Back to Journals » Clinical Ophthalmology » Volume 17
Brolucizumab for Neovascular Age-Related Macular Degeneration (BEL Study)
Authors Van Cleemput L, Peeters F, Jacob J
Received 24 January 2023
Accepted for publication 21 March 2023
Published 8 April 2023 Volume 2023:17 Pages 1077—1085
DOI https://doi.org/10.2147/OPTH.S402090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Liesbeth Van Cleemput,1,2 Freya Peeters,1,2 Julie Jacob1,2
1Department of Ophthalmology, University Hospital Leuven, Leuven, Belgium; 2Research Group Ophthalmology, Department of Neurosciences, KU Leuven, Leuven, Belgium
Correspondence: Liesbeth Van Cleemput, Department of Ophthalmology, University Hospital Leuven, Herestraat 49, Leuven, 3000, Belgium, Tel +32 470 86 72 56, Fax +32 16 33 23 67, Email [email protected]
Purpose: This retrospective observational study reports early results on a cohort of neovascular age-related macular degeneration (nAMD) patients switched to brolucizumab, a recently approved anti-vascular endothelial growth factor (anti-VEGF).
Patients and Methods: We evaluated best-corrected visual acuity (BCVA), treatment interval, central subfield retinal thickness (CST) and the presence of intra-retinal (IRF), subretinal (SRF) and/or sub-retinal pigment epithelium (sub-RPE) fluid on optical coherence tomography (OCT). Concurrently, patients were carefully examined for signs of intra-ocular inflammation (IOI) and other adverse events.
Results: Seventeen patients (19 eyes) were included. The difference in BCVA at baseline compared to the last examination following brolucizumab injection was not statistically significant (Wilcoxon signed-rank test, p=0.247). Mean CST decrease was − 5.16 ± 48.28 μm (p=0.647). A morphological improvement in IRF was observed in four eyes, with a complete resolution in 50% (n=2) and a decrease in 50% (n=2). Regarding SRF (total n=15), resolution was seen in 46.67% (n=7), decrease in 26.67% (n=4) and stabilization in 13.33% (n=2). Increase in SRF was observed in 13.33% (n=2). Of 14 eyes with sub-RPE fluid, 7.14% (n=1) demonstrated a resolution, 42.86% (n=6) a decrease, 50% (n=7) a stabilization and none an increase in fluid. Mean treatment interval was increased by 4.08 ± 1.40 weeks (p< 0.001). Treatment was discontinued in seven eyes (41.18%), including four cases due to IOI. In all four cases, inflammation was mild and resolved under corticosteroid treatment. No cases of vasculitis were observed.
Conclusion: This study provides additional data suggesting that brolucizumab is a beneficial alternative for patients refractory to other anti-VEGF therapies. It can provide a morphological reduction in fluid and prolong the treatment interval, while maintaining a stable BCVA and CST. However, as a higher occurrence of IOI is probable, patients should be informed, selected and monitored carefully. Signs of inflammation should be detected early and treated promptly.
Keywords: macular degeneration, vascular endothelial growth factor A, intravitreal injections, treatment outcome, inflammation
Introduction
Age-related macular degeneration (AMD) is a chronic progressive retinal disease and a leading cause of blindness in developed countries.1 Neovascular AMD (nAMD) is currently managed by repetitive intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF), inhibiting the pathological growth of new blood vessels and stabilizing or improving visual loss.1,2 This requires frequent clinic visits for injections and disease monitoring, causing a high burden for patients and health-care systems.3,4
Brolucizumab (Beovu®, Novartis) is a new type of anti-VEGF that was approved for the treatment of nAMD by the FDA on October 7 2019, and by the European Medicines Agency on February 13 2020. It consists of a single-chain antibody fragment that inhibits vascular-endothelial growth factor A (VEGF-A). Due to its small molecular weight of 26kDa, it allows for the delivery of a higher molar dose and thus greater concentration gradient. It consequently has the potential for more effective tissue penetration and increased drug distribution at the target site. This would lead to a more efficient inhibition of vascular leakage and sustained duration of action.5–7 Dugel et al5 performed two pivotal multicenter randomized Phase 3 trials (HAWK and HARRIER), comparing brolucizumab to aflibercept in the treatment of nAMD. They reported a non-inferiority of brolucizumab regarding best-corrected visual acuity (BCVA), better anatomical fluid outcomes assessed on optical coherence tomography (OCT) and a prolonged and more predictable duration of effect.5,8 Unfortunately, a higher occurrence of intra-ocular inflammation (IOI) was also observed in the HAWK and HARRIER as well as other clinical trials. In most cases, the IOI was mild to moderate and treatable with corticosteroids, but there were also cases of occlusive retinal vasculitis, raising serious concerns for the safety profile of brolucizumab.5,8–16
This study aims to report real-world experience with brolucizumab regarding efficacy, durability and safety as observed in a single-center trial. We evaluated effects on visual acuity, anatomical efficacy (resolution or decrease in fluid on OCT) and progression-free interval duration. Concurrently, a close monitoring of adverse events with meticulous attention to signs of IOI was performed.
Material and Methods
A clinical retrospective observational study was conducted at the University Hospital of Leuven, Belgium (NCT05220085). All patients were treated with brolucizumab between December 2020 and November 2021. The participants provided oral informed consent before study initiation. The BEL study (Beovu Experience Leuven) protocol was approved by an independent Ethics Committee and complied with the tenets of the Declaration of Helsinki.
Subjects
All patients were previously diagnosed with nAMD in at least one eye and treated with another type of anti-VEGF (bevacizumab, ranibizumab or aflibercept), but there was a need for “switch” or “extend” of this treatment. A need for “switch” was defined as a persistence of intraretinal (IRF), subretinal (SRF) or sub-retinal pigment epithelium (sub-RPE) fluid, despite 4-weekly anti-VEGF injections. In the “extend” group, there was an inability to prolong the 4-weekly interval of the current treatment due to disease recurrence in case of interval extension. Patients with any form of active IOI, any history of uveitis or previous inflammation, a history of a prior inflammatory reaction to anti-VEGF injections and monophthalmic patients were excluded. Additionally, in the first 6 months, no bilateral treatment was administered. Every patient treated with brolucizumab in our center (University Hospital Leuven) between December 2020 and November 2021 was included in the study.
Study Interventions
Baseline examinations consisted of BCVA assessment (using ETDRS-charts, reported in logMAR), slit-lamp examination, wide-field color picture imaging (CFP), wide-field fluorescein-angiography (FA), OCT and OCT angiography (OCTA). Included patients received an intravitreal injection of brolucizumab (6mg, 0.05mL) in a loading dose of 3 injections every 4 weeks. After loading dose, the interval could be prolonged in case of decreased or stable disease activity (treat and extend regimen), as decided by a trained retina specialist during follow-up visits (consisting of BCVA, CFP, OCT and OCTA). Concurrently, patients were carefully examined for adverse events with thorough attention to signs of IOI and vasculitis. FA was performed during follow-up visits in case of any clinical signs of inflammation (symptoms, cells in the anterior chamber or vitreous, fundoscopic signs of vitritis, chorioretinitis or vasculitis).
Assessment of Efficacy, Durability and Safety
Drug efficacy was monitored using BCVA, OCT and OCT-A. A qualitative assessment of retinal fluid was performed consisting of a thorough observation of the presence, reduction, increase or stabilization of IRF, SRF or sub-RPE fluid on OCT. The central subfield thickness (CST) was also measured and compared between the first and last visits. Durability was assessed by progression-free interval duration. During follow-up visits, disease activity and progression were carefully monitored by a trained retina specialist before intravitreal injection.
Patients were instructed to monitor clinical symptoms of IOI (decrease in visual acuity, pain, redness, floaters, scotomata) and to contact our hospital urgently for a reassessment in case of occurrence of these symptoms. Nevertheless, a thorough inspection for adverse events, signs of IOI and vasculitis was performed during clinic visits and before every intravitreal injection (using slit-lamp examination, CFP, FA, OCT and OCTA). When inflammation would occur, the patient would be closely monitored and treated promptly.17
Statistical Analysis
Data were collected retrospectively until November 2021. Descriptive statistics including mean, median, standard deviation (± SD) and range ([minimum-maximum]) were calculated for continuous variables. Additionally, paired samples t-test was used to measure mean differences, with a p-value <0.05 considered as statistically significant. Normality was assessed using the Shapiro–Wilk test, the Wilcoxon signed-rank test was used if sample data were not distributed normally (eg BCVA). All statistical analyses were performed using SPSS (IBM SPSS Statistics 28.0.0.0), and graphs were generated using Matplotlib 3.5.1.18
Results
Cohort Characteristics
A total of 17 patients (19 eyes) were included and observed between 9 December 2020 and 30 November 2021. We included 7 males (41.18%) and 10 females (58.82%). Mean age was 73.59 ±6.84 [65–86] years old. The indication for inclusion was a need for switch (n=12, 70.59%) or extend (n=5, 29.42%) using the current anti-VEGF regimen. The mean number of previous intravitreal injections was 43.82 ±23.58 [12–91], the mean treatment interval was 4.21 ±0.58 [3.5–5.5] weeks. A total of 88 brolucizumab injections were administered, the mean number of injections per patient was 5.18 ±2.6 [1–9]. Table 1 summarizes baseline characteristics. A more detailed overview (including treatment history) of the study population characteristics is also provided in Addendum Supplementary Table 1.
 |
Table 1 Cohort Characteristics at Baseline Visit |
Median follow-up time was 24 weeks [12–50]. In seven patients (41.18%), the treatment was stopped, 10 patients are still ongoing (58.82%). In two patients (11.76%), the other eye was also included after 6 months of successful response. The baseline of this eye was set at the visit before its first injection, and results were included in our analyses.
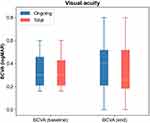
Visual Acuity
Mean (±SD and range) BCVA at baseline was 0.38 ±0.21 [0.16–1.0] logMAR and 0.35 ±0.22 [0–0.8] logMAR at the last examination following brolucizumab injection (Wilcoxon signed-rank test, p=0.247) for the total cohort of 17 patients. In the group of ongoing patients (n=10), the mean BCVA was 0.38 ±0.24 [0.16–1.0] and 0.32 ± 0.25 [0–0.8] for the first and last visits, respectively (Wilcoxon signed-rank test, p=0.063). A graphical representation is provided in Figure 1.
Anatomic Outcome
A qualitative assessment of fluid distribution as observed on OCT imaging was performed. Out of four eyes with IRF at the baseline visit, a resolution in fluid was seen in 2 (50%) and a stabilization in 2 (50%) eyes after brolucizumab injections. In the 15 eyes with SRF, 7 eyes (46.67%) showed a resolution, 4 (26.67%) a decrease and 2 (13.33%) a stabilization in fluid. An increase in SRF was observed in 2 (13.33%) eyes. 14 eyes showed sub-RPE fluid during baseline visit, after brolucizumab treatment 1 (7.14%) demonstrated a resolution, 6 (42.86%) a decrease, 7 (50%) a stabilization and none an increase in fluid. A graphical representation is provided in Figure 2.
The mean CST (shown in Figure 3) was 242 ±54.58 [161–400] µm at baseline and decreased to 236.84 ±51.30 [163–348] µm after the last brolucizumab administration (p=0.647). In the ongoing group, mean CST at the last follow-up was 214.92 ±34.62 [163–249] µm. The mean difference compared to baseline was lower, but statistically insignificant (p=0.230).
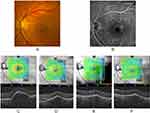
Additionally, Figure 4 demonstrates an example of a case in which the SRF is resolved and sub-RPE fluid is reduced after brolucizumab treatment.
Treatment Interval
The mean treatment interval of injections was 4.21 ±0.58 [3.5–5.5] weeks at baseline. In the ongoing group (n=10), the mean treatment interval at the last visit was 8.25 ±1.12 [6–10] weeks. This is a significant increase in mean 4.08 ±1.40 additional weeks (p<0.001). Patients who discontinued treatment were switched back to their previously used anti-VEGF drug and regimen.
Safety
Out of 17 patients, treatment was discontinued in 7 patients (41.18%) of which 4 cases (23.53%) were due to IOI. In total, 10 patients (58.82%) are still ongoing, with a bilateral treatment in 2 patients (11.76%).
Intra-Ocular Inflammation
In four cases, treatment with brolucizumab was stopped due to intra-ocular inflammation. Table 2 provides a descriptive overview of the management and outcome of these patients. A mild anterior chamber inflammation was observed in three eyes, with an associated mild vitritis in two eyes. All patients were immediately treated with topical steroids, after which inflammation resided quickly. In one eye, mild anterior inflammation 7 days after brolucizumab was accompanied by conjunctival and peri-ocular chemosis combined with extreme itching of the entire body and a diffuse maculopapular rash. The rash was treated successfully with oral antihistamines and local steroid ointments, and additional allergic testing was performed. The systemic reaction was most likely due to a delayed hypersensitivity reaction to fluorescein (two exams were performed within a 1-week interval to rule out vasculitis). However, 4 weeks later signs of vitritis were present, and a sub-tenon triamcinolone injection was administered, resulting in a complete resolution of vitritis after 1 week.
 |
Table 2 Descriptive Overview of the Four Patients Showing Signs of Intra-Ocular Inflammation During Brolucizumab Treatment |
None of the subjects showed signs of vasculitis during brolucizumab treatment.
Additional Exclusions and Observations
One patient reported a subjectively better vision during treatment with aflibercept. Another patient had an event of acute arterial hypertension and wished to discontinue brolucizumab treatment, but a causal relation was not established. A third patient had disease recurrence (with an increase in IRF and SRF) despite 6-weekly brolucizumab injections and was switched back to aflibercept. During the course of this study, Novartis® has recommended an interval duration of at least 8 weeks (after the loading dose) following an early termination of the MERLIN study due to an increased occurrence of IOI at 4-weekly intervals.16
In three patients (33.33% of phakic eyes), we observed an increase in lens opacifications in the treated eye. One patient underwent a successful cataract surgery during brolucizumab treatment. The other two patients showed mild-to-moderate lens opacities and are managed conservatively.
Discussion
This study reports the early real-world data on a cohort of 17 nAMD patients (19 eyes) switched to brolucizumab after an insufficient response to previous anti-VEGF agents. The efficacy, durability and safety profile of intravitreal brolucizumab injections were examined using retrospective data analysis.
When comparing baseline values to the last visit following brolucizumab injections, we observed a stable BCVA and CST in the total cohort. Differences in BCVA and CST between the first and last visits were statistically insignificant. Furthermore, our study confirms an effective further reduction in SRF, IRF and sub-RPE fluid in the majority of patients switched to brolucizumab. In only one eye a morphological increase in fluid (SRF) was observed on OCT-imaging. These findings mirror similar results by other reports. However, the previously reported significant reduction in CST was not achieved in our study population.5–8,19–21 Our study does confirm brolucizumab’s longer duration of effect as a prolonged treatment interval was possible for all 10 ongoing patients. In essence, this data confirms the potential of brolucizumab as a viable alternative for patients refractory to other anti-VEGF drugs as it can provide a morphological advantage and more sustainable duration of action while maintaining a stable vision.
Brolucizumab was discontinued in four eyes due to IOI. In most cases inflammation was mild and well controlled with topical steroids. One eye showed anterior chamber inflammation followed by vitritis that was also well-managed under sub-tenon corticoid injections. None of the patients showed signs of occlusive or non-occlusive vasculitis. Since the approval of brolucizumab, post-marketing reports disclosed an increased occurrence rate of IOI and retinal vasculitis, including retinal occlusive vasculitis with consequent severe visual loss.10–13 Moreover, a post-hoc review of the phase 3 HAWK and HARRIER trials conducted by an independent Safety Review Committee established by Novartis reported similar findings.8,14 After these safety concerns were raised, researchers advised to cease brolucizumab treatment in case of IOI, as the drug was now contra-indicated for patients with active IOI.14,15 Most cases of inflammation were observed within the first 6 months after initiation, however some occurred even after 12 months, advocating for a sustained vigilance for IOI beyond the loading phase.14 In our study, the inflammation occurred within the first 2 injections of brolucizumab in two eyes (50%). Furthermore, as all patients were screened with meticulous care for the occurrence of IOI and were instructed to urgently return to our clinic in case of clinical symptoms, we were able to detect and adequately treat the cases of inflammation at an early stage. Our data therefore support a close monitoring and patient counselling for signs of IOI. It also confirms that an early detection and treatment of IOI in brolucizumab-treated patients is pivotal for a good outcome.9,10,14,15,19,20
The immunogenic mechanism behind this increased occurrence of IOI and vasculitis is not yet clear as data is still limited.9,10 Some studies have suggested that female gender for example might provide a higher risk for the occurrence of IOI.10–12,20,21 In our study, however, 50% of IOI patients were female. Preliminary results from the BASICHR0049 study, a non-interventional study examining the blood samples of 11 patients treated with brolucizumab (5 with a subsequent retinal vasculitis/occlusion event and 6 controls), suggest a treatment-emergent B- and T-cell mediated immune response to brolucizumab in brolucizumab-associated retinal vasculitis/occlusion.22 More studies examining the underlying pathogenesis and risk factors are necessary, as they might provide essential information to better predict, prevent and manage inflammation in brolucizumab-treated patients.
Finally, a relatively high occurrence of increased lens opacifications was observed, but none of the intravitreal injection procedures were complicated with lens-touch. Excluding traumatic cataract induced by intravitreal injections, we found no other studies that reported an increased incidence of lens opacification after brolucizumab. As data is limited, it is currently uncertain that this finding is coincidental.
Limitations of the study include a small sample size, a short follow-up period and the absence of a control group. Data were collected retrospectively, and the study was performed in a single-center. It is observational and exploratory and aims to provide early results in a real-world setting, but the series is too small to make any definitive statements regarding effectivity or safety. While a non-inferiority of brolucizumab’s efficacy compared to aflibercept has been established in multiple reports,5,8 we cannot make any statements about this as our study was not comparative in nature. The strengths of this study include its description of results in a real-world setting concerning a group of patients with a high unmet need as they are refractory to other anti-VEGF therapies. For our patients, brolucizumab proved to be a durable alternative to their previous regimen. Additional research with larger cohorts and longer follow-up is necessary to provide additional data regarding safety, efficacy and durability.
Conclusion
The results of this cohort lead to conclude that brolucizumab is a viable alternative for patients refractory to other anti-VEGF drugs. It effectively reduces fluid in different retinal compartments and prolongs the treatment interval in nAMD patients while maintaining a stable vision. An increased occurrence of IOI was observed in our cohort. However, this study suggests that inflammation is predominantly mild and well-manageable under corticosteroid use, if detected and treated early. Patients should be informed, selected and monitored carefully. Further research examining the mechanisms behind the increased rate of inflammation as well as additional long-term analyses including larger population samples could provide more useful information regarding the efficacy, safety profile and durability of brolucizumab.
Data Sharing Statement
All data acquired during this study are included in the article and its online Supplementary Material. Further inquiries can be administered to the corresponding author.
Statement of Ethics
The study protocol was reviewed and approved by Ethics Committee Research UZ/KU Leuven, approval number S66240. As this was a retrospective study, the committee decided that written informed consent was not needed. The study complied with the tenets of the Declaration of Helsinki.
Acknowledgments
Novartis Pharma has provided the brolucizumab samples used in this study. There was no additional funding or role in this study. The company had no input into the content of this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
J.J. is a consultant for Novartis, Bayer, Roche and MONA.health. F.P. is a consultant for Novartis, Bayer, AbbVie, Horus Pharma and MONA.health. The authors report no other conflicts of interest in this work.
References
1. Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi:10.1016/S0140-6736(12)60282-7
2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
3. Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220–226. doi:10.1136/bjophthalmol-2014-305327
4. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–e16. doi:10.1016/S2214-109X(13)70145-1
5. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi:10.1016/j.ophtha.2019.04.017
6. Holz FG, Dugel PU, Weissgerber G, et al. Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration. Ophthalmology. 2016;123(5):1080–1089. doi:10.1016/j.ophtha.2015.12.030
7. Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124(9):1296–1304. doi:10.1016/j.ophtha.2017.03.057
8. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER. Ophthalmology. 2021;128(1):89–99. doi:10.1016/j.ophtha.2020.06.028
9. Sharma A, Kumar N, Parachuri N, et al. Brolucizumab and immunogenicity. Eye. 2020;34(10):1726–1728. doi:10.1038/s41433-020-0853-9
10. Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345–1359. doi:10.1016/j.ophtha.2020.04.017
11. Haug SJ, Hien DL, Uludag G, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18:100680. doi:10.1016/j.ajoc.2020.100680
12. Witkin AJ, Hahn P, Murray TG, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Retin Dis. 2020;4(4):269–279. doi:10.1177/2474126420930863
13. Jain A, Chea S, Matsumiya W, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep. 2020;2020:18.
14. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion–related events with brolucizumab. Ophthalmology. 2021;128(7):1050–1059. doi:10.1016/j.ophtha.2020.11.011
15. Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5(6):519–527. doi:10.1016/j.oret.2020.09.020
16. Novartis. Novartis reports one year results of Phase III MERLIN study evaluating Beovu® every four week dosing and provides update on Beovu clinical program; 2021. Available from: https://www.novartis.com/news/media-releases/novartis-reports-one-year-results-phase-iii-merlin-study-evaluating-beovu-every-four-week-dosing-and-provides-update-beovu-clinical-program.
17. Holz FG, Heinz C, Wolf A, Hoerauf H, Pleyer U. Intraokulare Entzündungen bei Brolucizumab-Anwendung. Der Ophthalmologe. 2021;118(3):248–256. doi:10.1007/s00347-021-01321-8
18. Hunter JD. Matplotlib: a 2D graphics environment. Comput Sci Eng. 2007;9(3):90–95. doi:10.1109/MCSE.2007.55
19. Bulirsch LM, Sassmannshausen M, Nadal J, Liegl R, Thiele S, Holz FG. Short-term real-world outcomes following intravitreal brolucizumab for neovascular AMD: SHIFT study. Br J Ophthalmol. 2021;106:1288–1294. doi:10.1136/bjophthalmol-2020-318672
20. Matsumoto H, Hoshino J, Mukai R, Nakamura K, Akiyama H. Short-term outcomes of intravitreal brolucizumab for treatment-naive neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci Rep. 2021;11(1):6759. doi:10.1038/s41598-021-86014-7
21. Enriquez AB, Baumal CR, Crane AM, et al. Early experience with brolucizumab treatment of neovascular age-related macular degeneration. JAMA Ophthalmol. 2021;139(4):441–448. doi:10.1001/jamaophthalmol.2020.7085
22. Schmouder R, Maciejewski B, Karle A, et al. Immunologic Features of Beovu®-Associated Retinal Vasculitis/Retinal Vascular Occlusion. Euretina Congress; 2021.
23. Jabs D, Nussenblatt R, Rosenbaum J, Becker M. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140:509–516.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.