
21. Indiani C, Rizzardi KF, Castelo PM, et al. Childhood obesity and firmicutes/bacteroidetes ratio in the gut microbiota: a systematic review. Child Obes. 2018;14(8):501–509. doi:10.1089/chi.2018.0040
22. Bach Knudsen KE, Laerke HN, Hedemann MS, et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients. 2018;10(10):1499. doi:10.3390/nu10101499
23. Tian L, Zhou X-Q, Jiang W-D, et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017;66:548–563. doi:10.1016/j.fsi.2017.05.049
24. Sharma S, Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J Nutr Biochem. 2019;63:101–108. doi:10.1016/j.jnutbio.2018.10.003
25. Zhou H, Sun L, Zhang S, et al. The crucial role of early-life gut microbiota in the development of type 1 diabetes. Acta Diabetol. 2021;58(3):249–265. doi:10.1007/s00592-020-01563-z
26. Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi:10.1016/j.cell.2016.05.041
27. McNabney SM, Henagan TM. Short Chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients. 2017;9(12):1348. doi:10.3390/nu9121348
28. Peng L, Li ZR, Green RS, et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625. doi:10.3945/jn.109.104638
29. Fodor RS, Georgescu AM, Cioc AD, et al. Time- and dose-dependent severity of lung injury in a rat model of sepsis. Rom J Morphol Embryol. 2015;56(4):1329–1337.
30. Guo F, Yan CY. Effect of SecinH3 on lung injury induced by sepsis of rats. Asian Pac J Trop Med. 2015;8(12):1049–1054. doi:10.1016/j.apjtm.2015.11.004
31. da Fonseca LM, Reboredo MM, Lucinda LM, et al. Emphysema induced by elastase enhances acute inflammatory pulmonary response to intraperitoneal LPS in rats. Int J Exp Pathol. 2016;97(6):430–437. doi:10.1111/iep.12214
32. Miao L, Gong Y, Li H, et al. Alterations in cecal microbiota and intestinal barrier function of laying hens fed on fluoride supplemented diets. Ecotoxicol Environ Saf. 2020;193:110372. doi:10.1016/j.ecoenv.2020.110372
33. Park CH, Seo JH, Lim JY, et al. Changing trend of neonatal infection: experience at a newly established regional medical center in Korea. Pediatr Int. 2007;49(1):24–30. doi:10.1111/j.1442-200X.2007.02310.x
34. Li Q, Wang C, Tang C, et al. Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Crit Care. 2015;19(1):37. doi:10.1186/s13054-015-0738-7
35. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
36. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11(8):e0161174. doi:10.1371/journal.pone.0161174
37. DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-Resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050. doi:10.1056/NEJMoa1910437
38. Prescott HC, Dickson RP, Rogers MA, et al. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. 2015;192(5):581–588. doi:10.1164/rccm.201503-0483OC
39. Ojima M, Motooka D, Shimizu K, et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig Dis Sci. 2016;61(6):1628–1634. doi:10.1007/s10620-015-4011-3
40. He J, Zhang P, Shen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17):6356. doi:10.3390/ijms21176356
41. Rabassa AA, Rogers AI. The role of short-chain fatty acid metabolism in colonic disorders. Am J Gastroenterol. 1992;87(4):419–423.
42. Nastasi C, Candela M, Bonefeld CM, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5:16148. doi:10.1038/srep16148
43. Macia L, Tan J, Vieira AT, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi:10.1038/ncomms7734
44. Vernia P, Fracasso PL, Casale V, et al. Topical butyrate for acute radiation proctitis: randomised, crossover trial. Lancet. 2000;356(9237):1232–1235. doi:10.1016/S0140-6736(00)02787-2
45. Vernia P, Annese V, Bresci G, et al. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest. 2003;33(3):244–248. doi:10.1046/j.1365-2362.2003.01130.x
46. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi:10.1038/nature11450
47. Luhrs H, Gerke T, Muller JG, et al. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37(4):458–466. doi:10.1080/003655202317316105
48. Maeda T, Towatari M, Kosugi H, et al. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96(12):3847–3856. doi:10.1182/blood.V96.12.3847
49. Okumura T, Nozu T, Ishioh M, et al. Centrally administered butyrate improves gut barrier function, visceral sensation and septic lethality in rats. J Pharmacol Sci. 2021;146(4):183–191. doi:10.1016/j.jphs.2021.04.005
50. Fu J, Li G, Wu X, et al. Sodium butyrate ameliorates intestinal injury and improves survival in a rat model of cecal ligation and puncture-induced sepsis. Inflammation. 2019;42(4):1276–1286. doi:10.1007/s10753-019-00987-2
51. Gao Y, Li S, Wang J, et al. Modulation of intestinal epithelial permeability in differentiated caco-2 cells exposed to aflatoxin M1 and ochratoxin A individually or collectively. Toxins. 2017;10(1):13. doi:10.3390/toxins10010013
52. Lou X, Xue J, Shao R, et al. Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances. Front Immunol. 2022;13:1063543. doi:10.3389/fimmu.2022.1063543
53. van der Beek CM, Bloemen JG, van den Broek MA, et al. Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J Nutr. 2015;145(9):2019–2024. doi:10.3945/jn.115.211193
54. Clausen MR, Mortensen PB. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995;37(5):684–689. doi:10.1136/gut.37.5.684
55. Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi:10.2337/db07-1403
56. Wang HB, Wang PY, Wang X, et al. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57(12):3126–3135. doi:10.1007/s10620-012-2259-4
57. Liu B, Qian J, Wang Q, et al. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS One. 2014;9(8):e106184. doi:10.1371/journal.pone.0106184
58. Ziegler K, Kerimi A, Poquet L, et al. Butyric acid increases transepithelial transport of ferulic acid through upregulation of the monocarboxylate transporters SLC16A1 (MCT1) and SLC16A3 (MCT4). Arch Biochem Biophys. 2016;599:3–12. doi:10.1016/j.abb.2016.01.018
59. Jirsova Z, Heczkova M, Dankova H, et al. The effect of butyrate-supplemented parenteral nutrition on intestinal defence mechanisms and the parenteral nutrition-induced shift in the gut microbiota in the rat model. Biomed Res Int. 2019;2019:7084734. doi:10.1155/2019/7084734
60. Meex RCR, Blaak EE. Mitochondrial dysfunction is a key pathway that links saturated fat intake to the development and progression of NAFLD. Mol Nutr Food Res. 2021;65(1):e1900942. doi:10.1002/mnfr.201900942
61. Kim CH. B cell-helping functions of gut microbial metabolites. Microb Cell. 2016;3(10):529–531. doi:10.15698/mic2016.10.536
62. Mayer K, Schaefer MB, Seeger W. Fish oil in the critically ill: from experimental to clinical data. Curr Opin Clin Nutr Metab Care. 2006;9(2):140–148. doi:10.1097/01.mco.0000214573.75062.0a
63. Martin JM, Stapleton RD. Omega-3 fatty acids in critical illness. Nutr Rev. 2010;68(9):531–541. doi:10.1111/j.1753-4887.2010.00313.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.