Back to Journals » Drug Design, Development and Therapy » Volume 17
Caged Polyprenylated Xanthones in Garcinia hanburyi and the Biological Activities of Them
Authors He R, Jia B , Peng D, Chen W
Received 20 June 2023
Accepted for publication 2 November 2023
Published 5 December 2023 Volume 2023:17 Pages 3625—3660
DOI https://doi.org/10.2147/DDDT.S426685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Ruixi He, Buyun Jia, Daiyin Peng, Weidong Chen
School of Pharmacy, Anhui University of Chinese Medicine, Hefei, People’s Republic of China
Correspondence: Ruixi He, School of Pharmacy, Anhui University of Chinese Medicine, No. 350 Longzihu Road, Xinzhan District, Hefei, 230012, People’s Republic of China, Tel +8613637080511, Fax +8655168129099, Email [email protected]
Abstract: The previous phytochemical analyses of Garcinia hanburyi revealed that the main structural characteristic associated with its biological activity is the caged polyprenylated xanthones with a unique 4-oxatricyclo [4.3.1.03,7] dec-2-one scaffold, which contains a highly substituted tetrahydrofuran ring with three quaternary carbons. Based on the progress in research of the chemical constituents, pharmacological effects and modification methods of the caged polyprenylated xanthones, this paper presents a preliminary predictive analysis of their drug-like properties based on the absorption, distribution, metabolism, excretion and toxicity (ADME/T) properties. It was found out that these compounds have very similar pharmacokinetic properties because they possess the same caged xanthone structure, the 9,10-double bond in a,b-unsaturated ketones are critical for the antitumor activity. The author believes that there is an urgent need to seek new breakthroughs in the study of these caged polyprenylated xanthones. Thus, the research on the route of administration, therapeutic effect, structural modification and development of such active ingredients is of great interest. It is hoped that this paper will provide ideas for researchers to develop and utilize the active ingredients derived from natural products.
Keywords: caged polyprenylated xanthones, pharmacological effects, antitumor, modification, ADME/T properties
Graphical Abstract:
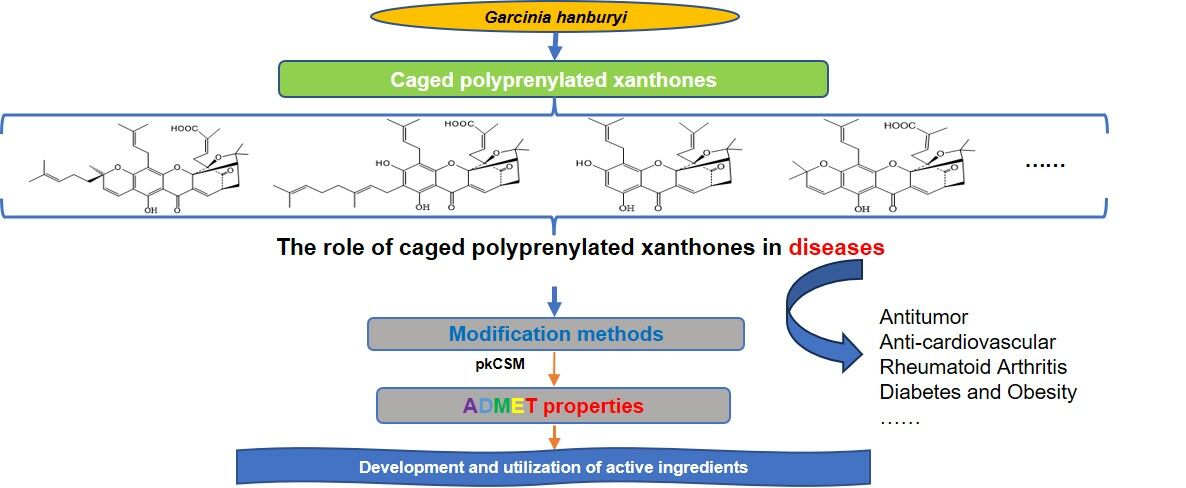
Introduction
Garcinia hanburyi is widely distributed in the tropical rainforests of Southeast Asia. Gamboge is the dried resin exuded from the stems of Garcinia hanburyi that can be used as a pigment and has also been used for a long time as a folk medicine.1 In China, people are increasingly concerned about the safety of toxic herbs. Garcinia hanburyi is regulated as a toxic drug for medical use, and it requires special modification for clinical use. The previous phytochemical analyses of Garcinia hanburyi revealed that the main structural characteristic associated with its biological activity is the caged polyprenylated xanthones with a specialized 4-oxatricyclo [4.3.1.03,7] dec-2-one scaffold,2–9 which contains a highly substituted tetrahydrofuran ring.10 We have compiled the relevant literature and found out that more than 50 different xanthones have now been extracted from gamboge. The available evidence suggests that gamboge has anticancer properties, with gambogenic acid (GNA) and gambogic acid (GA) being the main components responsible for these activities. They have been demonstrated to exert cytotoxic activities through a variety of mechanisms.11–14 Among them, GA has been applied to treat a wide range of cancers, such as breast, liver, gastric, lung, colon, and skin cancers. Its therapeutic effects have been well established,15 and it has been shown to exert anticancer effects through apoptosis induction, cell cycle arrest, telomerase and angiogenesis inhibition.16,17 In recent years, increasing evidence has demonstrated that GNA exhibits higher antitumor activity and lower toxicity compared to GA, and the extraction process is simple and less costly.18–21 Despite these advantages, GNA has not been approved for clinical application mainly due to its poor aqueous solubility and low bioavailability.22 With the aim of overcoming these limitations, researchers have carried out various studies such as on the technology nanocarrier drug delivery, to improve the bioavailability of GNA.
Due to the diversity and potent activities of caged polyprenylated xanthones extracted from Garcinia hanburyi, a series of in-depth studies have been conducted by researchers all over the world. Our team has conducted study on these bioactive ingredients. Our work covers many aspects, including compound isolation, pharmacokinetics, and formulation. In our previous studies, several highly fascinating methods were found to improve the therapeutic efficacy of GNA, including the use of solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs),23 liquid crystal dispersions,24 PEGylated liposomes, PEGylated nonionic surfactant vesicles,25 and folic acid-modified nonionic surfactant vesicles.26 More than 50 different types of caged polyprenylated xanthones have been extracted from Garcinia hanburyi, but researchers have mostly focused more on GNA and GA. However, the basic properties of the other ingredients, particularly their absorption, distribution, metabolism, excretion and toxicity (ADME/T) properties, all of which are the key factors affecting the efficacy of drugs in vivo, are unclear. However, in the development of new drugs, the evaluation of the physicochemical properties, such as ADME/T properties, is limited by a number of factors, such as the high economic cost and the long time required. Therefore, these properties are usually considered at the stage of clinical research. According to statistics, 40% of drug candidates are eliminated from further development due to poor bioavailability, pharmacokinetic properties or toxicity.27 Based on a collection of reliable experimental data as reported, a computer program was developed to effectively predict the ADME/T properties of bioactive ingredients.28 Compared with the traditional experiments in vivo, computer programs can process multiple active ingredients in batches and predict their ADME/T properties by simply providing the structure of the compound, thus making this process more efficient and less costly.29 PkCSM software is a distance-based graphical feature that can be used to predict and optimize the pharmacokinetic properties and toxicity of small molecules. It consists of 30 predictors divided into five major classes: absorption (7 predictors), distribution (4 predictors), metabolism (7 predictors), excretion (2 predictors), and toxicity (10 predictors).30 To start forecasting, only with the SMILES code of the compound, it enables easy and rapid early stage assessment of compounds. Under the pkCSM program, the ADME/T properties of 51 caged polyprenylated xanthones derived from Garcinia hanburyi were predicted and summarized. It is hoped that this will promote the development and utilization of natural products.
Chemical Structures of the Xanthones in Garcinia hanburyi
To predict and compare the ADME/T properties of the caged polyprenylated xanthones derived from Garcinia hanburyi more comprehensively, we summarized 51 of them that had been isolated to date. The caged polyprenylated xanthones currently known derived from Garcinia hanburyi are listed in Table 1. As typical caged polyprenylated xanthones in Garcinia hanburyi, GA (Compound 9) and GNA (Compound 36) are of great interest for their proliferation inhibitory effects on a variety of tumor cells.
 |
Table 1 Chemical Structures of the Caged Polyprenylated Xanthones Derived from Garcinia hanburyi |
The Role of Caged Polyprenylated Xanthones in Diseases
Antitumor
In recent years, in vitro and in vivo experiments have shown that the caged polyprenylated xanthones extracted from Garcinia hanburyi exhibits anti-tumor effects, the anti-tumor effects of GA and GNA are mainly achieved through induction of apoptosis, cell cycle arrest and inhibition of tumor cell invasion and migration, and the compounds of gambogefic acid, 7-methoxygambogellic acid, 7-methoxygambogic acid, 7-methoxyepigambogic acid, 8,8a-dihydro-8-hydroxymorellic acid, 8,8a-dihydro-8-hydroxygambogenic acid, oxygambogic acid, gambogenific acid, 7-methoxyisomorellinol, 8,8a-dihydro-8-hydroxygambogic acid also have inhibitory effects on cancer cells.4
Induction of Apoptosis
It was demonstrated that GA induces apoptosis in non-small cell lung cancer (NSCLC) A549 cells by upregulating the expression of pro-apoptotic genes BAX and PUMA and downregulating the expression of anti-apoptotic gene BCL-2 through transcription factor P53.35 In addition, GA can increase the sensitivity of MCF-7 to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and promoted TRAIL-induced apoptosis in breast cancer cells by enhancing the activity of caspase-3 and caspase-8.36 GA can also induce apoptosis in glioblastoma by increasing the levels of the pro-apoptotic proteins BAX and apoptosis-inducing factor (AIF), and this is a non-caspase-related apoptotic pathway.37,38 The Janus kinase-signal transducers and activators of transcription (JAK-STAT) signaling pathway are closely related to tumor cell apoptosis, and it was shown that GA could induce apoptosis of esophageal cancer cells by inhibiting the JAK-STAT signaling pathway.39 The mechanism of GNA-induced apoptosis in tumor cells has also received considerable attention in recent years, studies have shown that GNA can induce apoptosis in breast cancer cells through the mitochondrial pathway, it can also induce apoptosis by inhibiting the expression of the anti-apoptotic protein BCL-2 and activating apoptosis-related proteins.40,41
Blocking the Cell Cycle
Cell cycle factor is an important target for tumor treatment. Several studies show that caged polyprenylated xanthones in Garcinia hanburyi can block the tumor cell cycle. GNA can induce apoptosis by downregulating the expression of cyclin-dependent kinases (CDKs) that arrest the cell cycle in the G1 phase and subsequently activate caspases.42 GA can induce mRNA expression of genes related to cell cycle arrest, thereby causing cells to arrest in the G0/G1 phase.43
Inhibit the Invasion and Metastasis of Tumor Cells
Invasion and metastasis of tumor cells are the main reasons for the poor prognosis of patients. Experimental results have shown that GA can reduce the invasion of breast cancer cells and colon cancer cells, follow-up mechanistic studies revealed that this may be related to the c-Jun N-terminal kinase (JNK) signaling pathway, which increases the secretion of matrix metalloproteinases (MMPs) in cancer cells, disrupts the extracellular matrix, decreases intercellular adhesion, and thus promotes invasion and metastasis of cancer cells.44 GNA and isomorellin were found to attenuate the migration and invasion of tumor cells by inhibiting the NF-κB pathway.45,46
Inhibit Angiogenesis
It was revealed that GNA could significantly reduce p-PI3K, p-AKT, and vascular endothelial growth factor (VEGF) expression, further experiments showed that GNA inhibits angiogenesis through the PTENPI3K/AKT/VEGF/eNOS pathway.47 In addition, it was also demonstrated that morellic acid, gambogenin and isogambogenic acid showed comparable antiangiogenic activities with less toxicities than GA.48
Induction of Cellular Autophagy
In recent years, autophagy has received extensive attention in numerous researches of antitumor drugs. GA could induce significant upregulation the expression of autophagy-related factors ATG7, BECLIN-1 and LC3-II in acute lymphoid leukemia cells, while inhibiting Wnt/β-catenin signaling, thus further inhibiting cell growth.49 It was observed that glioma cells treated with GNA showed increased expression of autophagic proteins and increased secretion of autophagic vesicles, suggesting that GNA can induce autophagy in tumor cells, thereby inhibiting tumor growth.50
Other Mechanism Studies
The accumulation of reactive oxygen species (ROS) has an important impact on the development of tumors. It was suggested that GA can induce the accumulation of ROS in tumor cells, which may be related to the ability of GA to inhibit cytosolic thioredoxin (TRX-1) and mitochondrial thioredoxin (TRX2) distributed in the cytoplasm and mitochondrion, which play a key role in maintaining ROS homeostasis.51–53 Recent studies have observed that GA kills cancer cells by inducing a vacuolization-associated cell death, and this phenomenon may be associated with GA-induced proteasomal inhibition leads to the endoplasmic reticulum (ER) dilation and ER stress in treated cancer cells.54
Anti-Cardiovascular Diseases
Currently, cardiovascular disease (CVD) is a great threat to human health. Previous studies have shown that the inflammation and risk of cardiovascular diseases have a strong consistent relationship, and this result has been proven by clinical trials and epidemiological studies.55 Studies have concluded that the activated pro-inflammatory cytokines, oxidative stress and inflammation and C-reactive protein (CRP) are key mechanisms in the development of CVD.56 Fu et al57 evaluated the role of neoglycyrrhetinic acid in sepsis-associated myocardial injury, and they discovered that GNA exerts anti-apoptotic, anti-fibrotic and anti-inflammatory effects in septic mice through inactivation of the MAPK/NF-κB pathway. Studies have shown that GA can inhibit cardiac hypertrophy and fibrosis induced by pressure or isoprenaline infusion by inhibiting the NF-κB pathways and proteasome, indicating that GA therapy may a new strategy for the treatment of cardiac hypertrophy and fibrosis.58
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic, systemic disease characterized by inflammatory synovitis. It is characterized by polyarticular, symmetric, aggressive joint inflammation of the small joints of the hands and feet, often accompanied by extra-articular organ involvement and positive serum rheumatoid factor, which can lead to deformities of the joints. There is no specific treatment for rheumatoid arthritis. The aim of treatment is to maintain joint mobility and coordinated function, and different therapies are used at different stages of the disease. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used to relieve pain and inflammation in the acute phase of RA. Early findings suggest that the ethyl acetate extract of gamboge appears to have a mechanism of action similar to NSAIDs rather than to steroids.59 Subsequent studies have demonstrated that GA is one of the NSAIDs that inhibit the development of RA by suppressing the levels of inflammatory molecules and cytokines.60 Wu et al61 revealed that the anti-inflammatory effect of GA in RA rats was mediated by modulation of the PI3K/Akt/mTOR signaling pathway.
Diabetes and Obesity
AMP-activated protein kinase (AMPK), an AMP-dependent protein kinase, is a key factor in the regulation of biological energy metabolism and also a key factor in the study of metabolism-related diseases, such as type 2 diabetes and obesity.62,63 Genetic and pharmacological studies have shown that AMPK is essential for the body to maintain glucose homeostasis. Zhao et al63 demonstrated for the first time that GA activates the AMPK signaling pathway by directly interacting with AMPK. Protein tyrosine phosphatase 1B (PTP1B) is involved in maintaining the balance of tyrosine protein phosphorylation and negatively regulates insulin signaling.34 The active compounds extracted from Garcinia hanburyi such as GA, moreollic acid, morellic acid, 10-methoxygambogenic acid, GNA, GA, morellinol, 10-methoxygambogin and desoxymorellin, were identified to be PTP1B inhibitors, they inhibit PTP1B in dose-dependent manner, the binding sites of GA and morellinol with PTP1B were studied, and it was revealed that the inhibitory activities are highly correlated with the caged motif and prenyl group in A ring.
Other Diseases
Recent research has shown that GNA ameliorates cardiac injury and dysfunction in LPS-induced septic mice by inhibiting cardiac apoptosis, fibrosis and inflammation through downregulation of p-JNK and p-NF-κB.57 In a recent work, binding affinities of xanthone compounds which including morellic acid, gambogellic acid, GNA, moreollic acid with SARS-CoV-2 main protease (Mpro) were predicted using the molecular docking technique, the results showed that morellic acid has a high-binding affinity towards SARS-CoV-2 Mpro, and this suggests that MA is a promising candidate for anti-COVID-19; however, this requires further detailed in vivo experimental estimation and clinical evaluation.64
Pharmacokinetic Studies and Countermeasures for Improving the Drug-Like Properties of Caged Polyprenylated Xanthones
To effectively utilize the curative effects of caged polyprenylated xanthones in clinic, an in vivo pharmacokinetic study of the drug is essential. In previous studies, GA was administered via intravenous injection; however, GA has an extremely short half-life in plasma.17,65 Administering GA intravenously to patients to treat tumors leads to side effects such as cardiotoxicity, liver damage and phlebitis.66 For these reasons, GA was not evaluated in Phase III clinical trials as an intravenous antitumor agent. In addition, the previous study found that, GA is poorly absorbed after gastrointestinal administration in rats and has low bioavailability in vivo; studies have shown that, after oral administration, GA is toxic to various rat organs.67,68 In view of the above-mentioned drawbacks, the clinical applications of GA administered via intravenous injection and orally are limited. Actually, many active ingredients of TCM with antitumor activity have strong hydrophobicity, and the conventional solubilization techniques cannot meet the needs of the development of insoluble drugs. To address these challenges, multiple studies have been conducted to refine the pharmacokinetic and pharmacodynamic performance of GA, chemical structure modifications, combination therapy and different types of nanoscale drug delivery systems (Table 2) and have been employed to modify or encapsulate GA while avoiding vascular irritation and organ toxicity in vivo. Rational medicinal modifications on GA will improve its physicochemical properties and drug-like characters. The chemical structural of GA is shown in Figure 1. Previous structure modifications of GA mainly focused on the 9,10-double carbon bond of a,b-unsaturated ketone, 6-hydroxyl group, isopentenyl groups and the 30-carboxyl group. It was found that the 9,10-double bond in a,b-unsaturated ketones is essential for the apoptosis-inducing activity, and the replacement of the acidic carboxyl group with ester and amide does not have much effect on the activity, it is also suggests that the hydrophilic face of GA may not have much relevance for its binding to biological targets. With the development of nano drug delivery systems, more and more elaborate and complex drug delivery systems are being designed, researchers have conducted studies on various nano-delivery systems of GA, including passive targeting, active targeting, tumor microenvironment response and bionic targeting, for example, the aqueous solubility of GA can be improved by chemical conjugation to a water-soluble polymer such as polyethylene glycol (PEG),69 Moreover, in order to control the release of GA, enhance its accumulation at tumor sites, and reduce side effects, multifunctional nanoparticles of GA with pH-sensitive and redox-responsive sensitivities as well as receptor-targeted responses were developed. However, more attention should be paid to the in vivo degradation and systemic toxicity of excipients used in these preparations, especially administered via intravenous injection. To date, no related oral or intravenous preparations of GA that have successfully passed clinical trials for market approval. In recent years, people have continued to explore new routes to deliver GA with improved bioavailability and reduced toxicity to serve as a breakthrough in tumor treatment. In addition to intravenous and oral administration, recent attention has been focused on improving the effectiveness of GNA through local delivery. Previously, researchers demonstrated that localized administration of GA with the help of chemical penetration enhancers could be a safe and effective therapy for the treatment of melanoma.70 In their follow-up study, compared with chemical penetration enhancers, ultrasound, and intravenous injection, GA exhibited the strongest antimelanoma activity with combined chemical penetration enhancers and ultrasound administration, as chemical penetration enhancers can increase the cavitation effect of US.71
 |
Table 2 GA Modifications |
 |
Figure 1 The chemical structure of GA. |
GNA is another major active ingredient extracted from the resin of gamboge, exhibits broader antitumor activity and less systemic toxicity than GA.72 To date, in vivo pharmacokinetic results in rats have shown that GNA is as poorly absorbed as GA after intragastric administration of Garcinia hanburyi extract. Additionally, the pharmacokinetic data of these two structurally similar xanthones are comparable, which means that slight changes in the position of the substituent on the alkyl side chain do not appreciably affect the in vivo pharmacokinetic properties of the compound.65,67,73–75 With the aim of overcoming the in vivo pharmacokinetic shortcomings of GNA for cancer therapy, recent studies have been trying to modify GNA with the aid of nanocarriers to improve its bioavailability and reduce its toxicity (Table 3). In 2013, our group prepared GNA-SLNs and compared the pharmacokinetic characteristics in rats after intraperitoneal injection of GNA solution and GNA-SLNs.76 Additionally, colloidal delivery systems were successfully fabricated for the targeted delivery of GNA. As demonstrated by the pharmacokinetic assay, after being encapsulated by the nano-delivery system, the residence time of GNA in the blood circulation was prolonged; in addition, the antitumor ability of the encapsulated GNA was significantly enhanced. In conclusion, the results of this study suggest that the nano-delivery system can potentially be used to deliver GNA. Notably, intravenous administration increases vascular damage and causes recurrent pain due to the vascular irritability of GA. As an attractive alternative, oral administration offers the following advantages, for instance, various dosage forms are available, relatively low production costs, ease of production and good patient compliance.77 Researchers have also designed oral dosage forms of GNA, such as, polydopamine nanoparticles were prepared for encapsulating and stabilizing GNA coated with sodium alginate after the modification of folic acid to achieve antitumor effect after oral administration and to improve the water solubility, bioavailability and tumor targeting of GNA.78 Our subject group also successfully isolated and extracted another component of Garcinia hanburyi, morellic acid (MA) (Compound 3, Table 1), which has also shown a good antitumor effect. As predicted, MA also has unfavorable pharmacokinetics; therefore, our group prepared MA NLCs to conquer this problem.79 However, the feasibility of producing this nano-delivery system, as well as the in vivo degradation and systemic toxicity of the excipients, need to be further investigated.
 |
Table 3 GNA Modifications |
In recent years, studies have shown that water processing could alter the bioavailability of five caged xanthones in Garcinia hanburyi, which could attenuate toxicity and increase their effects.80 Apart from the abovementioned studies, very few pharmacokinetic studies of other caged xanthones have been reported.
Interpretation of the Prediction Model
Physical and Chemical Properties
The pkCSM prediction results of physical and chemical properties (Table 4) show that the molecular weights of these caged polyprenylated xanthones are all greater than 500 with the exception of forbesione (Compound 46), the number of hydrogen bond acceptors is less than 10, the number of hydrogen bond donors is less than 5, and the logP values are between 5.0 and 8.5. The above parameters partially conform to the rule of Lipinski,174 and among these parameters, their drug-like properties are mainly limited by their larger molecular weight and higher lipid solubility.
 |
Table 4 Predicted Physical and Chemical Properties for the Caged Polyprenylated Xanthones in Garcinia hanburyi Provided by pkCSM |
Absorption
The absorption parameter (Table 5) results show that these caged polyprenylated xanthones have poor water solubility, and lipid-soluble drugs are not absorbed as well as those that are water-soluble, especially after administration via the gastrointestinal tract.175 Additionally, these compounds have different degrees of Caco-2 cell permeability. In the pkCSM predictive model, high Caco-2 permeability would give predicted values greater than 0.90. Thus, GA and GNA are predicted to have high Caco-2 permeability. The intestinal absorption rates of these compounds ranged from 65% to 100%, well above the low intestinal absorption threshold of 30%, and they are considered to be well absorbed. These compounds also have certain skin permeability (they are not easily absorbed through the skin if the value is greater than −2.5).176 Notably, most of these compounds are substrates or inhibitors of p-glycoprotein (P-gp), suggesting that they may be excreted from cells by P-gp, which would lead to drug resistance.177 As P-gp inhibitors, these compounds may have significant pharmacokinetic implications for P-gp substrates, and may either be exploited for specific therapeutic advantages or result in contraindication.
 |
Table 5 Predicted Absorption Properties for the Caged Polyprenylated Xanthones in Garcinia hanburyi Provided by pkCSM |
Distribution
In terms of distribution (Table 6), the steady-state volume of distribution (VDss) values of these xanthones are between −0.478 and 0.755, with those of GA and GNA being −0.154 and −0.478, respectively. The higher the VDss is, the more a drug is distributed in tissue rather than plasma.178 This means that the compound would be cleared quickly with a short retention time in vivo if the VDss value is less than −0.15. It can be seen from the unbound fraction179,180 that most of these compounds bind to serum proteins. The VDss values and unbound fraction jointly predict that these caged polyprenylated xanthones have a short residence time, are eliminated quickly, and do not accumulate easily in vivo. The predicted values of blood‒brain barrier (BBB) permeability (logBB) for these compounds are less than 0.3, which means that none of these compounds can easily cross the BBB. Notably, the logBB values for GNA and GA are less than −1, which means that they are poorly distributed to the brain. The predicted extent of BBB permeability and central nervous system (CNS) permeability suggest that these compounds do not easily penetrate the BBB or enter the CNS, and therefore, they do not produce side effects on the brain.
 |
Table 6 Predicted Distribution Properties for the Caged Polyprenylated Xanthones in Garcinia hanburyi Provided by pkCSM |
Metabolism
In terms of metabolism (Table 7), cytochrome P450s (CYP450s) are an important class of enzymes involved in the metabolism of exogenous substances and mainly found in the liver. The two main isoforms responsible for drug metabolism are 2D6 and 3A4. Many drugs are deactivated by CYP450s; however, some are activated by these enzymes, which may lead to excessive drug accumulation if the compound is a CYP450 inhibitor.181 The prediction results indicated that these caged polyprenylated xanthones are substrates of CYP3A4 with the exception of forbesione (Compound 46), and some of them, including GNA, are also inhibitors of CYP3A4. This implies that when these xanthones are co-administered with drugs that are CYP3A4 substrates, they will interfere with metabolism and may induce drug accumulation in vivo, leading to toxicity.
 |
Table 7 Predicted Metabolism Properties for the Caged Polyprenylated Xanthones in Garcinia hanburyi Provided by pkCSM |
Excretion
The excretion section (Table 8) describes the total clearance of the caged polyprenylated xanthones and whether they are organic cation transporter 2 (OCT2) substrates. Total clearance is related to bioavailability, which is important when determining dosing rates so that steady-state concentrations can be achieved. OCT2 is a renal uptake transporter that plays important roles in the disposition and renal clearance of drugs and endogenous compounds.182,183 From the predicted excretion data, these caged polyprenylated xanthones are not substrates of OCT2 and thus have a low risk of nephrotoxicity.
 |
Table 8 Predicted Excretion Properties for the Caged Polyprenylated Xanthones in Garcinia hanburyi Provided by pkCSM |
Toxicity
In terms of toxicity (Table 9), these caged polyprenylated xanthones are not hERG inhibitors and therefore have no cardiotoxicity, were predicted to be negative in the AMES test and skin sensitivity test, and thus have no mutagenicity and do not irritate the skin; however, these compounds have certain Tetrahymena pyriformis and minnow toxicity. T. pyriformis is a protozoan bacterium with nutritional requirements, subcellular organelles and biochemical pathways similar to those of mammalian cells.184 This organism is commonly used to predict drug toxicity, and a predicted value greater than −0.5 is considered toxic. The value of minnow toxicity represents the concentration of a molecule that is necessary to cause the death of 50% of flathead minnows. This predicted value was below −0.3 for all of the caged polyprenylated xanthones, indicating that they may have aquatic toxicity.
 |
Table 9 Predicted Toxicity for the Caged Polyprenylated Xanthones in Garcinia hanburyi Provided by pkCSM |
Conclusion
In recent years, a lot of researches have been conducted on the pharmacological effects and formulation of caged polyprenylated xanthones in Garcinia hanburyi, with plenty of results achieved. However, the pharmacological study of the caged polyprenylated xanthones is still not deep enough, and there is no systematic research conducted on the quality standards and in vivo processes of active ingredients in Garcinia hanburyi. Based on the progress in research of the chemical constituents, pharmacological effects and modification methods of the caged polyprenylated xanthones, this paper presents a preliminary predictive analysis of their drug-like properties based on the ADME/T properties. These compounds have disadvantageous physical and chemical properties, including a large molecular weight, poor water solubility and low bioavailability in vivo, which is an obstacle to developing new drugs through the use of active ingredients contained in natural products. For the caged xanthones in Garcinia hanburyi, the author believes that subsequent studies could be carried out by considering the following points. (1) The new dosage forms and routes of administration. Currently, these compounds are mainly considered for injectable formulations. For example, based on the predicted results, these compounds have a certain degree of skin permeability, and it might be worth considering the possibility of dermal delivery. (2) The focus on researches for other indications. In addition to their use in cancer treatment, the caged xanthones can be studied and developed for other indications. Notably, gamboge has been used in traditional medicine as a potent purgative and to treat infected wounds. (3) The chemical modifications based on streamlined structure. Previous studies have shown that the 9,10-double bond in a,b-unsaturated ketones is essential for the antitumor activity and the acidic carboxyl group of GA without much effect on apoptosis-inducing activity. In terms of drug-likeness, the large molecular weights of these caged xanthones cause certain difficulties in both formulation studies and industrialization, and attempts can be made to simplify their structures while retaining the pharmacophores in the research and development of these ingredients. (4) The systematic studies on other caged xanthones. In addition to GA and GNA, we can also fully compare and explore the properties of other caged xanthones in Garcinia hanburyi, such as forbesione, which has been found to have a therapeutic effect on cholangiocarcinoma.185–187
Acknowledgments
This work was supported by the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2009ZX09103-399) and the National Natural Science Foundation of China (82073923).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen Y, He S, Tang C, Li J, Yang G. Caged polyprenylated xanthones from the resin of Garcinia hanburyi. Fitoterapia. 2016;109:106–112. doi:10.1016/j.fitote.2015.12.002
2. Asano J, Chiba K, Tada M, Yoshii T. Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry. 1996;41:815–820. doi:10.1016/0031-9422(95)00682-6
3. Sukpondma Y, Rukachaisirikul V, Phongpaichit S. Antibacterial caged-tetraprenylated xanthones from the fruits of Garcinia hanburyi. Chem Pharm Bull (Tokyo). 2005;53:850–852. doi:10.1248/cpb.53.850
4. Tao SJ, Guan SH, Wang W, et al. Cytotoxic polyprenylated xanthones from the resin of Garcinia hanburyi. J Nat Prod. 2009;72:117–124. doi:10.1021/np800460b
5. Han QB, Yang L, Wang YL, et al. A pair of novel cytotoxic polyprenylated xanthone epimers from gamboges. Chem Biodivers. 2006;3:101–105. doi:10.1002/cbdv.200690000
6. Wang LL, Li ZL, Xu PY, et al. A new cytotoxic caged polyprenylated xanthone from the resin of Garcinia hanburyi. Chin Chem Lett. 2008;19:1221–1223. doi:10.1016/j.cclet.2008.06.013
7. Tao SJ, Guan SH, Li XG, et al. A highly rearranged pentaprenylxanthonoid from the resin of Garcinia hanburyi, Helv. Chim Acta. 2010;93:1395–1400. doi:10.1002/hlca.200900415
8. Ren YL, Yuan CH, Chai HB, et al. Absolute configuration of (−)-gambogic acid, an antitumor agent. J Nat Prod. 2011;74:460–463. doi:10.1021/np100422z
9. Deng YX, Pan SL, Zhao SY, et al. Cytotoxic alkoxylated xanthones from the resin of Garcinia hanburyi. Fitoterapia. 2012;83:1548–1552. doi:10.1016/j.fitote.2012.08.023
10. Anantachoke N, Tuchinda P, Kuhakarn C, et al. Prenylated caged xanthones: chemistry and biology. Pharm Biol. 2012;50:78–91.
11. Han QB, Xu HX. Caged Garcinia xanthones: development since 1937. Curr Med Chem. 2009;16:3775–3796. doi:10.2174/092986709789104993
12. Lei QM, Liu JM. A review and outlook on the anti-cancer effects of Garcinia hanburyi. Chin J Cancer Prev Treat. 2003;10:216–219.
13. Lei QM, Liu JM, Gong DE. Anti-cancer experimental study of Garcinia hanburyi. Chin J Oncol. 1985;7:282.
14. Cao JM, Chen FH, Zhang T. Experimental observation of the inhibitory effect of Garcinia Cambogia extract (736-1) on human liver cancer cell lines. Jiangxi Med J. 1980;3:1–2+63+65–66.
15. Hatami E, Jaggi M, Chauhan SC, et al. Gambogic acid: a shining natural compound to nanomedicine for cancer therapeutics. Biochim Biophys Acta Rev Cancer. 2020;1874:188381. doi:10.1016/j.bbcan.2020.188381
16. Kashyap D, Mondal R, Tuli HS, et al. Molecular targets of gambogic acid in cancer: recent trends and advancements. Tumour Biol. 2016;37:12915–12925. doi:10.1007/s13277-016-5194-8
17. Tang WL, Tang WH, Szeitz A, et al. Systemic study of solvent-assisted active loading of gambogic acid into liposomes and its formulation optimization for improved delivery. Biomaterials. 2018;166:13–26. doi:10.1016/j.biomaterials.2018.03.004
18. Lu GB, Yang XX, Huang QS. Isolation and structure of Gambogenic Acid from Garcinia hanburyi. Acta Pharm Sin. 1984;19:636–639.
19. Qu BX, Hao XG, Li DH. Experimental study on the anti-cancer effect of Garcinia Cambogia II. Chin J Clin Oncol. 1991;1:52–54.
20. Qu BX, Ji YJ, Zhang P, et al. The Cytocidal Kinetic Effects of Gamboge II on L1210 Leukemic Cells. Chin J Clin Oncol. 1991;01:55–58.
21. Cheng H, Peng DY, Wang XS, et al. Antitumor effects of neogambogic acid both in vivo and in vitro. Chin Trad Herbal Drugs. 2008;39:236–240.
22. Yu Q, Zhang B, Zhou Y, et al. Co-delivery of gambogenic acid and VEGF-siRNA with anionic liposome and polyethylenimine complexes to HepG2 cells. J Liposome Res. 2018;1:34–42.
23. Lin T, Huang X, Wang Y, et al. Long circulation nanostructured lipid carriers for gambogenic acid: formulation design, characterization, and pharmacokinetic. Xenobiotica. 2017;47:793–799. doi:10.1080/00498254.2016.1229084
24. Luo Q, Lin T, Zhang CY, et al. A novel glyceryl monoolein-bearing cubosomes for gambogenic acid: preparation, cytotoxicity and intracellular uptake. Int J Pharm. 2015;493:30–39. doi:10.1016/j.ijpharm.2015.07.036
25. Lin T, Fang Q, Peng D, et al. PEGylated nonionic surfactant vesicles as drug delivery systems for Gambogenic acid. Drug Deliv. 2013;20:277–284. doi:10.3109/10717544.2013.836618
26. Lin TY, Chang JL, Xun Y, et al. Folic acid-modified nonionic surfactant vesicles for gambogenic acid targeting: preparation, characterization, and in vitro and in vivo evaluation. Kaohsiung J Med Sci. 2020;36:344–353. doi:10.1002/kjm2.12162
27. Waring MJ, Arrowsmith J, Leach AR, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov. 2015;14:475–486. doi:10.1038/nrd4609
28. Yang M, Chen J, Xu L, et al. A novel adaptive ensemble classification framework for ADME prediction. RSC Adv. 2018;8:11661–11683. doi:10.1039/C8RA01206G
29. Hou T, Wang J, Zhang W, et al. Recent advances in computational prediction of drug absorption and permeability in drug discovery. Curr Med Chem. 2006;13:2653–2667. doi:10.2174/092986706778201558
30. Pires DE, Blundell TL, Ascher DB. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem. 2015;58:4066–4072. doi:10.1021/acs.jmedchem.5b00104
31. Yang J, Ding L, Hu L, et al. Rapid characterization of caged xanthones in the resin of Garcinia hanburyi using multiple mass spectrometric scanning modes: the importance of biosynthetic knowledge based prediction. J Pharm Biomed Anal. 2012;60:71–79. doi:10.1016/j.jpba.2011.10.035
32. Zhou Y, Liu X, Yang J, et al. Analysis of caged xanthones from the resin of Garcinia hanburyi using ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Anal Chim Acta. 2008;629:104–118. doi:10.1016/j.aca.2008.09.044
33. Li SL, Song JZ, Han QB, et al. Improved high-performance liquid chromatographic method for simultaneous determination of 12 cytotoxic caged xanthones in gamboges, a potential anticancer resin from Garcinia hanburyi. Biomed Chromatogr. 2008;22:637–644. doi:10.1002/bmc.981
34. Tan XF, Uddin Z, Park C, et al. Competitive protein tyrosine phosphatase 1B (PTP1B) inhibitors, prenylated caged xanthones from Garcinia hanburyi and their inhibitory mechanism. Bioorg Med Chem. 2017;25:2498–2506. doi:10.1016/j.bmc.2017.03.010
35. Guo XT, Wei YL, Lei JJ, et al. Effects of gambogic acid on apoptosis and expression of Bcl-2, Bax and P53 in non-small cell lung cancer A549 cells. Chin J Gerontol. 2018;38:911–914.
36. Wang S, Xu Y, Li C, et al. Gambogic acid sensitizes breast cancer cells to TRAIL-induced apoptosis by promoting the crosstalk of extrinsic and intrinsic apoptotic signalings. Food Chem Toxicol. 2018;119:334–341. doi:10.1016/j.fct.2018.02.037
37. Jang JH, Kim JY, Sung EG, et al. Gambogic acid induces apoptosis and sensitizes TRAIL-mediated apoptosis through downregulation of cFLIPL in renal carcinoma Caki cells. Int J Oncol. 2016;48:376–384. doi:10.3892/ijo.2015.3249
38. Thida M, Kim DW, Tran TTT, et al. Gambogic acid induces apoptotic cell death in T98G glioma cells. Bioorg Med Chem Lett. 2016;26:1097–1101. doi:10.1016/j.bmcl.2015.11.043
39. Yu JR, Yang S, Zhong HB, et al. Effect of gambogic acid on proliferation and apoptosis of esophageal cancer cell KYSE450 by inhibiting JAK-STAT pathway. J Pract Oncol. 2021;36:228–233.
40. Huang T, Zhang H, Wang X, et al. Gambogenic acid inhibits the proliferation of small‑cell lung cancer cells by arresting the cell cycle and inducing apoptosis. Oncol Rep. 2019;41:1700–1706. doi:10.3892/or.2018.6950
41. Hahnvajanawong C, Boonyanugomol W, Nasomyon T, et al. Apoptotic activity of caged xanthones from Garcinia hanburyi in cholangiocarcinoma cell lines. World J Gastroenterol. 2010;16:2235–2243. doi:10.3748/wjg.v16.i18.2235
42. Shen D, Wang Y, Niu H, et al. Gambogenic acid exerts anticancer effects in cisplatin‑resistant non‑small cell lung cancer cells. Mol Med Rep. 2020;21:1267–1275. doi:10.3892/mmr.2020.10909
43. Xia Z, Tang Z. Network pharmacology analysis and experimental pharmacology study explore the mechanism of gambogic acid against endometrial cancer. ACS Omega. 2021;6:10944–10952. doi:10.1021/acsomega.1c00696
44. Liu T, Liu J, Lu XX. Effects of gambogic acid on biological behavior and JNK signaling pathway of human colon cancer HT-29 cells. Progr Anat Sci. 2020;26:193–195.
45. Zhou S, Zhao N, Wang J. Gambogenic acid suppresses bladder cancer cells growth and metastasis by regulating NF-κB signaling. Chem Biol Drug Des. 2020;96:1272–1279. doi:10.1111/cbdd.13737
46. Hahnvajanawong C, Sahakulboonyarak T, Boonmars T, et al. Inhibitory effect of isomorellin on cholangiocarcinoma cells via suppression of NF-κB translocation, the phosphorylated p38 MAPK pathway and MMP-2 and uPA expression. Exp Ther Med. 2021;21:151. doi:10.3892/etm.2020.9583
47. Gao J, Cheng H, Li QL. Gambogenic acid intervenes against angiogenesis in human umbilical vein endothelial cells via the PTEN-PI3K/AKT/VEGF/eNOS signaling pathway. J Anhui Univ Chin Med. 2021;40:81–87.
48. Yang J, He S, Li S, et al. In vitro and in vivo antiangiogenic activity of caged polyprenylated xanthones isolated from Garcinia hanburyi Hook. f. Molecules. 2013;18:15305–15313. doi:10.3390/molecules181215305
49. Wang T, Du J, Kong D, et al. Gambogic acid inhibits proliferation and induces apoptosis of human acute T‑cell leukemia cells by inducing autophagy and downregulating β‑catenin signaling pathway: mechanisms underlying the effect of Gambogic acid on T‑ALL cells. Oncol Rep. 2020;44:1747–1757. doi:10.3892/or.2020.7726
50. Wang QX, Cheng H, Li QL. Gambogic acid induces the autophagy of glioma U87 cells. J Anhui Univ Chin Med. 2019;38:72–76.
51. Nie F, Zhang X, Qi Q, et al. Reactive oxygen species accumulation contributes to gambogic acid-induced apoptosis in human hepatoma SMMC-7721 cells. Toxicology. 2009;260:60–67. doi:10.1016/j.tox.2009.03.010
52. Yang J, Li C, Ding L, et al. Gambogic acid deactivates cytosolic and mitochondrial thioredoxins by covalent binding to the functional domain. J Nat Prod. 2012;75:1108–1116. doi:10.1021/np300118c
53. Yang LJ, Chen Y, He J, et al. Effects of gambogic acid on the activation of caspase-3 and downregulation of SIRT1 in RPMI-8226 multiple myeloma cells via the accumulation of ROS. Oncol Lett. 2012;3:1159–1165. doi:10.3892/ol.2012.634
54. Seo MJ, Lee DM, Kim IY, et al. Gambogic acid triggers vacuolization-associated cell death in cancer cells via disruption of thiol proteostasis. Cell Death Dis. 2019;10:187. doi:10.1038/s41419-019-1360-4
55. Costa S, Reina-Couto M, Albino-Teixeira A, et al. Statins and oxidative stress in chronic heart failure. Rev Port Cardiol. 2016;35:41–57. doi:10.1016/j.repc.2015.09.006
56. Urbieta Caceres VH, Lin J, Zhu XY, et al. Early experimental hypertension preserves the myocardial microvasculature but aggravates cardiac injury distal to chronic coronary artery obstruction. Am J Physiol Heart Circ Physiol. 2011;300:H693–701.
57. Fu W, Fang X, Wu L, et al. Neogambogic acid relieves myocardial injury induced by sepsis via p38 MAPK/NF-κB pathway. Korean J Physiol Pharmacol. 2022;26:511–518. doi:10.4196/kjpp.2022.26.6.511
58. Liu S, Zhao C, Yang C, et al. Gambogic acid suppresses pressure overload cardiac hypertrophy in rats. Am J Cardiovasc Dis. 2013;3:227–238.
59. Panthong A, Norkaew P, Kanjanapothi D, et al. Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hook f. J Ethnopharmacol. 2007;111:335–340. doi:10.1016/j.jep.2006.11.038
60. Cascão R, Vidal B, Raquel H, et al. Potent anti-inflammatory and antiproliferative effects of gambogic acid in a rat model of antigen-induced arthritis. Mediators Inflamm. 2014;2014:195327. doi:10.1155/2014/195327
61. Wu X, Long L, Liu J, et al. Gambogic acid suppresses inflammation in rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2017;16:7112–7118. doi:10.3892/mmr.2017.7459
62. Zhao B, Shen H, Zhang L, et al. Gambogic acid activates AMP-activated protein kinase in mammalian cells. Biochem Biophys Res Commun. 2012;424:100–104. doi:10.1016/j.bbrc.2012.06.078
63. Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9:407–416. doi:10.1016/j.cmet.2009.03.012
64. El-Seedi HR, Ibrahim HMS, Yosri N, et al. Naturally occurring xanthones; biological activities, chemical profiles and in silico drug discovery. Curr Med Chem. 2024;31:62–101.
65. Zheng Z, Ou W, Zhang X, et al. UHPLC-MS method for determination of gambogic acid and application to bioavailability, pharmacokinetics, excretion and tissue distribution in rats. Biomed Chromatogr. 2015;29:1581–1588. doi:10.1002/bmc.3462
66. Zhengtao Z, Jinwan W. Phase I human tolerability trial of gambogic acid. Chin J New Drugs. 2007;16:79–83.
67. Hua X, Liang C, Dong L, et al. Simultaneous determination and pharmacokinetic study of gambogic acid and gambogenic acid in rat plasma after oral administration of Garcinia hanburyi extracts by LC-MS/MS. Biomed Chromatogr. 2015;29:545–551. doi:10.1002/bmc.3311
68. Qi Q, You Q, Gu H, et al. Studies on the toxicity of gambogic acid in rats. J Ethnopharmacol. 2008;117:433–438. doi:10.1016/j.jep.2008.02.027
69. Nguyen A, Rouhollahi E, Böttger R, et al. Interplay between the linker and polymer molecular weight of a self-assembling prodrug on the pharmacokinetics and therapeutic efficacy. Biomater Sci. 2022;10:3122–3136. doi:10.1039/D1BM01947C
70. Zhang D, Wang W, Hou T, et al. New delivery route of gambogic acid via skin for topical targeted therapy of cutaneous melanoma and reduction of systemic toxicity. J Pharm Sci. 2021;110:2167–2176. doi:10.1016/j.xphs.2020.12.024
71. Zhang D, Chen B, Mu Q, et al. Topical delivery of gambogic acid assisted by the combination of low-frequency ultrasound and chemical enhancers for chemotherapy of cutaneous melanoma. Eur J Pharm Sci. 2021;166:105975. doi:10.1016/j.ejps.2021.105975
72. Chen X, Zhang X, Cai H, et al. Targeting USP9x/SOX2 axis contributes to the anti-osteosarcoma effect of neogambogic acid. Cancer Lett. 2020;469:277–286. doi:10.1016/j.canlet.2019.10.015
73. Hao K, Zhao XP, Liu XQ, Wang GJ. Determination of gambogic acid in dog plasma by high-performance liquid chromatography for a pharmacokinetic study. Biomed Chromatogr. 2007;21:279–283. doi:10.1002/bmc.752
74. Hao K, Liu XQ, Wang GJ, et al. Pharmacokinetics, tissue distribution and excretion of gambogic acid in rats. Eur J Drug Metab Pharmacokinet. 2007;32(2):63–68. doi:10.1007/BF03190993
75. Chen JP, Wang DL, Yang LL, et al. Ultra-high-performance liquid chromatography tandem mass spectrometry method for the determination of gambogenic acid in dog plasma and its application to a pharmacokinetic study. Biomed Chromatogr. 2014;28(12):1854–1859. doi:10.1002/bmc.3231
76. Huang X, Chen YJ, Peng DY, et al. Solid lipid nanoparticles as delivery systems for Gambogenic acid. Colloids Surf B Biointerfaces. 2013;102:391–397. doi:10.1016/j.colsurfb.2012.08.058
77. Martínez-Martínez M, Rodríguez-Berna G, Bermejo M, et al. Covalently crosslinked organophosphorous derivatives-chitosan hydrogel as a drug delivery system for oral administration of camptothecin. Eur J Pharm Biopharm. 2019;136:174–183. doi:10.1016/j.ejpb.2019.01.009
78. Wang B, Yuan T, Zha L, et al. Oral delivery of gambogenic acid by functional polydopamine nanoparticles for targeted tumor therapy. Mol Pharm. 2021;18:1470–1479. doi:10.1021/acs.molpharmaceut.1c00030
79. Jia B, Li S, Hu X, et al. Recent research on bioactive xanthones from natural medicine: garcinia hanburyi. AAPS PharmSciTech. 2015;16:742–758. doi:10.1208/s12249-015-0339-4
80. Pan LY, Wang YS, Liu XH, et al. Pharmacokinetic comparison of five xanthones in rat plasma after oral administration of crude and processed Garcinia hanburyi extracts. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1126–1127:121737. doi:10.1016/j.jchromb.2019.121737
81. Kuemmerle J, Jiang S, Tseng B, et al. Synthesis of caged 2,3,3a,7a-tetrahydro-3,6-methanobenzofuran-7(6H)-ones: evaluating the minimum structure for apoptosis induction by gambogic acid. Bioorg Med Chem. 2008;16:4233–4241. doi:10.1016/j.bmc.2008.02.084
82. Zhang HZ, Kasibhatla S, Wang Y, et al. Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg Med Chem. 2004;12:309–317. doi:10.1016/j.bmc.2003.11.013
83. Wang J, Zhao L, Hu Y, et al. Studies on chemical structure modification and biology of a natural product, gambogic acid (I): synthesis and biological evaluation of oxidized analogues of gambogic acid. Eur J Med Chem. 2009;44:2611–2620. doi:10.1016/j.ejmech.2008.09.034
84. Wang J, Ma J, You Q, et al. Studies on chemical modification and biology of a natural product, gambogic acid (II): synthesis and bioevaluation of gambogellic acid and its derivatives from gambogic acid as antitumor agents. Eur J Med Chem. 2010;45:4343–4353. doi:10.1016/j.ejmech.2010.04.037
85. Wang X, Lu N, Yang Q, et al. Spectacular modification of Gambogic acid on microwave irradiation in methanol: isolation and structure identification of two products with potent anti-tumor activity. Bioorg Med Chem Lett. 2010;20:2438–2442. doi:10.1016/j.bmcl.2010.03.021
86. Wang X, Lu N, Yang Q, et al. Studies on chemical modification and biology of a natural product, gambogic acid (III): determination of the essential pharmacophore for biological activity. Eur J Med Chem. 2011;46:1280–1290. doi:10.1016/j.ejmech.2011.01.051
87. Sun HP, Liu ZL, Xue X, et al. Studies on chemical structure modification and structure-activity relationship of derivatives of gambogic acid at C(39). Chem Biodivers. 2012;9:1579–1590. doi:10.1002/cbdv.201100415
88. Zhang XJ, Li X, Yang YR, et al. Studies on chemical-structure modification and structure-activity relationship of gambogic acid derivatives at carbon (34). Chem Biodivers. 2012;9:2295–2308. doi:10.1002/cbdv.201200081
89. Saxena V, Hussain MD. Poloxamer 407/TPGS mixed micelles for delivery of gambogic acid to breast and multidrug-resistant cancer. Int J Nanomedicine. 2012;7137:713–721.
90. Zhang ZH, Wang XP, Ayman WY, et al. Studies on lactoferrin nanoparticles of gambogic acid for oral delivery. Drug Deliv. 2013;20:86–93. doi:10.3109/10717544.2013.766781
91. Cai L, Qiu N, Xiang M, et al. Improving aqueous solubility and antitumor effects by nanosized gambogic acid-mPEG₂₀₀₀ micelles. Int J Nanomedicine. 2014;9:243–255. doi:10.2147/IJN.S54050
92. Yu F, He C, Waddad AY, et al. N-octyl-N-arginine-chitosan (OACS) micelles for gambogic acid oral delivery: preparation, characterization and its study on in situ intestinal perfusion. Drug Dev Ind Pharm. 2014;40:774–782. doi:10.3109/03639045.2013.786723
93. Yin D, Yang Y, Cai H, et al. Gambogic acid-loaded electrosprayed particles for site-specific treatment of hepatocellular carcinoma. Mol Pharm. 2014;11:4107–4117. doi:10.1021/mp500214a
94. Yao J, Li Y, Sun X, et al. Nanoparticle delivery and combination therapy of gambogic acid and all-trans retinoic acid. Int J Nanomedicine. 2014;9:3313–3324. doi:10.2147/IJN.S62793
95. Li J, Wang X, Shao Y, et al. A novel exploration of a combination of gambogic acid with TiO2 nanofibers: the photodynamic effect for HepG2 cell proliferation. Materials (Basel). 2014;7:6865–6878. doi:10.3390/ma7096865
96. Zhang W, Qiao L, Wang X, et al. Inducing cell cycle arrest and apoptosis by dimercaptosuccinic acid modified Fe3O4 magnetic nanoparticles combined with nontoxic concentration of bortezomib and gambogic acid in RPMI-8226 cells. Int J Nanomedicine. 2015;10:3275–3289. doi:10.2147/IJN.S80795
97. Doddapaneni R, Patel K, Owaid IH, et al. Tumor neovasculature-targeted cationic PEGylated liposomes of gambogic acid for the treatment of triple-negative breast cancer. Drug Deliv. 2016;23:1232–1241. doi:10.3109/10717544.2015.1124472
98. Tian L, Chen BA, Cheng J, et al. Effects of magnetic nanoparticles of Fe3O4 combinated with gambogic acid on apoptosis of SMMC-7721 cells. Onco Targets Ther. 2015;8:2285–2290. doi:10.2147/OTT.S86494
99. Ding Y, Wang Y, Opoku-Damoah Y, et al. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials. 2015;72:90–103. doi:10.1016/j.biomaterials.2015.08.051
100. Fang X, Xu Y, Wang S, et al. Pluronic F68-linoleic acid nano-spheres mediated delivery of gambogic acid for cancer therapy. AAPS PharmSciTech. 2017;18:147–155. doi:10.1208/s12249-015-0473-z
101. Liu L, Qi XJ, Zhong ZK, et al. Nanomedicine-based combination of gambogic acid and retinoic acid chlorochalcone for enhanced anticancer efficacy in osteosarcoma. Biomed Pharmacother. 2016;83:79–84. doi:10.1016/j.biopha.2016.06.001
102. Yu F, Tang X. Novel long-circulating liposomes consisting of PEG modified β-sitosterol for gambogic acid delivery. J Nanosci Nanotechnol. 2016;16:3115–3121. doi:10.1166/jnn.2016.12405
103. He M, Ro L, Liu J, et al. Folate-decorated arginine-based poly(ester urea urethane) nanoparticles as carriers for gambogic acid and effect on cancer cells. J Biomed Mater Res A. 2017;105:475–490. doi:10.1002/jbm.a.35924
104. Zhang Y, Yang Z, Tan X, et al. Development of a more efficient albumin-based delivery system for gambogic acid with low toxicity for lung cancer therapy. AAPS PharmSciTech. 2017;18:1987–1997. doi:10.1208/s12249-016-0670-4
105. Xu Y, Wang C, Ding Y, et al. Nanoparticles with optimal ratiometric co-delivery of docetaxel with gambogic acid for treatment of multidrug-resistant breast cancer. J Biomed Nanotechnol. 2016;12:1774–1781. doi:10.1166/jbn.2016.2282
106. Saini P, Ganugula R, Arora M, et al. The next generation non-competitive active polyester nanosystems for transferrin receptor-mediated peroral transport utilizing gambogic acid as a ligand. Sci Rep. 2016;6:29501. doi:10.1038/srep29501
107. Dahmani FZ, Xiao Y, Zhang J, et al. Multifunctional polymeric nanosystems for dual-targeted combinatorial chemo/angiogenesis therapy of tumors [retracted in: adv Healthc Mater. 2022 Apr;11(8):e2200329]. Adv Healthc Mater. 2016;5:1447–1461. doi:10.1002/adhm.201600169
108. Zhang Z, Qian H, Yang M, et al. Gambogic acid-loaded biomimetic nanoparticles in colorectal cancer treatment. Int J Nanomedicine. 2017;12:1593–1605. doi:10.2147/IJN.S127256
109. Zhang D, Zou Z, Ren W, et al. Gambogic acid-loaded PEG-PCL nanoparticles act as an effective antitumor agent against gastric cancer. Pharm Dev Technol. 2018;23:33–40. doi:10.1080/10837450.2017.1295068
110. Yan X, Yang Y, He L, et al. Gambogic acid grafted low molecular weight heparin micelles for targeted treatment in a hepatocellular carcinoma model with an enhanced anti-angiogenesis effect. Int J Pharm. 2017;522:110–118. doi:10.1016/j.ijpharm.2017.02.051
111. Wang S, Shao M, Zhong Z, et al. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017;24:1791–1800. doi:10.1080/10717544.2017.1406558
112. Xu Y, Wang S, Chan HF, et al. Triphenylphosphonium-modified poly(ethylene glycol)-poly(ε-caprolactone) micelles for mitochondria- targeted gambogic acid delivery. Int J Pharm. 2017;522:21–33. doi:10.1016/j.ijpharm.2017.01.064
113. Ji Y, Shan S, He M, et al. Inclusion complex from cyclodextrin-grafted hyaluronic acid and pseudo protein as biodegradable nano-delivery vehicle for gambogic acid. Acta Biomater. 2017;62:234–245. doi:10.1016/j.actbio.2017.08.036
114. Yu F, Jiang F, Tang X, et al. N-octyl-N-arginine-chitosan micelles for gambogic acid intravenous delivery: characterization, cell uptake, pharmacokinetics, and biodistribution. Drug Dev Ind Pharm. 2018;44:615–623. doi:10.1080/03639045.2017.1405973
115. Kang Y, Lu L, Lan J, et al. Redox-responsive polymeric micelles formed by conjugating gambogic acid with bioreducible poly(amido amine)s for the co-delivery of docetaxel and MMP-9 shRNA. Acta Biomater. 2018;68:137–153. doi:10.1016/j.actbio.2017.12.028
116. Yang Y, Liu Y, Cheng C, et al. Rational design of GO-modified Fe3O4/SiO2 nanoparticles with combined rhenium-188 and gambogic acid for magnetic target therapy. ACS Appl Mater Interfaces. 2017;9:28195–28208. doi:10.1021/acsami.7b07589
117. Yang Y, Zhu W, Dong Z, et al. 1D coordination polymer nanofibers for low-temperature photothermal therapy. Adv Mater. 2017;29:10. doi:10.1002/adma.201703588
118. Ganugula R, Arora M, Saini P, et al. Next generation precision-polyesters enabling optimization of ligand-receptor stoichiometry for modular drug delivery. J Am Chem Soc. 2017;139:7203–7216. doi:10.1021/jacs.6b13231
119. Wang Y, Liang X, Tong R, et al. Gambogic acid-loaded polymeric micelles for improved therapeutic effect in breast cancer. J Biomed Nanotechnol. 2018;14:1695–1704. doi:10.1166/jbn.2018.2626
120. Huang R, Li J, Kebebe D, et al. Cell penetrating peptides functionalized gambogic acid-nanostructured lipid carrier for cancer treatment. Drug Deliv. 2018;25:757–765. doi:10.1080/10717544.2018.1446474
121. Sang MM, Liu FL, Wang Y, et al. A novel redox/pH dual-responsive and hyaluronic acid-decorated multifunctional magnetic complex micelle for targeted gambogic acid delivery for the treatment of triple negative breast cancer. Drug Deliv. 2018;25:1846–1857. doi:10.1080/10717544.2018.1486472
122. Lyu L, Huang LQ, Huang T, et al. Cell-penetrating peptide conjugates of gambogic acid enhance the antitumor effect on human bladder cancer EJ cells through ROS-mediated apoptosis. Drug Des Devel Ther. 2018;12:743–756. doi:10.2147/DDDT.S161821
123. Ke Z, Yang L, Wu H, et al. Evaluation of in vitro and in vivo antitumor effects of gambogic acid-loaded layer-by-layer self-assembled micelles. Int J Pharm. 2018;545:306–317. doi:10.1016/j.ijpharm.2018.04.016
124. Feng Z, Wang Z, Yang Y, et al. Development of a safety and efficacy nanoemulsion delivery system encapsulated gambogic acid for acute myeloid leukemia in vitro and in vivo. Eur J Pharm Sci. 2018;125:172–180. doi:10.1016/j.ejps.2018.10.001
125. Zhang Z, Qian H, Huang J, et al. Anti-EGFR-iRGD recombinant protein modified biomimetic nanoparticles loaded with gambogic acid to enhance targeting and antitumor ability in colorectal cancer treatment. Int J Nanomedicine. 2018;13:4961–4975. doi:10.2147/IJN.S170148
126. Zhang Y, Tan X, Ren T, et al. Folate-modified carboxymethyl-chitosan/polyethylenimine/bovine serum albumin based complexes for tumor site-specific drug delivery. Carbohydr Polym. 2018;198:76–85. doi:10.1016/j.carbpol.2018.06.055
127. Yang Y, Cai H, Yuan X, et al. Efficient targeting drug delivery system for Lewis lung carcinoma, leading to histomorphological abnormalities restoration, physiological and psychological statuses improvement, and metastasis inhibition. Mol Pharm. 2018;15:2007–2016. doi:10.1021/acs.molpharmaceut.8b00161
128. Liu F, Huang X, Han L, et al. Improved druggability of gambogic acid using core-shell nanoparticles. Biomater Sci. 2019;7:1028–1042. doi:10.1039/C8BM01154K
129. Xu W, Wang H, Dong L, et al. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int J Nanomedicine. 2019;14:4649–4666. doi:10.2147/IJN.S201110
130. Song K, Wang Z, Liu X, et al. A novel dual sensitive polymer-gambogic acid conjugate: synthesis, characterization, and in vitro evaluation. Nanotechnology. 2019;30:505701. doi:10.1088/1361-6528/ab40ee
131. Fang W, Dai YJ, Wang T, et al. Aminated β-cyclodextrin-grafted Fe3O4-loaded gambogic acid magnetic nanoparticles: preparation, characterization, and biological evaluation. RSC Adv. 2019;9:27136–27146. doi:10.1039/C9RA04955J
132. Arora M, Ganugula R, Kumar N, et al. Next-generation noncompetitive nanosystems based on gambogic acid: in silico identification of transferrin receptor binding sites, regulatory shelf stability, and their preliminary safety in healthy rodents. ACS Appl Bio Mater. 2019;2:3540–3550. doi:10.1021/acsabm.9b00419
133. Kebebe D, Wu Y, Zhang B, et al. Dimeric c(RGD) peptide conjugated nanostructured lipid carriers for efficient delivery of Gambogic acid to breast cancer. Int J Nanomedicine. 2019;Volume 14:6179–6195. doi:10.2147/IJN.S202424
134. Wang W, Li X, Wang Z, et al. A novel “mosaic-type” nanoparticle for selective drug release targeting hypoxic cancer cells. Nanoscale. 2019;11:2211–2222. doi:10.1039/C8NR06452K
135. Chen Z, Hong G, Liu Z, et al. Synergistic antitumor efficacy of doxorubicin and gambogic acid-encapsulated albumin nanocomposites. Colloids Surf B Biointerfaces. 2020;196:111286. doi:10.1016/j.colsurfb.2020.111286
136. Na K, Liu K, Yu J, et al. A solvent-assisted active loading technology to prepare gambogic acid and all-trans retinoic acid co-encapsulated liposomes for synergistic anticancer therapy. Drug Deliv Transl Res. 2020;10:146–158. doi:10.1007/s13346-019-00669-4
137. Zhang D, Chu Y, Qian H, et al. Antitumor activity of thermosensitive hydrogels packaging gambogic acid nanoparticles and tumor-penetrating peptide iRGD against gastric cancer. Int J Nanomedicine. 2020;15:735–747. doi:10.2147/IJN.S231448
138. Xu X, Liu K, Jiao B, et al. Mucoadhesive nanoparticles based on ROS activated gambogic acid prodrug for safe and efficient intravesical instillation chemotherapy of bladder cancer. J Control Release. 2020;324:493–504. doi:10.1016/j.jconrel.2020.03.028
139. Han L, Wang Y, Huang X, et al. Specific-oxygen-supply functionalized core-shell nanoparticles for smart mutual-promotion between photodynamic therapy and gambogic acid-induced chemotherapy. Biomaterials. 2020;257:120228. doi:10.1016/j.biomaterials.2020.120228
140. Nguyen A, Ando H, Böttger R, et al. Utilization of click chemistry to study the effect of poly(ethylene)glycol molecular weight on the self-assembly of PEGylated gambogic acid nanoparticles for the treatment of rheumatoid arthritis. Biomater Sci. 2020;8:4626–4637. doi:10.1039/D0BM00711K
141. Xu Q, Chu CC. Development of ROS-responsive amino acid-based poly(ester amide) nanoparticle for anticancer drug delivery. J Biomed Mater Res A. 2021;109:524–537. doi:10.1002/jbm.a.37035
142. Shao F, Zhang M, Xu L, et al. Multiboosting of cancer immunotherapy by a core-shell delivery system. Mol Pharm. 2020;17(1):338–348. doi:10.1021/acs.molpharmaceut.9b01113
143. Jin R, Xie J, Yang X, et al. A tumor-targeted nanoplatform with stimuli-responsive cascaded activities for multiple model tumor therapy. Biomater Sci. 2020;8:1865–1874. doi:10.1039/C9BM01992H
144. Ganugula R, Arora M, Zou D, et al. A highly potent lymphatic system-targeting nanoparticle cyclosporine prevents glomerulonephritis in mouse model of lupus. Sci Adv. 2020;6:123eabb3900. doi:10.1126/sciadv.abb3900
145. Ma Z, Li N, Zhang B, et al. Dual drug-loaded nano-platform for targeted cancer therapy: toward clinical therapeutic efficacy of multifunctionality. J Nanobiotechnology. 2020;18:123. doi:10.1186/s12951-020-00681-8
146. Ganugula R, Arora M, Lepiz MA, et al. Systemic anti-inflammatory therapy aided by double-headed nanoparticles in a canine model of acute intraocular inflammation. Sci Adv. 2020;6:eabb7878. doi:10.1126/sciadv.abb7878
147. Kwan HY, Xu Q, Gong R, et al. Targeted Chinese medicine delivery by A new family of biodegradable pseudo-protein nanoparticles for treating triple-negative breast cancer: in vitro and in vivo study. Front Oncol. 2021;10:600298. doi:10.3389/fonc.2020.600298
148. Yang R, Lu M, Ming L, et al. 89Zr-labeled multifunctional liposomes conjugate chitosan for PET-trackable triple-negative breast cancer stem cell targeted therapy. Int J Nanomedicine. 2020;15:9061–9074. doi:10.2147/IJN.S262786
149. Sun J, Li Y, Teng Y, et al. NIR-controlled HSP90 inhibitor release from hollow mesoporous nanocarbon for synergistic tumor photothermal therapy guided by photoacoustic imaging. Nanoscale. 2020;12(27):14775–14787. doi:10.1039/D0NR02896G
150. Li J, Zhu D, Ma W, et al. Rapid synthesis of a Bi@ZIF-8 composite nanomaterial as a near-infrared-II (NIR-II) photothermal agent for the low-temperature photothermal therapy of hepatocellular carcinoma. Nanoscale. 2020;12:17064–17073. doi:10.1039/D0NR03907A
151. Wang F, Dong L, Wei X, et al. Effect of gambogic acid-loaded Porous-Lipid/PLGA microbubbles in combination with ultrasound-triggered microbubble destruction on human glioma. Front Bioeng Biotechnol. 2021;9:711787. doi:10.3389/fbioe.2021.711787
152. Du Q, Lv F, Huang J, et al. A multiple environment-sensitive prodrug nanomicelle strategy based on chitosan graftomer for enhanced tumor therapy of gambogic acid. Carbohydr Polym. 2021;267:118229. doi:10.1016/j.carbpol.2021.118229
153. Park SC, Heo H, Jang MK. Polyethylenimine grafted-chitosan based Gambogic acid copolymers for targeting cancer cells overexpressing transferrin receptors. Carbohydr Polym. 2022;277:118755. doi:10.1016/j.carbpol.2021.118755
154. Dang W, Guo P, Song X, et al. Nuclear targeted peptide combined with gambogic acid for synergistic treatment of breast Cancer. Front Chem. 2022;9:821426. doi:10.3389/fchem.2021.821426
155. Ma Z, Pi J, Zhang Y, et al. Enhanced anticancer efficacy of dual drug-loaded self-assembled nanostructured lipid carriers mediated by pH-responsive folic acid and human-derived cell penetrating peptide dNP2. Pharmaceutics. 2021;13:600. doi:10.3390/pharmaceutics13050600
156. Yang L, Hou X, Zhang Y, et al. NIR-activated self-sensitized polymeric micelles for enhanced cancer chemo-photothermal therapy. J Control Release. 2021;339:114–129. doi:10.1016/j.jconrel.2021.09.017
157. Shan X, Zhang X, Wang C, et al. Molecularly engineered carrier-free co-delivery nanoassembly for self-sensitized photothermal cancer therapy. J Nanobiotechnology. 2021;19:282. doi:10.1186/s12951-021-01037-6
158. Sun Q, Tang K, Song L, et al. Covalent organic framework based nanoagent for enhanced mild-temperature photothermal therapy. Biomater Sci. 2021;9:7977–7983. doi:10.1039/D1BM01245B
159. Luo Z, An J, Shi W, et al. One step assembly of ginsenoside Rb1-based nanovehicles with fast cellular transport in photothermal-chemical combined cancer therapy. Nanotechnology. 2021;32:195103. doi:10.1088/1361-6528/abe1f0
160. Tian B, Wang C, Du Y, et al. Near infrared-triggered theranostic nanoplatform with controlled release of HSP90 inhibitor for synergistic mild photothermal and enhanced nanocatalytic therapy with hypoxia relief. Small. 2022;18:e2200786. doi:10.1002/smll.202200786
161. Yang J, Zeng W, Fu X, et al. Targeted intelligent mesoporous polydopamine nanosystems for multimodal synergistic tumor treatment. J Mater Chem B. 2022;10:5644–5654. doi:10.1039/D2TB00973K
162. Hatami E, Nagesh PKB, Chauhan N, et al. In situ nanoparticle self-assembly for combination delivery of therapeutics to non-small cell lung cancer. ACS Appl Bio Mater. 2022;5:1104–1119. doi:10.1021/acsabm.1c01158
163. Dai Y, Du W, Gao D, et al. Near-infrared-II light excitation thermosensitive liposomes for photoacoustic imaging-guided enhanced photothermal-chemo synergistic tumor therapy. Biomater Sci. 2022;10:435–443. doi:10.1039/D1BM01669E
164. Huang P, Yang LL, Wang CY, et al. Preparation and characterization of folate targeting magnetic nanomedicine loaded with gambogenic acid. J Nanosci Nanotechnol. 2015;15:4774–4783. doi:10.1166/jnn.2015.9849
165. Yuan H, Li X, Zhang C, et al. Nanosuspensions as delivery system for gambogenic acid: characterization and in vitro/in vivo evaluation. Drug Deliv. 2016;23:2772–2779. doi:10.3109/10717544.2015.1077294
166. Chen F, Zhang XH, Hu XD, et al. Enhancement of radiotherapy by ceria nanoparticles modified with neogambogic acid in breast cancer cells. Int J Nanomedicine. 2015;10:4957–4969. doi:10.2147/IJN.S82980
167. Liu SJ, Liu JW, Cong JZ, et al. Preparation of neogambogic acid nanoliposomes and its pharmacokinetics in rats. J Coll Physicians Surg Pak. 2018;28:937–940.
168. Tang X, Sun J, Ge T, et al. PEGylated liposomes as delivery systems for Gambogenic acid: characterization and in vitro/in vivo evaluation. Colloids Surf B Biointerfaces. 2018;172:26–36. doi:10.1016/j.colsurfb.2018.08.022
169. Wang B, Cheng W, Zhang C, et al. Self-assembled micelles based on gambogenic acid-phospholipid complex for sustained-release drug delivery. J Microencapsul. 2019;36:566–575. doi:10.1080/02652048.2019.1656294
170. Yu Q, Zhang B, Zhou Y, et al. Co-delivery of gambogenic acid and VEGF-siRNA with anionic liposome and polyethylenimine complexes to HepG2 cells. J Liposome Res. 2019;29:322–331. doi:10.1080/08982104.2018.1473423
171. Cheng W, Wang B, Zhang C, et al. Preparation and preliminary pharmacokinetics study of GNA-loaded zein nanoparticles. J Pharm Pharmacol. 2019;71:1626–1634. doi:10.1111/jphp.13151
172. Lin TY, Zhu TT, Xun Y, et al. A novel drug delivery system of mixed micelles based on poly(ethylene glycol)-poly(lactide) and poly(ethylene glycol)-poly(ɛ-caprolactone) for gambogenic acid. Kaohsiung J Med Sci. 2019;35:757–764. doi:10.1002/kjm2.12110
173. Zha L, Qian J, Wang B, et al. In vitro/in vivo evaluation of pH-sensitive Gambogenic acid loaded Zein nanoparticles with polydopamine coating. Int J Pharm. 2020;587:119665. doi:10.1016/j.ijpharm.2020.119665
174. Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi:10.1016/S0169-409X(00)00129-0
175. Ali J, Camilleri P, Brown MB, et al. Revisiting the general solubility equation: in silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J Chem Inf Model. 2012;52:420–428. doi:10.1021/ci200387c
176. Han Y, Zhang J, Hu CQ, et al. In silico ADME and Toxicity Prediction of Ceftazidime and Its Impurities. Front Pharmacol. 2019;10:434. doi:10.3389/fphar.2019.00434
177. Aszalos A. Drug-drug interactions affected by the transporter protein, P-glycoprotein (ABCB1, MDR1) I. Preclinical aspects. Drug Discov Today. 2007;12:833–837. doi:10.1016/j.drudis.2007.07.022
178. Berellini G, Lombardo F. An accurate in vitro prediction of human VDss based on the Øie-Tozer equation and primary physicochemical descriptors. 3. analysis and assessment of predictivity on a large dataset. Drug Metab Dispos. 2019;47:1380–1387. doi:10.1124/dmd.119.088914
179. Hongjiu L, Yanrong H. Comparison of two methods forecasting binding rate of plasma protein. Comput Math Methods Med. 2014;2014:957154. doi:10.1155/2014/957154
180. Hong Y, Shaw PJ, Tattam BN, et al. Plasma protein distribution and its impact on pharmacokinetics of liposomal amphotericin B in paediatric patients with malignant diseases. Eur J Clin Pharmacol. 2007;63:165–172. doi:10.1007/s00228-006-0240-x
181. Kuhnke L, Ter Laak A, Göller AH. Mechanistic reactivity descriptors for the prediction of Ames mutagenicity of primary aromatic amines. J Chem Inf Model. 2019;59:668–672. doi:10.1021/acs.jcim.8b00758
182. Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447:666–676. doi:10.1007/s00424-003-1089-9
183. Mandíková J, Volková M, Pávek P, et al. Entecavir interacts with influx transporters hOAT1, hCNT2, hCNT3, but not with hOCT2: the potential for renal transporter-mediated cytotoxicity and drug-drug interactions. Front Pharmacol. 2016;6:304. doi:10.3389/fphar.2015.00304
184. Wang Z, Chen L, Al-Kasir R, et al. In vitro toxicity of melamine against Tetrahymena pyriformis cells. J Vet Sci. 2011;12:27–34. doi:10.4142/jvs.2011.12.1.27
185. Boueroy P, Hahnvajanawong C, Boonmars T, et al. Synergistic effect of forbesione from garcinia hanburyi in combination with 5-fluorouracil on cholangiocarcinoma. Asian Pac J Cancer Prev. 2017;18:3343–3351. doi:10.22034/APJCP.2017.18.12.3343
186. Boueroy P, Hahnvajanawong C, Boonmars T, et al. Antitumor effect of forbesione isolated from Garcinia hanburyi on cholangiocarcinoma in vitro and in vivo. Oncol Lett. 2016;12:4685–4698. doi:10.3892/ol.2016.5284
187. Hahnvajanawong C, Wattanawongdon W, Chomvarin C, et al. Synergistic effects of isomorellin and forbesione with doxorubicin on apoptosis induction in human cholangiocarcinoma cell lines. Cancer Cell Int. 2014:14–68. doi:10.1186/1475-2867-14-14
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
