Back to Journals » Journal of Pain Research » Volume 12
C7 slope and its association with serum lipid levels and Modic changes in patients with cervical spondylotic myelopathy
Authors Lv B , Xu T, Wan B, Ding H, Yao X , Chen J , Ji P, Zhao Y, Luo Y, Zhou Z, Yang S, Jiang Q, Yuan J , Yin G
Received 26 September 2018
Accepted for publication 21 February 2019
Published 30 May 2019 Volume 2019:12 Pages 1767—1776
DOI https://doi.org/10.2147/JPR.S188823
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Bin Lv1,*, Tao Xu2,*, Bowen Wan2,*, Hua Ding,1 Xiang Yao,1 Jian Chen,2 Peng Ji,1 Yilei Zhao,1 Yongjun Luo,2 Zhimin Zhou,2 Shengquan Yang,3 Qinyi Jiang,1 Jishan Yuan,1 Guoyong Yin2
1Department of Orthopedics, The Affiliated People’s Hospital of Jiangsu University, Zhenjiang, Jiangsu Province 212002, People’s Republic of China; 2Department of Orthopedics, The First Affiliated Hospital with Nanjing Medical University, Nanjing, Jiangsu Province 210029, People’s Republic of China; 3Department of Orthopedics, Yancheng No.1 People’s Hospital, Yancheng, Jiangsu Province 224000, People’s Republic of China
*These authors contributed equally to this work
Background: Several studies have substituted the T1 slope (T1S) with the C7 slope (C7S) because the C7 endplate is clearer on radiographs. Further, abnormal serum lipid levels have been proven to be related with the development of disc degeneration. The aim of this study was to explore the relationship between C7S, serum lipid levels, cervical parameters related to cervical sagittal balance and Modic changes (MCs) in patients with multisegment cervical spondylotic myelopathy (CSM).
Methods: Between January 2014 and January 2017, 75 patients with multisegment CSM were enrolled in our retrospective study. Gender, age, history of smoking status and alcohol consumption, and laboratory test data were recorded. The cervical sagittal balance parameters C7S, T1S, cervical lordosis (CL), neck tilt (NT), thoracic inlet angle (TIA), C2–C7 sagittal vertical axis (SVA), and T1S-CL were analyzed with Spearman correlation tests and multiple linear regression analysis. We diagnosed MCs through computed tomography or magnetic resonance imaging of the cervical spine. Patients were divided into four subgroups according to the presence or absence of MCs and their C7S values.
Results: 75 patients were included in our study. Age, gender, C7S, and T1S were significantly different between the two groups. However, there was no statistical difference with regard to smoking status, alcohol consumption, lipoprotein(a), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, albumin, globulin, triglycerides, total cholesterol, Ca,2+ CL, T1S, TIA, NT, and T1S−CL. The correlation between HDL-C, LDL-C, ALB, GLB, Ca,2+ C7S, T1S, MCs, NT, TIA, and C2–C7 SVA was statistically significant.
Conclusion: Significant correlations were observed between MCs and TG (as well as other preoperative sagittal parameters), which may accelerate the development of degeneration of the cervical spine. Therefore, alcohol consumption, TG, and sagittal parameters, such as C7S, and T1S could be a promising candidate for the assessment of cervical sagittal balance and predicting neck pain.
Keywords: C7 slope, Modic change, serum lipid, cervical myelopathy, neck pain, cervical sagittal alignment
Introduction
Cervical spondylotic myelopathy (CSM) is a common degenerative disorder that results in neurological dysfunction secondary to narrowing of the spinal canal, which causes functional decline and neck pain. A close relationship between the cervical sagittal range of motion and the severity of neck pain was demonstrated by previous studies,22 and they indicate that cervical sagittal alignment plays a vital role in the progression of CSM. Disc degeneration is considered to be the causative factor for abnormal sagittal alignment, which eventually leads to spinal cord dysfunction and neck pain. Further, investigations on Finnish male patients have indicated the potential relationship of sciatica with higher total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, and triglyceride (TG) levels.14 Based on these previous findings, we hypothesized that serum lipid levels are correlated with CSM or neck pain.
Modic classified signal intensity changes of the vertebral endplate on magnetic resonance imaging (MRI) scans into three types:18 MC I indicates bone marrow inflammation and edema; MC II manifests as fatty involution of the marrow and bone; and MC III appears as endplate sclerotic changes.19 A strong correlation between MCs and axial neck pain in patients with neck symptoms has been demonstrated.26 However, no report so far has focused on the relationship between Modic changes (MCs) and cervical sagittal balance in patients with CSM.
The T1 slope is known as an indicator of cervical and global sagittal alignment. The cervical sagittal alignment includes neck tilt (NT), cervical lordosis (CL), thoracic inlet angle (TIA), C2–C7 Cobb angle (C2–C7 Cobb), T1 slope (T1S), and C2–C7 sagittal vertical axis (C2–C7 SVA), which are similar to pelvic tilt (PT). C7 slope was defined as the angle between a horizontal reference line and a line parallel to the upper endplate of C7.6 A higher C7 slope (C7S) is related with lordotic changes and functional recovery.23 However, no study has reported the relationship between C7S and MCs and its impact on cervical sagittal balance in patients with axial neck pain. In the present study, we have hypothesized that serum lipids levels are associated with MCs and that C7S could be a potential indicator of cervical segments with MCs and cervical sagittal instability in patients with CSM.
Previous studies have demonstrated that TG levels are related with low back pain in patients with atherothrombotic disease.8 Moreover, TG and TC have recently been reported to be associated with symptomatic lumbar disc herniation.15 A positive association has also been found between lumbar disc degeneration and the presence of MCs.4 An association between MCs, cervical sagittal balance parameters, and improvement in quality of life has been reported too.7,21 Based on all these findings, we believe that a synergistic effect involving serum lipid levels and sagittal balance parameters is vital to the progression of disc degeneration, as well as MCs.
Methods
Patient population
The study was approved by the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University. All patients signed informed consents form when enrolled in our study. The study included a total of 75 patients with CSM who suffered from myelopathy, radiculopathy, or axial neck pain. All the patients underwent multilevel posterior cervical fusion or laminectomy for cervical stenosis by ossification of posterior longitudinal ligament.
Data collection
We got the value of high-density lipoprotein (HDL), LDL, lipoprotein-a (Lpa), albumin (ALB), globulin (GLB), and TC, and TG from laboratory tests before surgery.
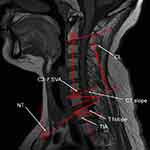
We measured the following sagittal parameters on the preoperative lateral radiographs according to previous literature28 (depicted in Figure 1).
C7 slope or C7S: The angle formed by the horizontal plane and the upper end plate (UEP) of C7.
T1 slope: The angle formed by the horizontal plane and the UEP of T1.
CL: This was judged based on the C2–C7 Cobb angle.
NT: The angle between the vertical line of the sternum tip and the line drawn from the sternum tip to the center of the T1 UEP.
TIA: The angle between the line connecting the center of the T1 UEP, the upper point of the sternum, and the perpendicular line off the T1 UEP.
C2–C7 SVA: The distance between the plumb line through the C2 center and the plumb line of the posterior C7 UEP.
C2–C7 Cobb angle: The angle formed by the inferior end plates of C2 and C7.
T1S-CL: T1 slope minus the C2–C7 Cobb angle.
MC II was assessed on MRI images. All measurement was performed by three individual surgeons. All measurement was performed by three surgeons. We measured the cervical sagittal parameters with the software Centricity PACS 4.0 (GE Healthcare, Barrington, IL, USA).
Statistical analysis
Our data collected are showed as mean (SD). The results were compared using the paired sample t test. We analyzed the correlations by Spearman correlation analyses. Differences were regarded as statistically significant when P<0.05. The SPSS software (version 19.0; IBM Corporation, Armonk, NY, USA) was used for statistical analyses.
Results
Demographic and clinical data
The demographic and laboratory data for the 75 patients with CSM enrolled in our study are shown in Table 1. The mean age of all patients was 55.63 (10.62) years. The patients were divided into four groups based on occurrence of MC and degree of C7S. Age showed significant difference between the four groups (P<0.001). Gender, smoking status, alcohol consumption, HDL-C, LDL-C, LPa, ALB, GLB, TG, TC, and Ca2+ did not differ significantly between the four groups. The values of TG and Ca2+ were obviously higher than normal in all the groups. There were total of 75 patients in the MC I and the MC II.
 | Table 1 Comparison of the enrolled populations based on demographic, laboratory variables, and MCs |
The mean and SD values of the cervical sagittal parameters are summarized in Table 2. Only C7S and T1S differed significantly between the groups.
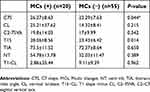 | Table 2 Comparison of preoperative sagittal parameters based on C7 slope and MCs |
The correlations between the sagittal and laboratory parameters are summarized in Table 3. The correlations between C7S, MCs, NT, TIA, CL, and C2–C7 SVA were statistically significant, as were the correlations between TG, TC, HDL-C, and LDL-C.
 | Table 3 Spearman correlation coefficient between factors that influence postoperative cervical sagittal parameters |
The results of linear regression analysis of the cervical sagittal parameters and laboratory parameters are shown in Figure 2. Presurgery MCs (R=0.288, P=0.012) was significantly correlated with presurgery TG. Presurgery T1S (R=−0.232, P=0.046) was significantly correlated with presurgery LDL-C. Presurgery C7S (R=−0.229, P=0.048) was significantly correlated with presurgery HDL-C. Presurgery NT was significantly correlated with presurgery ALB (R=−0.274, P=0.018) and Ca2+ (R=−0.228, P=0.049). Presurgery T1S (R=0.227, P=0.050), C7 S (R=0.304, P=0.008), GLB (R=0.232, P=0.045), and Ca2+ (R=−0.236, P=0.041) were significantly correlated with presurgery TIA (Table 4).
 | Table 4 Spearman correlation co-efficient between factors that influence postoperative cervical sagittal parameters |
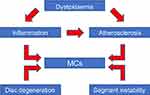 | Figure 2 The pathogenetic mechanism underlying the relationships between MCs, dyslipidemia, inflammation, atherosclerosis, disc degeneration, and segment instability.Abbreviation: MCs, Modic changes. |
Discussion
This study is the first to demonstrate the potential relationship between serum lipid levels and MCs in patients with CSM. In addition, the findings indicate that age and TG may be risk factors for the progression of MCs for strong correlation between them (Figure 2). Our investigation also demonstrated that the prevalence of OPLL in patients with CSM was higher in the MCs (+) group than in the MCs (−) group (25.00% vs 28.57%). Our results confirmed that cervical sagittal balance parameters, T1S, differed significantly according to the presence of MCs. In addition, within the cervical sagittal parameters themselves, C7S was found to be strongly associated with TIA, CL, and C2–C7 SVA (P<0.05).
The precise mechanisms that lead to a CSM are not fully understood. Combinations of several factors act a part in the progression of CSM. The association between CSM, ALB, GLB, and serum lipid levels has been investigated. Our results demonstrate that there was no significant difference between the two groups (according to the presence of MCs) with regard to levels of the serum lipid. Nonetheless, we show here for the first time that MCs are significantly correlated with TG levels (Figure 3). Serum lipid levels may lead to decreased vascularity through its associations with risk factors for vascular disease.11 This indicates that the risk of MCs could be reduced by controlling serum lipid levels. MCs are also well known to destroy the endplate vascular architecture and the metabolic pathway between the vertebral body and disc.20 This could mean that disc degeneration can also be controlled by inhibiting the progression of MCs. It is believed that the sagittal parameters of the cervical spine can predict the development of degenerative CSM. These findings indicate that a higher T1S is associated with the progression of MCs. This is not consistent with the results of previous studies, which show that a smaller C7-T1 lordotic angle was an independent characteristic of patients with cervical imbalance.
Several studies have focused on the potential relationship between T1S and cervical sagittal balance in patients with cervical spondylosis.13 Moreover, high T1S may stimulate the progression of MCs caused by impaired sagittal balance.16 However, no study has demonstrated the relationship between T1S, cervical sagittal balance, serum lipid levels, and MCs in patients with multisegment CSM. Therefore, in the present study, for the first time, we demonstrate that cervical segments with MCs are significantly less likely to have CSM. We believe that this is a consequence of less angular motion in segments with MCs; this is in agreement with previous studies which have suggested that MCs are correlated with loss of mobility and disc degeneration.6
Patients with MCs are more likely to suffer from disc herniation at the C6/7 levels.17 Further, the current literature indicates that cervical balance may be impaired when T1S is greater than 25°, which also accelerates disc degeneration and MCs at the C5-6 level. Our findings extend this previous finding to patients with hyperlipemia, in whom cervical stability was observed to change at the C4-C5 and C5-C6 levels when CL increased. On the contrary, other studies have found that patients with stress concentration and decreased CL may experience cervical disc degeneration and spondylotic changes instead of impaired sagittal balance.29 Nonetheless, there is evidence to show that the loss of CL is compensated by the reduction of C7S; this suggests that the take-off point from the cervical spine may be a predictive factor in patients with myelopathy.
Here, we elucidated the relationship between C7S and MCs in patients with hyperlipemia following accurate measurements on MRI scans, in order to avoid the limitations of radiographs and filled the vacancy of the correlative research about MCs, serum lipid levels, and cervical parameters. Our study confirmed that patients in the high-C7S group had higher preoperative CL,T1S, and C2-7 SVA than those in the low-C7S group, which is in agreement with previous research.2 The demographic characteristics of the patients may explain this difference between the groups. Sagittal lordotic segmental alignment has been proved to be a convinced predictor of adjacent-level degeneration.3 We found that patients with high C7S had more kyphotic alignment changes. Thus, high C7S may be required to maintain energy efficient, pain free, and upright posture patients with hyperlipemia.
Previous studies confirm that there is significant correlation between T1S in degenerative cervical spine and cervical sagittal balance. In particular, T1S is of clinical value in the diagnosis of degenerative cervical spondylosis.12 T1S, NT, and TIA are obscured on MRI due to the body position.25 C7S is used as a substitute for T1S as a result of the strong association between the C7 slope, T1 slope, and cervical sagittal balance.28 Previous studies have confirmed that T1S and TIA in patients with MCs can interact with each other and ultimately contribute to the development of the sagittal alignment of the cervical spine.9 Thus, T1S and TIA may be efficient for the evaluation of sagittal balance of the cervical spine. The C7 slope is associated with C2–C7 cervical lordotic compensation, thus connecting the occipitocervical and thoracolumbar spine.24 Here, we have demonstrated that not only T1S and TIA but also other cervical sagittal parameters, including C2–C7 SVA and CL, can interact with each other, with C7S as a mediator.
T1S is not correlated with cranial offset, which is a parameter related to C2–C7 SVA. Fortunately, we observed a correlation between C7S and C2–C7 SVA, thus demonstrating that the take-off point of the cervical spine from the thoracic spine may have more predictive value than SVA in degenerative patients. This may explain the strong correlation of cranial tilt with SVA, as this value increases with the combination of increased T1S and simultaneous loss of CL. In addition, we believe that there was a significant correlation between C7S, cranial tilt, and C2–C7 SVA. Kim et al demonstrated that C2–C7 SVA had a significant relationship with the C2–C7 Cobb angle in asymptomatic persons.10 However, C2–C7 SVA did not have a significant correlation with C2–C7 angle in our degenerative group. This is probably because in our symptomatic patient population, compensatory mechanisms, such as NT or thoracic hypokyphosis, caused a decrease in C7S so as to maintain normal SVA in response to loss of CL.
Limitations
The main limitations of this study are the lack of a control group and the dynamic relationship between MCs and C7S in the cervical spine. In addition, we could not control for confounding factors as a result of the small sample size. The retrospective nature of our study is another limitation. In the future, prospective studies should be carried out to assess the relationship between C7S, MC types, and clinical outcome. Finally, we did not assess the potential relationship between cervical, thoracolumbar, and spinopelvic parameters, or the influence of high C7S on surgical outcomes.
Conclusion
Age, gender, and TG were associated with the progression of MCs in patients with CSM. Significant correlations exist between TIA, CL, T1-CL, C2–C7 SVA, NT, CL, and C7S. Despite its limitations, our study is the first to validate the relationship between Ca2+, TG, and MCs.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7b–12b. doi:10.1016/S0002-9149(98)00031-9
2. Cho JH, Ha J-K, Kim DG, et al. Does preoperative T1 slope affect radiological and functional outcomes after cervical laminoplasty? Spine. 2014;39:E1575–E1581. doi:10.1097/BRS.0000000000000614
3. Faldini C, Pagkrati S, Leonetti D, Miscione MT, Giannini S, Giannini S. Sagittal segmental alignment as predictor of adjacent-level degeneration after a cloward procedure. Clin Orthop Relat Res®. 2011;469:674–681. doi:10.1007/s11999-010-1614-z
4. Farshad-Amacker NA, Hughes A, Herzog RJ, Seifert B, Farshad M. The intervertebral disc, the endplates and the vertebral bone marrow as a unit in the process of degeneration. Eur Radiol. 2017;27:2507–2520. doi:10.1007/s00330-016-4584-z
5. Georgy MMA, Vaida FA, Stern MA, Murphy KA. Association between type 1 Modic changes and propionibacterium acnes infection in the cervical spine: an observational study. Am J Neuroradiol. 2018;39:1764–1767. doi:10.3174/ajnr.A5741
6. Hayashi T, Suzuki A, Shiba K, et al. Effect of Modic changes on spinal canal stenosis and segmental motion in cervical spine. Eur Spine J. 2014;23:1737–1742. doi:10.1007/s00586-014-3406-8
7. Hayashi T, Daubs MD, Suzuki A, Phan K, Shiba K, Wang JC. Effect of Modic changes on spinal canal stenosis and segmental motion in cervical spine. Eur Spine J. 2014;23:1737–1742. doi:10.1007/s00586-014-3406-8
8. Hemingway H, Shipley M, Stansfeld S, et al. Are risk factors for atherothrombotic disease associated with back pain sickness absence? The Whitehall II Study. J Epidemiol Community Health. 1999;53:197–203. doi:10.1136/jech.53.4.197
9. Jun HS, Chang I, Song JH, et al. Is it possible to evaluate the parameters of cervical sagittal alignment on cervical computed tomographic scans? Spine. 2014;39:E630–E636. doi:10.1097/BRS.0000000000000281
10. Kim HS, Kim TH, Park MS, et al. K-line tilt as a novel radiographic parameter in cervical sagittal alignment. Eur Spine J. 2018;27:2023–2028. doi:10.1007/s00586-018-5634-9
11. Kim KS, Owen WL, Williams D, Adams-Campbell LL. A comparison between BMI and Conicity index on predicting coronary heart disease: the Framingham Heart Study. Ann Epidemiol. 2000;10:424–431.
12. Kim T-H, Lee SY, Kim YC, Park MS, Kim SW. T1 slope as a predictor of kyphotic alignment change after laminoplasty in patients with cervical myelopathy. Spine. 2013;38:E992–E997. doi:10.1097/BRS.0b013e3182972e1b
13. Knott PT, Mardjetko SM, Techy F. The use of the T1 sagittal angle in predicting overall sagittal balance of the spine. Spine J. 2010;10:994–998. doi:10.1016/j.spinee.2010.08.031
14. Leino-Arjas P, Kauppila L, Kaila-Kangas L, et al. Serum lipids in relation to sciatica among Finns. Atherosclerosis. 2008;197:43–49. doi:10.1016/j.atherosclerosis.2007.07.035
15. Longo UG, Denaro L, Spiezia F, Forriol F, Maffulli N, Denaro V. Symptomatic disc herniation and serum lipid levels. Eur Spine J. 2011;20:1658–1662. doi:10.1007/s00586-011-1737-2
16. Ma Z, Liu P, Liu J, et al. Kinematic analysis of the relationship between Modic changes and sagittal balance parameters in the cervical spine. Am J Neuroradiol. 2017;96. doi:10.1097/MD.0000000000007699
17. Mann E, Peterson C, Hodler J. Degenerative marrow (modic) changes on cervical spine magnetic resonance imaging scans: prevalence, inter- and intra-examiner reliability and link to disc herniation. Spine. 2011;36:1081–1085. doi:10.1097/BRS.0b013e3181ef6a1e
18. Modic MT, Masaryk T, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168:177–186. doi:10.1148/radiology.168.1.3289089
19. Modic MT, Steinberg P, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi:10.1148/radiology.166.1.3336678
20. Mok FP, Samartzis D, Karppinen J, Fong DY, Luk KD, Cheung KM. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016;16:32–41. doi:10.1016/j.spinee.2015.09.060
21. Mok FP, Samartzis D, Karppinen J, Fong DY, Luk KD, Cheung KM. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016;16:32–41. doi:10.1016/j.spinee.2015.09.060
22. Nicholson KJ, Millhouse PW, Pflug E, et al. Cervical sagittal range of motion as a predictor of symptom severity in cervical spondylotic myelopathy. Spine. 2018;43:883–889. doi:10.1097/BRS.0000000000002478
23. Nori S, Shiraishi T, Aoyama R, et al. Muscle-preserving selective laminectomy maintained the compensatory mechanism of cervical lordosis after surgery. Spine. 2018;43:542–549. doi:10.1097/BRS.0000000000002359
24. Nunez-Pereira S, Hitzl W, Bullmann V, Meier O, Koller H. Sagittal balance of the cervical spine: an analysis of occipitocervical and spinopelvic interdependence, with C-7 slope as a marker of cervical and spinopelvic alignment. J Neurosurg Spine. 2015;16–23. doi:10.3171/2014.11.SPINE14368
25. Pesenti SA, Blondel B, Peltier E, Choufani E, Bollini G, Jouve JL. Interest of T1 parameters for sagittal alignment evaluation of adolescent idiopathic scoliosis patients. Eur Spine J. 2016;25:424–429. doi:10.1007/s00586-015-4244-z
26. Sheng-Yun L, Letu S, Jian C, et al. Comparison of modic changes in the lumbar and cervical spine, in 3167 patients with and without spinal pain. PLoS One. 2014;9:e114993. doi:10.1371/journal.pone.0114993
27. Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–1217. doi:10.1038/nm1102-1211
28. Tamai K, Buser Z, Paholpak P, Sessumpun K, Nakamura H, Wang JC. Can C7 slope substitute the T1 slope?: an analysis using cervical radiographs and kinematic MRIs. Spine. 2018;43:520–525. doi:10.1097/BRS.0000000000002371
29. Yang B-S, Lee S-K, Song K-S, et al. The use of T1 sagittal angle in predicting cervical disc degeneration. Asian Spine J. 2015;9:757. doi:10.4184/asj.2015.9.5.757
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.