Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Burning and Scaling Probably Associated with Dupilumab Therapy: A Case Report
Authors Luo N , Wang Q , Lei M, Li Z, Li T, Hao P
Received 20 May 2022
Accepted for publication 12 August 2022
Published 17 August 2022 Volume 2022:15 Pages 1659—1662
DOI https://doi.org/10.2147/CCID.S373997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Nana Luo,1,2 Qiuyue Wang,1 Min Lei,1 Zhiyong Li,1 Tianhao Li,2 Pingsheng Hao2
1School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Department of Dermatology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
Correspondence: Tianhao Li; Pingsheng Hao, Hospital of Chengdu University of Traditional Chinese Medicine, No. 39 Shi-er-qiao Road, Chengdu, Sichuan Province, People’s Republic of China, Tel +86-13880986337 ; +86-13881965024, Fax +86-28-87732407, Email [email protected]; [email protected]
Case Report: We present the case of a 51-year-old male who experienced temporary desquamation and recurrent burning sensation in primary skin lesions after the injection of dupilumab. The scaling lasted for 1 week and subsided, while the burning became aggravated with each injection of dupilumab, which gradually subsides after 8 weeks, and there was no recurrence since then.
Conclusion: Dupilumab is an emerging and efficacious biologics medication for AD. The burning sensation and scaling we report may be the adverse events of dupilumab. Rare adverse reactions to biologics deserve the attention of physicians.
Keywords: dupilumab, atopic dermatitis, burning, scaling, adverse event
Introduction
Dupilumab, the first biologic treatment for moderate to severe atopic dermatitis approved by the Food and Drug Administration, targets the pathogenic type 2 inflammatory by blocking the effects of both IL-4 and IL-13.1 Various evidence supports dupilumab is a successful therapy for AD. Adverse effects such as nasopharyngitis, injection site reactions, conjunctivitis, headache, and herpes simplex virus infection have been reported in Phase III clinical trials, while a small percentage of patients discontinue dupilumab because of an intolerance to conjunctivitis.2 Other rare related adverse reactions like facial and neck erythema, rosacea, psoriasis, dry skin, etc. have been included by the FDA.3 Biological agents are gradually becoming increasingly used in dermatological diseases, and it is important to assess the safety and efficacy of dermatological biologics in real-world practice.4,5 We present the case of an AD patient who experienced temporary descaling and recurrent burning sensation in skin lesions during dupilumab administration.
Case Report
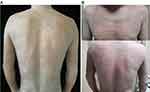
A 51-year-old Chinese male with severe AD affecting the face, neck, trunk, and extremities for 5 years received dupilumab after he failed to respond to conventional therapy: methotrexate, antihistamine, and potent topical steroids. In addition, two dermatology professors at our hospital diagnosed the patient with AD according to Hanifin and Rajka criteria and the scoring atopic dermatitis (SCORAD) was 63.95 points. The patient has a 20-year history of well-controlled psoriasis and denied a history of other chronic conditions, including mental illness. There were no significant abnormalities in his auxiliary tests (including antinuclear antibody profile, male tumor markers, hepatitis, syphilis, urinalysis, stool analysis, etc.), except for elevated eosinophils to 1.1*10^9/L, an elevation in eosinophil percentage to 12%, and an increase in immunoglobulin E to 5050 IU/mL. Then he was initiated on dupilumab with a 600 mg loading dose followed by 300 mg biweekly maintenance doses, and the lesion and itching were gradually relieved. While, there were desquamation and a mild burning sensation in the original lesion after the third injection of dupilumab (Figure 1A and B), and scaling worsened on the third day (Supplementary Picture). The patient was recommended to increase the frequency of moisturizing, and the scaling and burning resolved on day 7. While the burning sensation aggravated after each dupilumab injection, especially for lesions that have remitted in different parts of the body. The burning was paroxysmal, pronounced during the day, and was aggravated by exposure to heat. During the treatment for dupilumab, the patient did not receive phototherapy or other drugs that may cause skin burning. Therefore, skin biopsy and oral methotrexate 10mg/week were offered to him, but he refused both, then he received systemic treatment with vitamin C, calcium gluconate and cetirizine, without significant relief of burning. The burning sensation gradually subsided after 8 weeks. During the writing of this report, the patient had completed 16 weeks of dupilumab injections with a SCORAD score of 18.2, with significant disease remission, no conjunctivitis, recurrent burning sensation, and other adverse reactions have been observed.
Discussion
Dupilumab is a fully human anti-IL-4 receptorαmonoclonal antibody that blocks both IL-4 and IL-13 signaling, which significantly improves the quality of life of patients suffering from moderate to severe AD with high safety profile.6 Among the cases that have been reported, two patients showed scaling and burning after intramuscular injection of a loading dose of dupilumab 600mg, symptoms and signs disappeared after 1 week, and there was no recurrence in the subsequent maintenance dose of 300 mg, so the burning and scaling were considered dose-related side effects.7 Several patients also showed temporary burning sensation in the face or neck in the published cases of dupilumab-associated facial and neck erythema8 and rosacea.9 In addition, histopathological and immunohistochemical testing of the skin of four patients, who developed head and neck dermatitis after receiving dupilumab injections, suggested that this particular erythema of the face and neck was caused by dupilumab; however, the mechanism remains unclear.10 In the published cases of burning sensation, the feeling is temporary and limited in scope. While, our patient was almost recurrent and widespread, and the severity gradually increases with each treatment. Therefore, we consider that burning may be the adverse effects of dupilumab. Peeling signifies the shedding of defective stratum corneum, which is then replaced by corneocytes with a normal barrier function. Alternately, peeling could be a side effect.7
Studies have demonstrated that eczematous drug eruption (EDE) is a reactive spongiotic skin reaction to systemic medication, with a prevalence of 2.2–12.1% in patients with psoriasis treated with anti-IL-17A biologics, and that EDE is the result of an imbalance in the Th2/Th22 response secondary to blockade of IL-17A activity.11 Likewise, scaling and burning sensations may be rare and paradoxical reactions to biologic therapy in the case of AD treated with dupilumab. Interestingly, the patient’s psoriatic lesions showed no recurrence throughout the treatment period.
IL-4 plays a protective role in neurological disorders and can be analgesic. Pain relief is achieved in two ways:12,13 (i) by blocking the production of pro-inflammatory cytokines (eg, IL-1β, tumor necrosis factor, prostaglandin E2), (ii) by applying IL-4 to damaged nerves, which can achieve analgesic effects through IL-4Rα-mediated release of opioid peptides from M1 macrophages; IL-4 can also transfer macrophages from the M1 phenotype to the M2 phenotype, resulting in the production of opioid peptides in damaged nerves for pain relief. IL-13 is a pleiotropic cytokine that can be produced by a variety of cells and is particularly well known in the field of allergy and asthma. IL-13 receptors are expressed on both macrophages and sensory neurons. In addition, it can act as an analgesic by stimulating macrophages to produce the analgesic factor IL-10.14 However, more in-depth studies on its analgesic properties are yet to be detected. Both IL-4 and IL-13 have analgesic effects, so we hypothesized that when dupilumab antagonized IL-4Rα and thus inhibited the IL-4, and IL-13 pathway, the skin showed pain sensitivity especially in the lesions that were improving because the barrier was being reconstructed. The recurrence of burning pain in our patient after each dupilumab injection may be the result.
Conclusion
Dupilumab is an emerging and quite effective biologics medication for AD, and rare adverse reactions should be brought to the attention of doctors. Through the case, we consider that burning sensation and scaling may be the adverse effects of dupilumab, while the underlying mechanisms and solutions require to be investigated in future research.
Consent Statement
Informed consent was obtained from the patient to publication of the report details and associated images. Institutional approval was not required to publish the case details.
Funding
Hospital of Chengdu University of TCM Scientific Research Capacity Enhancement “Hundred Talents Program” (Grant No. 20B04).
Disclosure
All authors have no conflicts of interest in this work.
References
1. Ständer S. Atopic dermatitis. N Engl J Med. 2021;384(12):1136–1143. doi:10.1056/NEJMra2023911
2. Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a Phase 3 open-label extension study. J Am Acad Dermatol. 2020;82(2):377–388. doi:10.1016/j.jaad.2019.07.074
3. Wang Y, Jorizzo JL. Retrospective analysis of adverse events with dupilumab reported to the United States Food and Drug Administration. J Am Acad Dermatol. 2021;84(4):1010–1014. doi:10.1016/j.jaad.2020.11.042
4. Ruggiero A, Fabbrocini G, Cinelli E, et al. Anti-interleukin-23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real-world practice. Clin Exp Dermatol. 2022;47(3):561–567. doi:10.1111/ced.14979
5. Megna M, Cinelli E, Gallo L, et al. Risankizumab in real life: preliminary results of efficacy and safety in psoriasis during a 16-week period. Arch Dermatol Res. 2022;314(6):619–623. doi:10.1007/s00403-021-02200-7
6. Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. doi:10.1001/jamadermatol.2019.3336
7. Al Hammadi A, Parmar NV. Erythema, pruritus, and diffuse peeling of skin during dupilumab therapy for atopic dermatitis in three adults. Int J Dermatol. 2019;58(1):e14–e15. doi:10.1111/ijd.14296
8. Jo CE, Finstad A, Georgakopoulos JR, et al. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84(5):1339–1347. doi:10.1016/j.jaad.2021.01.012
9. Heibel HD, Hendricks AJ, Foshee JP, et al. Rosacea associated with dupilumab therapy. J Dermatolog Treat. 2021;32(1):114–116. doi:10.1080/09546634.2019.1624683
10. Dybała A, Sernicola A, Gomes V, et al. Dupilumab facial redness: histologic characterization on a series of four cases. Immunotherapy. 2022;14(4):183–188. doi:10.2217/imt-2021-0122
11. Megna M, Caiazzo G, Parisi M, et al. Eczematous drug eruption in patients with psoriasis under anti-interleukin-17A: does interleukin-22 play a key role? Clin Exp Dermatol. 2022;47(5):918–925. doi:10.1111/ced.15052
12. Labuz D, Celik M, Seitz V, et al. Interleukin-4 induces the release of opioid peptides from M1 macrophages in pathological pain. J Neurosci. 2021;41(13):2870–2882. doi:10.1523/JNEUROSCI.3040-20.2021
13. Celik M, Labuz D, Keye J, et al. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight. 2020;5(4). doi:10.1172/jci.insight.133093
14. Singh SK, Krukowski K, Laumet GO, et al. CD8+ T cell-derived IL-13 increases macrophage IL-10 to resolve neuropathic pain. JCI Insight. 2022;7(5). doi:10.1172/jci.insight.154194
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.