Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Budesonide/Glycopyrrolate/Formoterol Fumarate Metered Dose Inhaler Improves Exacerbation Outcomes in Patients with COPD without a Recent Exacerbation History: A Subgroup Analysis of KRONOS
Authors Martinez FJ , Ferguson GT , Bourne E , Ballal S, Darken P , Aurivillius M, Dorinsky P , Reisner C
Received 14 October 2020
Accepted for publication 7 January 2021
Published 28 January 2021 Volume 2021:16 Pages 179—189
DOI https://doi.org/10.2147/COPD.S286087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Fernando J Martinez,1 Gary T Ferguson,2 Eric Bourne,3 Shaila Ballal,4 Patrick Darken,5 Magnus Aurivillius,6 Paul Dorinsky,3 Colin Reisner4
1Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York, NY, USA; 2Pulmonary Research Institute of Southeast Michigan, Farmington Hills, MI, USA; 3AstraZeneca, Durham, NC, USA; 4AstraZeneca, Morristown, NJ, USA; 5AstraZeneca, Wilmington, DE, USA; 6AstraZeneca, Gothenburg, Sweden
Correspondence: Fernando J Martinez
Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York, NY 10065, USA
Tel +1 646 962 2748
Email [email protected]
Purpose: In the Phase III, 24-week KRONOS study (NCT02497001), triple therapy with budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI) reduced exacerbation rates versus glycopyrrolate/formoterol fumarate (GFF) MDI in patients with moderate-to-very severe chronic obstructive pulmonary disease (COPD) and no requirement for a history of exacerbations. We report a post hoc analysis investigating whether the benefits observed were driven by patients with ≥ 1 exacerbation in the 12 months prior to the study.
Patients and Methods: Patients received BGF MDI 320/18/9.6 μg, GFF MDI 18/9.6 μg, budesonide/formoterol fumarate (BFF) MDI 320/9.6 μg, or budesonide/formoterol fumarate dry powder inhaler (BUD/FORM DPI) 400/12 μg twice-daily. Post hoc analyses were conducted on exacerbation and lung function results from patients with and without a documented exacerbation in the 12 months prior to the study.
Results: Overall, 74% (1411/1896) of the modified-intent-to-treat (mITT) population had no moderate/severe exacerbations in the 12 months prior to the study. BGF MDI reduced exacerbation rates versus GFF MDI in the prior (58%; unadjusted p=0.0003) and no prior (48%; unadjusted p=0.0001) exacerbations subgroups. The magnitude of reduction in exacerbation rates was generally similar within subgroups for BGF MDI versus BFF MDI and BUD/FORM DPI. In the prior exacerbations subgroup, risk during treatment for time to first exacerbation was lower with BGF MDI versus GFF MDI (p=0.0022) and BFF MDI (p=0.0110); excluding the first 30 days of data yielded similar results. The magnitude of reduction in exacerbation rates for BGF MDI compared with GFF MDI increased with eosinophil count.
Conclusion: In patients with or without a history of exacerbations in the 12 months prior to the study, BGF MDI reduced exacerbation rates versus GFF MDI, suggesting results observed in the overall population were not driven by the small subgroup with a prior history of exacerbations.
Keywords: fixed-dose combination, COPD, exacerbations of COPD
Introduction
Bronchodilator therapy with long-acting β2-agonists (LABA) and/or long-acting muscarinic antagonists (LAMA) is the mainstay of treatment for chronic obstructive pulmonary disease (COPD).1 However, for patients who develop further exacerbations of COPD, despite dual combination therapy, use of triple combination therapy with an inhaled corticosteroid (ICS), LAMA, and LABA is recommended.1 Importantly, real-world evidence has demonstrated that ICS are often prescribed inappropriately, for example, in patients with less severe disease without a known or established exacerbation risk.2,3 To date, most studies of triple versus dual fixed-dose therapies have been performed in patient populations with high exacerbation risk.4–9
The Phase III KRONOS study (NCT02497001) enrolled symptomatic patients (COPD Assessment Test [CAT] score ≥10) with moderate-to-very-severe COPD without any requirement for experiencing an exacerbation in the previous year, which was the case for the majority of patients randomized.10 The KRONOS study evaluated the efficacy and safety of the fixed-dose combination ICS/LAMA/LABA budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI), administered via an Aerosphere™ inhaler, and reported benefits on lung function and exacerbation rates versus dual therapies (LAMA/LABA and ICS/LABA) in these patients.10
Given the high proportion of patients with no prior exacerbations enrolled in the study, we undertook a post hoc analysis to investigate whether or not the exacerbation benefit in the overall population of the KRONOS study was driven by the small subset of patients included in the study reporting ≥1 exacerbation in the year prior to the study, or was also true for patients with no prior exacerbations.
Methods
Study Design
KRONOS (NCT02497001) was a 24-week, double-blind, parallel-group randomized controlled study and the design has been previously described.10 Eligible patients were 40–80 years of age, with a smoking history of ≥10 pack-years (current or former smokers), a confirmed diagnosis of moderate-to-very severe COPD, as defined by a post-bronchodilator forced expiratory volume in 1 s (FEV1) of 25–80% predicted, and were symptomatic (CAT score ≥10) despite treatment with ≥2 inhaled maintenance therapies for at least 6 weeks before screening. A current diagnosis of asthma was exclusionary. Patients were not required to have an exacerbation in the previous 12 months. A COPD exacerbation was defined by modified Anthonisen criteria11 or physician justification.10
After randomization, patients received BGF MDI 320/18/9.6 µg (ICS/LAMA/LABA triple therapy), glycopyrrolate/formoterol fumarate (GFF) MDI 18/9.6 μg (LAMA/LABA dual therapy), or budesonide/formoterol fumarate (BFF) MDI 320/9.6 μg (ICS/LABA dual therapy) all delivered via an Aerosphere™ inhaler, or open-label budesonide/formoterol fumarate dry powder inhaler (BUD/FORM DPI) 400/12 μg (Symbicort® Turbuhaler®; ICS/LABA), as two inhalations, twice daily for 24 weeks. The doses of glycopyrrolate and formoterol fumarate are equivalent to 14.4 μg glycopyrronium and 10 μg formoterol fumarate dihydrate. Doses are the sum of two inhalations and represent half of the total daily dose.
All participants provided written informed consent and independent ethics committee/institutional review board approvals were obtained prior to the start of the study. Details of individual independent ethics committees and institutional review boards are provided in the Supplemental Appendix. The studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation/Good Clinical Practice and applicable regulatory requirements.
Assessments
The primary endpoints of FEV1 area under the curve from 0 to 4 h (AUC0–4) over 24 weeks (BGF MDI vs BFF MDI and BGF MDI vs BUD/FORM DPI) and change from baseline in morning pre-dose trough FEV1 over 24 weeks (BGF MDI vs GFF MDI and BFF MDI vs BUD/FORM DPI [non-inferiority]) have been reported previously.10 Secondary endpoints, including the rate of moderate/severe COPD exacerbations, have also been reported.10 In this manuscript, lung function endpoints and rate of exacerbations during the KRONOS study were analyzed post hoc for data from the two subgroups of patients with and without a documented exacerbation in the year prior to the study.
At each visit from randomization onwards (0, 4, 8, 12, 16, 20, and 24) spirometry was conducted, as previously reported, and occurrences of COPD exacerbations, medication changes, and adverse events (AEs) were recorded in an electronic case report form by study personnel.10 Criteria for identifying and reporting exacerbations have been published previously.10
Exacerbations were categorized as: moderate (led to treatment with systemic corticosteroids, antibiotics, or both, for ≥3 days, or ≥1 depot injectable dose of corticosteroids); or severe (led to hospital admission or a visit to a healthcare facility, eg an emergency department, that lasted ≥24 hours, or COPD-related death). Exacerbations with start and end dates that were ≤7 days apart were considered one event and assigned the maximum severity between the two events. A clinically consistent definition of pneumonia was implemented to standardize the diagnosis of pneumonia.10 An independent clinical endpoint committee reviewed all AEs reported as pneumonia or that potentially met criteria for major adverse cardiovascular events, and additionally evaluated cause-specific mortality. Treatment-emergent adverse events (TEAEs) were also evaluated.
Statistical Analyses
Post hoc analyses of lung function and exacerbation rates during the KRONOS study were conducted on data from the two subgroups of patients with and without an exacerbation within 12 months preceding the study. Efficacy analyses were conducted in the modified intent-to-treat (mITT) population (all data obtained before discontinuation from treatment from randomized patients receiving any dose of the study drug) using an efficacy estimand. Exacerbation rates were analyzed using negative binomial regression. Time at risk of experiencing an exacerbation was an offset variable in the model. Treatment comparisons were adjusted for baseline post-bronchodilator percent predicted FEV1, baseline eosinophil count, country, ICS use at screening, and exacerbation history (for the overall population). Model-adjusted rates and rate ratios were calculated. Time during an exacerbation, or in the 7 days following an exacerbation, was not included in the calculation of time at risk.
The change from baseline in morning pre-dose trough FEV1 and FEV1 AUC0–4 over 24 weeks were analyzed using linear models with repeated measures. The models included treatment, visit, treatment by visit interaction, and ICS use at screening as categorical covariates, and baseline FEV1, percent reversibility to salbutamol, and baseline eosinophil counts as continuous covariates. Analyses presented were not included under the Type I error control plan for the study, and therefore the p-values reported within the results are unadjusted. Patients in the safety population are those who received at least one dose of the study treatment.
Results
Patient Demographics
In total, 74.4% (1411/1896) of patients in the KRONOS mITT population had no documented moderate/severe exacerbations in the previous 12 months (Table 1; mean age 65.5 years; 72.8% male; 69.9% used ICS at screening). The remaining 25.6% (485/1896) had at least one documented moderate/severe exacerbation in the previous year (mean age 64.2 years; 66.6% male; 77.3% used ICS at screening), and 5.6% (107/1896) of patients had a severe exacerbation(s) in the previous year.
 |
Table 1 Patient Demographics and Characteristics by Reported History of Exacerbations |
Moderate/Severe Exacerbations
In patients without an exacerbation in the previous year, the adjusted annualized rate of moderate/severe exacerbations (number of events) was 0.41 (n=85), 0.80 (n=147), 0.42 (n=42), and 0.47 (n=47) for BGF MDI, GFF MDI, BFF MDI, and BUD/FORM DPI, respectively (Table 2). In those with prior exacerbations, the adjusted annualized rate of moderate/severe exacerbations (number of events) was 0.63 (n=47), 1.50 (n=81), 1.05 (n=32), and 0.84 (n=30) for BGF MDI, GFF MDI, BFF MDI, and BUD/FORM DPI, respectively (Table 2).
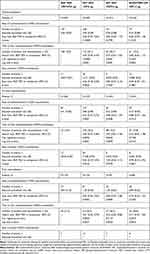 |
Table 2 Exacerbation Outcomes by Reported History of Exacerbations in the Previous 12 Months (Efficacy Estimand, mITT Population) |
BGF MDI reduced the rate of moderate/severe exacerbations versus GFF MDI by 48% in the no prior exacerbations subgroup (unadjusted p=0.0001) and in the prior exacerbations subgroup by 58% (unadjusted p=0.0003) (Table 2 and Figure 1). Moderate/severe exacerbation rates for BGF MDI versus BFF MDI and BGF MDI versus BUD/FORM DPI were generally similar within subgroups (Table 2 and Figure 1). There was also a numerical reduction in the rate of moderate/severe exacerbations with BGF MDI versus GFF MDI in the subgroup of patients in the no prior exacerbations subgroup who did not receive prior ICS, though the sample size of this post hoc analysis was too small to allow definitive conclusions to be made (Table S1).
In the prior exacerbations subgroup, for time to first moderate/severe COPD exacerbation, the risk during treatment with BGF MDI was lower versus GFF MDI (49%; unadjusted p=0.0022) and versus BFF MDI (48%; unadjusted p=0.0110) (Table 2).
Based on the results for BGF MDI versus GFF MDI, the number needed to treat (NNT) [95% CI] for 1 year to prevent one additional moderate/severe COPD exacerbation was 3 [2, 6] in the no prior exacerbations subgroup and 2 [1, 3] in the prior exacerbations subgroup. For comparison, in the overall KRONOS population, NNT [95% CI] for 1 year to prevent one additional moderate/severe COPD exacerbation was 3 [2, 4].
The impact of corticosteroid therapy was noted across all budesonide-containing preparations as a function of baseline eosinophil count, both in patients with exacerbations in the year prior (Figure 2A) and in those without prior exacerbations (Figure 2B). Rates of moderate/severe exacerbations were lower for BGF MDI versus GFF MDI in both prior and no prior exacerbations subgroups.
To assess the impact of acute ICS withdrawal on the findings, we conducted analyses of exacerbations excluding the first 30 days of data. When the first 30 days of data were excluded for patients with ICS use in the 30 days before screening, the moderate/severe exacerbation rate ratio was similar to the complete dataset for the overall population for BGF MDI versus GFF MDI (0.43 [0.31, 0.60] versus 0.48 [0.37, 0.64], respectively; unadjusted p<0.0001 for both) (Table S2 and Figure S1); moderate/severe exacerbation rates for BGF MDI versus BFF MDI or BUD/FORM DPI were generally similar to the complete dataset. When excluding the first 30 days in the complete prior exacerbation population, for time to first moderate/severe COPD exacerbation, the risk during treatment with BGF MDI was lower versus GFF MDI (unadjusted p=0.0201) and versus BFF MDI (unadjusted p=0.0193) (Table S3,10 Figure S2).
Severe Exacerbations
While not reported previously for the overall population, the adjusted annualized rate of severe exacerbations [number of events] was 0.047 [n=17], 0.131 [n=40], 0.055 [n=9], and 0.068 [n=12] for BGF MDI, GFF MDI, BFF MDI, and BUD/FORM DPI, respectively. BGF MDI reduced the rate of severe exacerbations versus GFF MDI by 64% (unadjusted p=0.0026).
In patients without exacerbations in the previous year, the adjusted annualized rate of severe exacerbations [number of events] was 0.046 [n=12], 0.108 [n=26], 0.043 [n=6], and 0.046 [n=6] for BGF MDI, GFF MDI, BFF MDI, and BUD/FORM DPI, respectively. BGF MDI reduced the rate of severe exacerbations versus GFF MDI by 58% (unadjusted p=0.0316) (Table 2).
Since only 5, 14, 3, and 6 severe exacerbations were reported for BGF MDI, GFF MDI, BFF MDI, and BUD/FORM DPI, respectively, in the prior exacerbations subgroup, the event numbers were too small to conduct meaningful analyses for severe exacerbations in this subgroup.
Lung Function
BGF MDI improved lung function versus BFF MDI over 24 weeks in patients with no prior exacerbation history (unadjusted p<0.001), and versus BUD/FORM DPI in both the no prior and prior exacerbation history populations (unadjusted p=0.0003 and p<0.0001, respectively). Analyses for both subgroups can be found in the supplementary information section (Table S4, Figure S3).
Safety
The TEAE profiles of the subgroups with and without an exacerbation in the previous 12 months were generally comparable. The incidence of confirmed pneumonia was low in both subgroups (Table 3).10
 |
Table 3 Comparison of TEAEs for No Prior Exacerbations and Prior Exacerbations Subgroups (Safety Population) |
Discussion
This subgroup analysis from the 24-week KRONOS study demonstrated that BGF MDI reduced the rate of moderate/severe COPD exacerbations versus GFF MDI in the subgroup with no history of moderate/severe exacerbations in the previous year. This suggests that the exacerbation reduction in KRONOS was not driven by the subset of patients with a prior history of exacerbations. Importantly, the effect of ICS treatment on exacerbation rates was seen in patients with a higher blood eosinophil count regardless of exacerbation history. The observed treatment effects on lung function, regardless of exacerbation history, are to be expected.1
Unlike many other randomized controlled studies of triple therapy versus dual therapies in patients with symptomatic COPD,4,5,8,9 KRONOS enrolled patients with no requirement for an exacerbation in the previous year.10 Therefore, we were able to conduct a sub-analysis to examine the effect of triple therapy in patients who were symptomatic, despite being on two or more inhaled maintenance therapies, but had no documented history of exacerbations in the year prior to the study. The analyses revealed that the effect of BGF MDI on exacerbation rates versus LAMA/LABA was consistent in those with and without prior exacerbations. In addition, the NNT for 1 year to prevent one additional moderate/severe COPD exacerbation in BGF MDI versus GFF MDI was similar in both subgroups (no prior history: 3; prior history: 2). While other triple therapy studies included patients with differing exacerbation risks (eg 1 versus 2 or more), with the exception of the FULFIL study,7,12 none included patients with no prior exacerbations.4,5,8,9 The 24-week FULFIL study was conducted in symptomatic patients with moderate-to-very severe COPD and showed that annual moderate/severe exacerbation rates were improved by triple therapy versus ICS/LABA regardless of a history of exacerbations (0/1 moderate exacerbations, ≥2 moderate exacerbations, or ≥1 severe exacerbation).12 Approximately one third of patients in the FULFIL study had no documented history of moderate/severe exacerbations in the previous year.7
ICS-containing regimens are not generally recommended for symptomatic patients who do not report exacerbations.1 However, in the current analysis, BGF MDI reduced moderate/severe and severe exacerbation rates versus LAMA/LABA in the subgroup of patients without a prior exacerbation. In seeking an explanation for the findings, we note that model-estimated rates of moderate/severe exacerbations showed that patients without a prior exacerbation in the previous 12 months had a substantial exacerbation rate when not receiving an ICS during the study (adjusted annualized rate for GFF MDI = 0.80, versus 0.41, 0.42, and 0.47 for BGF MDI, BFF MDI, and BUD/FORM DPI, respectively). This subgroup also experienced severe exacerbations during the study, although overall numbers were low. It is possible that exacerbation risk was unmasked in those patients no longer receiving ICS (more than 70% of these patients were receiving ICS prior to the study). Exacerbation characteristics and exacerbation risk can fluctuate over time and can affect treatment decisions for individual patients. For example, in the 3-year SPIROMICS study, exacerbation incidence frequently varied from year to year.13 Therefore, it is possible that patients on ICS had experienced exacerbations prior to the 12-month period when exacerbation history was collected.
Regarding prior ICS use, the rate ratio for BGF MDI versus GFF MDI exacerbation rates was similar to that of the overall population in the subgroup of patients with ICS use in the 30 days before screening. Moreover, the results for moderate/severe exacerbations were similar when the first 30 days of data were excluded. Taken together, this supports the view that the findings are not driven by acute ICS withdrawal. Furthermore, when the first 30 days of data were excluded from the prior exacerbations subgroup analysis, results for exacerbation risk and time to moderate/severe exacerbation were similar to the complete dataset.
The reasons for prescribing an ICS prior to the KRONOS study were not captured and, as real-world studies show, ICS are commonly prescribed over a wide spectrum of COPD phenotypes.14–18 This suggests that a more careful assessment of exacerbation histories, analysis of blood eosinophil count, and details on why ICS were prescribed may need to be part of future studies.
In the KRONOS study, the contribution of ICS in BGF MDI was demonstrated by the magnitude of risk reduction relative to GFF MDI in a population where the majority of patients had no prior exacerbations in the previous year.10 The 52-week WISDOM study suggested ICS can be removed in many patients with a prior exacerbation history based on eosinophil levels. However, WISDOM was a randomized withdrawal study, in which approximately 30% of patients were not receiving an ICS-containing treatment prior to the study, and all patients received triple therapy in the 6-week run-in before ICS withdrawal.19 These factors could have influenced the low exacerbation rates observed when ICS was withdrawn. The FLAME study also suggested that ICS can be removed in many patients with a prior exacerbation history.20 However, the exclusion of patients with eosinophil levels >600 cells/µL may have contributed to these results.21 It should be acknowledged that in FLAME the benefits of LAMA/LABA compared with ICS/LABA were greater in those with eosinophils <150 cells/µL than at higher levels where rate ratios approached unity.21 Furthermore, the study was not designed to investigate the benefit of adding ICS to LAMA/LABA.20
In this post hoc analysis of the KRONOS study, the benefits of BGF MDI relative to GFF MDI on rates of moderate/severe exacerbation increased as eosinophil counts increased, regardless of prior exacerbation history. These results were similar to those observed for the overall population.10 The relationship between eosinophil count and ICS treatment benefit has been previously documented22–24 and is supported by the Global Initiative for Chronic Obstructive Lung Disease 2020 recommendations,1 indicating the potential of using eosinophil counts as key biomarkers to inform future treatment guidelines. Given the suggested benefits of a triple regimen in KRONOS over both dual therapies, the role of dual therapy in preventing exacerbations in COPD may be questioned.
Preventing exacerbations is a priority as exacerbations are associated with lung function decline, morbidity, and mortality in COPD.25 A single exacerbation may be associated with a significant decline in lung function26 and patients experiencing frequent exacerbations are likely to be at the greatest risk for lung function decline over time.27,28 Finally, the increased mortality risk associated with COPD exacerbations, particularly severe COPD exacerbations, is further evidence of the importance of exacerbation prevention in the overall management of COPD.29–31
Within the current study, most patients were receiving an ICS; therefore, the generalizability of results could be limited. Also, the potential for under-reporting exacerbations cannot be excluded. Therefore, better characterization of exacerbation risk, based on expanded, documented, prior exacerbation history (eg over 2 years), including information on prior ICS treatment and rationale, could allow clarification of these findings in future studies.
Conclusions
This subgroup analysis of the KRONOS study showed the benefits of BGF MDI in reducing moderate/severe and severe exacerbation rates relative to GFF MDI, even among COPD patients with no history of exacerbations in the prior year. These findings suggest that benefits on exacerbation rates observed in the KRONOS study in symptomatic patients with moderate-to-very severe COPD were not driven by the patient subgroup with a history of prior exacerbations.
Abbreviations
AE, adverse event; AUC0–4, area under curve from 0 to 4 hours; BFF, budesonide/formoterol fumarate; BGF, budesonide/glycopyrrolate/formoterol fumarate; BUD/FORM DPI, budesonide/formoterol fumarate dry powder inhaler; CAT, COPD Assessment Test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; FEV1, forced expiratory volume in 1 s; GFF, glycopyrrolate/formoterol fumarate; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists; LSM, least-squares mean; MDI, metered dose inhaler; mITT, modified intent-to-treat; SE, standard error; TEAE, treatment-emergent adverse event.
Data Sharing Statement
The data sets used and analyzed during the current study will be available on reasonable request in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Acknowledgments
The authors thank all the patients and their families, and the team of investigators, research nurses, and operations staff involved in this study. The abstract of this paper was presented in part at the ACCP/CHEST 2019 Congress as an oral presentation. The oral presentation’s abstract was published in a supplement of the CHEST journal: CHEST 2019;156(4):A2276-2277; https://doi.org/10.1016/j.chest.2019.08.309
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by AstraZeneca. The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report. Medical writing support, under the direction of the authors, was provided by Audrey Gillies, MPhys, CMC Connect, McCann Health Medical Communications Ltd, funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines.32
Disclosure
FJM reports grants from AstraZeneca during the conduct of the study; personal fees and non-financial support from AbbVie, Afferent Pharmaceuticals Inc/Merck, Bayer, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, DevPro, Gala, Genentech, Gilead, GlaxoSmithKline, IQVIA, Nitto, Novartis, Promedior/Roche, ProterrixBio, Raziel, Patara/Respivant, Sanofi/Regeneron, Sunovion, Teva, TwoXAR, Veracyte, Verona, Zambon; and grants from National Institutes of Health, outside of the submitted work. GTF reports grants, personal fees, and non-financial support from AstraZeneca during the conduct of the study; grants, personal fees, and non-financial support from AstraZeneca, Boehringer Ingelheim, Novartis, and Sunovion; grants and personal fees from Theravance; and personal fees from Circassia, Galderma, GlaxoSmithKline, Innoviva, Mylan, Orpheris, Sanofi, Teva, and Verona, grants from Altavant and Knopp, outside of the submitted work. PDa, MA, and PDo are employees of AstraZeneca and hold stock and/or stock options in the company. SB is a former employee of AstraZeneca. EB and CR are former employees of AstraZeneca and hold stock and/or stock options in the company. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. 2020 Report. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.
2. Price D, Miravitlles M, Pavord I, et al. First maintenance therapy for COPD in the UK between 2009 and 2012: a retrospective database analysis. NPJ Prim Care Respir Med. 2016;26(1):16061. doi:10.1038/npjpcrm.2016.61
3. Wei YF, Kuo PH, Tsai YH, et al. Factors associated with the prescription of inhaled corticosteroids in GOLD group A and B patients with C. Int J Chron Obstruct Pulmon Dis. 2015;10(10):1951–1956. doi:10.2147/COPD.S88114
4. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi:10.1016/S0140-6736(18)30206-X
5. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi:10.1016/S0140-6736(16)31354-X
6. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi:10.1016/S0140-6736(17)30188-5
7. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC
8. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
9. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
10. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
11. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi:10.7326/0003-4819-106-2-196
12. Halpin DMG, Birk R, Brealey N, et al. Single-inhaler triple therapy in symptomatic COPD patients: FULFIL subgroup analyses. ERJ Open Res. 2018;4(2):00119–2017. doi:10.1183/23120541.00119-2017
13. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
14. Ding B, Small M, Holmgren U. A cross-sectional survey of current treatment and symptom burden of patients with COPD consulting for routine care according to GOLD 2014 classifications. Int J Chron Obstruct Pulmon Dis. 2017;12:1527–1537. doi:10.2147/COPD.S133793
15. Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217. doi:10.2147/COPD.S91694
16. Savran O, Godtfredsen N, Sørensen T, Jensen C, Ulrik CS. COPD patients prescribed inhaled corticosteroid in general practice: based on disease characteristics according to guidelines? Chron Respir Dis. 2019;16:1479973119867949. doi:10.1177/1479973119867949
17. Graf J, Jörres RA, Lucke T, Nowak D, Vogelmeier CF, Ficker JH. Medical Treatment of COPD: an analysis of guideline-adherent prescribing in a large national cohort (COSYCONET). Dtsch Arztebl Int. 2018;155(37):599–605. doi:10.3238/arztebl.2018.0599
18. Lane DC, Stemkowski S, Stanford RH, Tao Z. Initiation of triple therapy with multiple inhalers in chronic obstructive pulmonary disease: an analysis of treatment patterns from a U.S. retrospective database study. J Manag Care Spec Pharm. 2018;24(11):1165–1172. doi:10.18553/jmcp.2018.24.11.1165
19. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa1407154
20. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
21. Roche N, Chapman KR, Vogelmeier CF, et al. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME trial. Am J Respir Crit Care Med. 2017;195(9):1189–1197. doi:10.1164/rccm.201701-0193OC
22. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi:10.1164/rccm.201502-0235LE
23. Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7(9):745–756. doi:10.1016/S2213-2600(19)30190-0
24. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X
25. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113–118. doi:10.1183/09059180.00002610
26. Halpin DMG, Decramer M, Celli BR, Mueller A, Metzdorf N, Tashkin DP. Effect of a single exacerbation on decline in lung function in COPD. Respir Med. 2017;128:85–91. doi:10.1016/j.rmed.2017.04.013
27. Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847
28. Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi:10.1164/rccm.201605-1014OC
29. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
30. Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464–471. doi:10.1164/rccm.201710-2029OC
31. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi:10.1136/thoraxjnl-2011-201518
32. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. doi:10.7326/M15-0288
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


