Back to Journals » Infection and Drug Resistance » Volume 16
Brain Nocardiosis and Pulmonary Talaromycosis Infection in a Patient with Anti-IFN-γ Autoantibodies: A Case Report
Authors Wu S , Guo T, Zhang H, He Z , Zhang J, Zeng W
Received 3 June 2023
Accepted for publication 16 August 2023
Published 21 August 2023 Volume 2023:16 Pages 5421—5425
DOI https://doi.org/10.2147/IDR.S424212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Siyao Wu,1,* Ting Guo,2,* Hui Zhang,1 Zhiyi He,1 Jianquan Zhang,3 Wen Zeng1
1Department of Pulmonary and Critical Care Medicine, the First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China; 2Department of Dermatology and Venereology, the First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, People’s Republic of China; 3Department of Respiratory and Critical Medicine, the Eighth Affiliated Hospital, Sun Yat-Sen University, Shenzhen, Guangdong, 518000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wen Zeng, Department of Pulmonary and Critical Care Medicine, the First Affiliated Hospital of Guangxi Medical University, No. 6 Shuangyong Road, Nanning, Guangxi Zhuang Autonomous Region, 530021, People’s Republic of China, Tel +86 18589883694, Email [email protected]
Abstract: Adult-onset acquired immunodeficiency caused by anti-IFN-γ autoantibodies is associated with severe opportunistic infection. Due to lack of specific symptoms and different manifestations, this form of infection can be easily misdiagnosed or overlooked. Herein, we present a case of Nocardia farcinica and Talaromyces marneffei co-infection in a patient with anti-IFN-γ autoantibodies (AIGAs). The patient, a 54-year-old man, presented with a 1-month history of fever, coughing and expectoration, dizziness, headache and gait imbalance. Laboratory workup revealed increased inflammatory markers, negative anti-HIV antibody and a high positive titer of AIGAs. Chest computed tomography (CT) showed multiple patches of high-density shadows in both lungs, and brain enhanced magnetic resonance imaging (MRI) showed an irregular lesion. The patient underwent a craniotomy for resection of the lesion. Pulmonary T. marneffei infection was diagnosed through sputum and bronchoalveolar lavage fluid culture, and brain nocardiosis was confirmed via purulent fluid culture of brain tissue. With regular antibiotic therapy, his symptoms improved and there was no recurrence during 18-month follow-up. This may be the first detailed case report detailing infection with these two distinct pathogens in disparate anatomical locations.
Keywords: nocardia, Talaromyces marneffei, adult-onset acquired immunodeficiency, anti-IFN-γ autoantibodies, brain abscess
Introduction
Talaromyces marneffei (TM) is a thermally dimorphic fungus that affects multiple organs, including lung, lymph nodes, skin and bones. In the past, TM infection was primarily reported in patients with HIV. However, there has been a surge in reported cases of TM infection among individuals with anti-IFN-γ autoantibodies (AIGAs) in Southeast Asia.1 Nocardia is an aerobic Gram-positive filamentous bacterium that causes a rare but potentially fatal disease. Elderly individuals with diabetes and chronic obstructive pulmonary disease, as well as those who have undergone solid organ or hematopoietic stem cell transplantation, or are infected with HIV, constitute the population at risk for nocardiosis.2 Despite increasing evidence showing that compromised cellular immunity is a particularly strong risk factor for nocardiosis and TM infection,3 early diagnosis and timely treatment remain challenging due to non-specific symptoms and diverse manifestations, especially in HIV-negative patients. Here, we report a case of nocardia and TM co-infection in distinct systems with immunodeficiency due to AIGAs, which was successfully treated.
Case Presentation
A 54-year-old male patient, a native and resident of Guangxi, China, was admitted to our hospital with a 1-month history of fever, coughing with expectoration, dizziness, headache and gait imbalance for 9 days. He was a smoker and had no history of organ transplants, leukemia, diabetes and autoimmune disease.
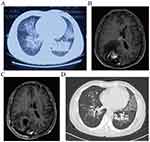
Basic laboratory workup revealed increased levels of leukocytes (10.12× 109/L), lymphocytes (3.55× 109/L) and erythrocyte sedimentation rate (ESR, 46mm/h). The CD4+ T-cell count was 1181 per mm3 (561–1,137 per mm3), and NK cell percentage was 33.56% (9.5–23.5%). The anti-HIV antibody was negative, and the absolute neutrophil count, IgG, IgM as well as C-reactive protein were all within normal limits. A chest computed tomography (CT) showed multiple high-density patches of shadows in both lungs (Figure 1A). Brain CT showed a cerebral infarction in the right occipital lobe, and brain enhanced magnetic resonance imaging (MRI) showed an irregular lesion measuring up to 3.5 cm × 2.5 cm × 3.2cm. (Figure 1B). T. marneffei isolated from sputum and bronchoalveolar lavage fluid (BALF) culture (Figure 2A), while blood cultures were negative. After receiving antifungal treatment with voriconazole (0.2g twice daily), the patient’s fever, coughing and expectoration symptoms improved 1 week later. However, he presented with progressive neurological symptoms characterized by exacerbating headache and bilateral visual impairment. As a result, the patient underwent a craniotomy for resection of the lesion.
Histological examination of brain tissue revealed no evidence of malignancy but suggested the presence of a chronic brain abscess with Gram-positive filamentous bacilli (Figure 2B). The diagnosis of Nocardia brain abscess was confirmed by the identification of Nocardia farcinica in purulent fluid culture, while testing for TM yielded negative results. Subsequently, the patient was diagnosed with brain nocardiosis and pulmonary talaromycosis. We initiated anti-infective therapy with oral sulfamethoxazole-trimethoprim (SMX 0.8g and TMP 0.16g three times daily) and moxifloxacin (0.4g once daily). With the implementation of the aforementioned treatment, there was a significant improvement in his symptoms including headache, gait imbalance and vision loss in both eyes, as well as complete resolution of the intracranial mass (Figure 1C). Serum sample was obtained to test the detection of AIGAs through an enzyme-linked immunosorbent assay kit (USCN Life Science Inc., Wuhan, China) according to the manufacturer’s protocols. We found that he had a high positive titer (15460ng/mL) and the positive titer value was based on our previous study.4
After 1-month treatment, CT scan showed improvement in lung lesions (Figure 1D), and there was no change in brain MRI. AIGAs turned negative (3021ng/mL) after 3 months. Overall, SMX-TMP and moxifloxacin were administered for 9 months, and the voriconazole was for 12 months. No relapse of either nocardia brain abscess or pulmonary TM infection had been observed during 18-month follow-up.
Discussion
Adult-onset acquired immunodeficiency, mediated by anti–IFN-γ autoantibodies, is strongly related to TM infection in HIV-negative patients.5 Nocardiosis is a potentially life-threatening disease that most commonly affects the lungs, central nervous system (CNS), and skin. Among them, CNS involvement is particularly common in immunocompromised patients.2 MRI imaging features of brain abscess mainly exhibit ring enhancement during the capsular phase. However, distinguishing between ring-enhancing glioblastoma and brain abscesses can sometimes be challenging.6 In our report, the patient was a middle-aged man without obvious immunosuppressive factors. He was initially diagnosed with a pulmonary TM infection. However, during successful antifungal therapy for TM infection, he developed neurological symptoms and was ultimately diagnosed with nocardia brain abscess. We obtained peripheral blood serum from the patient and detected the presence of AIGAs, which yielded a positive result. The inhibitory effect of AIGAs on CD4+T cell differentiation into Th1 cells led to persistent impairment of cell-mediated immune responses. Therefore, we identified AIGAs as the most significant risk factor, which contributed to the patient’s susceptibility to intracellular pathogens. Patients with AIGAs are susceptible to multiple pathogens, including nontuberculous mycobacteria (NTM), Varicella-zoster virus, T. marneffei, Salmonella spp, Cryptococcus spp and others. Zhang et al reported nocardiosis in patients with AIGAs previously, but there was no specific detail.7 This may be the first detailed case report of infection with these two different pathogens in different sites.
Initial therapy with amphotericin B deoxycholate (dAmB) has been the standard treatment for TM infection.8 However, because of its strong toxicity, voriconazole has been successfully used in the treatment of TM infection with or without HIV, and its response rates in both the primary and follow-up evaluations were similar to those in dAmB.9 Moreover, voriconazole is more effective than dAmB in patients with central fungal infection.10 The optimal duration of voriconazole treatment for HIV-negative patients with TM remains uncertain. Based on the current reports, antifungal therapy is recommended to be maintained for at least 1 year to prevent recurrence.11 SMX-TMP can cross the blood–brain barrier and most authorities recommend it as the keystone of nocardiosis treatment. However, N. farcinica has been identified as the most frequently isolated species in China, with a resistance rate to SMX-TMP reaching 53.8%.12,13 Due to the successful use of moxifloxacin against cerebral abscess caused by N. farcinica,14 our patient was treated with a combination therapy of SMX-TMP and moxifloxacin. The recommended duration for nocardiosis treatment is at least 6 months.15,16 The patient had a high positive titer of AIGAs, which was a high-risk factor for relapse. Therefore, we discharged our patient with a prolonged course of treatment. Surgical intervention is necessary when abscesses are larger than 2.5cm in diameter, located in critical areas of the brain or causing significant mass effect.6 The patient’s brain abscess was 3.5cm in diameter, and he has obvious symptoms of elevated intracranial pressure; thus, the surgery is of great significance. In summary, with a 9-month therapy for nocardiosis and a 12-month regimen for TM, the patient remained recurrence-free and seroconverted to negative AIGAs during an 18-month follow-up. The study reported patients with high-titer AIGAs are more likely to have relapsed infections, but the recurrence rate could be reduced by decreasing the titer of AIGAs.4 Effective control of relapsed infection through reduction of AIGA titers has been achieved with anti-CD20 monoclonal antibody or intravenous cyclophosphamide therapy, but a minimum duration of 6 months of antibacterial treatment should be considered prior to initiating these therapies.17,18 Additionally, clinicians should remain vigilant for other pathogens when patients test positive for AIGAs titer, not just NTM or TM infections.
Conclusion
This is a rare report referring to pulmonary TM and brain nocardia co-infection in patients with AIGAs. Patients with AIGAs are susceptible to various intracellular infections, however, given the limited specificity of clinical manifestations, this type of infection may be easily overlooked. Early clinical suspicion, diagnosis, and timely treatment are critical for immunocompromised patients to avoid poor prognosis.
Abbreviations
TM, Talaromyces marneffei; AIGAs, anti-IFN-γ autoantibodies; CT, computed tomography; MRI, magnetic resonance imaging; ESR, erythrocyte sedimentation rate; BALF, bronchoalveolar lavage fluid; SMX-TMP, sulfamethoxazole-trimethoprim; CNS, central nervous system; NTM, nontuberculous mycobacteria; dAmB, amphotericin B deoxycholate.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This work was approved by the Ethical Review Committee of the First Affiliated Hospital of Guangxi Medical University (2023-E020-01). Written informed consent to have the case details and any accompanying images published have been provided by the patient.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by a Scientific research project funded by the Guangxi Health Commission (Z-A20230462).
Disclosure
The authors have no competing interests to declare.
References
1. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367(8):725–734. doi:10.1056/NEJMoa1111160
2. Margalit I, Lebeaux D, Tishler O, et al. How do I manage nocardiosis. Clin Microbiol Infect. 2021;27(4):550–558. doi:10.1016/j.cmi.2020.12.019
3. Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia. 2019;184(6):709–720. doi:10.1007/s11046-019-00410-2
4. Zeng W, Qiu Y, Tang S, Zhang J, Pan M, Zhong X. Characterization of Anti-Interferon-γ Antibodies in HIV-Negative Patients Infected With Disseminated Talaromyces marneffei and Cryptococcosis. Open Forum Infect Dis. 2019;6(10):ofz208. doi:10.1093/ofid/ofz208
5. Guo J, Ning XQ, Ding JY, et al. Anti-IFN-γ autoantibodies underlie disseminated Talaromyces marneffei infections. J Exp Med. 2020;217(12). doi:10.1084/jem.20190502
6. Zhu JW, Zhou H, Jia WQ, You J, Xu RX. A clinical case report of brain abscess caused by Nocardia brasiliensis in a non-immunocompromised patient and a relevant literature review. BMC Infect Dis. 2020;20(1):328. doi:10.1186/s12879-020-05052-0
7. Qiu Y, Fang G, Ye F, et al. Pathogen spectrum and immunotherapy in patients with anti-IFN-γ autoantibodies: a multicenter retrospective study and systematic review. Front Immunol. 2022;13:1051673. doi:10.3389/fimmu.2022.1051673
8. Le T, Kinh NV, Cuc N, et al. A Trial of Itraconazole or Amphotericin B for HIV-Associated Talaromycosis. N Engl J Med. 2017;376(24):2329–2340. doi:10.1056/NEJMoa1613306
9. Huang W, Li T, Zhou C, Wei F, Cao C, Jiang J. Voriconazole Versus Amphotericin B as Induction Therapy for Talaromycosis in HIV/AIDS Patients: a Retrospective Study. Mycopathologia. 2021;186(2):269–276. doi:10.1007/s11046-021-00533-5
10. Nesky MA, McDougal EC, Peacock JE. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin Infect Dis. 2000;31(3):673–677. doi:10.1086/314042
11. Ouyang Y, Cai S, Liang H, Cao C. Administration of Voriconazole in Disseminated Talaromyces (Penicillium) Marneffei Infection: a Retrospective Study. Mycopathologia. 2017;182(5–6):569–575. doi:10.1007/s11046-016-0107-3
12. Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94(2):165–172. doi:10.1016/j.diagmicrobio.2018.12.007
13. Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407. doi:10.1016/j.mayocp.2011.11.016
14. Miksits K, Stoltenburg G, Neumayer HH, et al. Disseminated infection of the central nervous system caused by Nocardia farcinica. Nephrol Dial Transplant. 1991;6(3):209–214. doi:10.1093/ndt/6.3.209
15. Wallace RJ, Septimus EJ, Williams TW, et al. Use of trimethoprim-sulfamethoxazole for treatment of infections due to Nocardia. Rev Infect Dis. 1982;4(2):315–325. doi:10.1093/clinids/4.2.315
16. Anagnostou T, Arvanitis M, Kourkoumpetis TK, Desalermos A, Carneiro HA, Mylonakis E. Nocardiosis of the central nervous system: experience from a general hospital and review of 84 cases from the literature. Medicine. 2014;93(1):19–32. doi:10.1097/MD.0000000000000012
17. Browne SK, Zaman R, Sampaio EP, et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood. 2012;119(17):3933–3939. doi:10.1182/blood-2011-12-395707
18. Zeng W, Tang M, Yang M, Fang G, Tang S, Zhang J. Intravenous Cyclophosphamide Therapy for Anti-IFN-γ Autoantibody-Associated Talaromyces marneffei Infection. Open Forum Infect Dis. 2022;9(12):ofac612. doi:10.1093/ofid/ofac612
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.