Back to Journals » OncoTargets and Therapy » Volume 13
Brain Metastases of Non-Small Cell Lung Cancer: Magnetic Resonance Spectroscopy for Clinical Outcome Assessment in Patients with Stereotactic Radiotherapy
Authors Jia C , Li Z, Guo D , Zhang Z, Yu J , Jiang G, Xing X, Ji S , Jin F
Received 15 October 2020
Accepted for publication 7 December 2020
Published 22 December 2020 Volume 2020:13 Pages 13087—13096
DOI https://doi.org/10.2147/OTT.S286893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Congli Jia,1 Zhengquan Li,2 Dong Guo,1 Zhen Zhang,3 Jingming Yu,4 Guangdong Jiang,1 Xiaobo Xing,3 Shengjun Ji,5 Feng Jin3
1Weifang Medical University, Weifang, People’s Republic of China; 2Department of Laboratory Pathology, People’s Liberation Army 80th Group Military Hospital, Weifang, People’s Republic of China; 3Department of Radiotherapy & Oncology, Qingdao Center Hospital, Qingdao, People’s Republic of China; 4Department of Radiotherapy & Oncology, Shandong Hospital, Jinan, People’s Republic of China; 5Department of Radiotherapy & Oncology, Nanjing Medical University Affiliated Suzhou Hospital, Suzhou, People’s Republic of China
Correspondence: Shengjun Ji
Department of Radiotherapy & Oncology, Nanjing Medical University Affiliated Suzhou Hospital, No. 16 Baita Road, Suzhou 215001, People’s Republic of China
Email [email protected]
Feng Jin
Department of Radiotherapy & Oncology, Qingdao Center Hospital, No. 127 Siliu Road, Qingdao 266042, People’s Republic of China
Email [email protected]
Background: Brain metastases (BM) are severe incidents among patients with non-small cell lung cancer (NSCLC) and have been associated with significant morbidity and decreased survival; thus, new methods are required to improve clinical management. Magnetic resonance spectroscopy (MRS) allows noninvasive measurements of biochemical information from tumor tissue, providing clinically useful imaging biomarkers. The primary aim of this study was to explore the application of MRS in the assessment of tumor prognosis after stereotactic radiotherapy in NSCLC patients with BM.
Patients and Methods: MRS was performed on NSCLC patients attending Qingdao Center Hospital with suspected BM, and 68 patients were included in the survival analysis. The qualitative and quantitative parameters of MRS metabolites, such as choline (Cho), creatine (Cr), and N-acetyl-aspartate (NAA), were recorded. To select a cutoff for MRS metabolite parameters in the tumor and to distinguish patients who had recurrence, we performed an ROC curve analysis. Univariate and multivariate Cox regression analyses were used to assess the association between MRS metabolite parameters and clinical cancer prognosis.
Results: The average age was 56 years. A total of 68 NSCLC patients underwent metabolic evaluation with single voxel proton MRS and were selected for retrospective analysis. According to the area under the curve (AUC) to predict recurrence, the MRS metabolite parameters were determined as Cho (AUC=0.550), Cr (AUC=0.415), NAA (AUC=0.524), NAA/Cr (AUC=0.600), Cho/Cr (AUC=0.723), and Cho/NAA (AUC=0.543). Cho and Cr predicted poor survival while Cho/Cr and NAA/Cr predicted improved survival (P< 0.05). In the multivariate model with adjustment to establish the potential role of MRS metabolite parameters, Cho/Cr showed a significant association with OS (P=0.009) and PFS (P=0.006) after stereotactic radiotherapy.
Conclusion: The positive results of this study indicate the predictive value of metabolic characteristics of BM detected with MRS for the outcome after stereotactic radiotherapy.
Keywords: brain metastases, magnetic resonance spectroscopy, stereotactic radiotherapy
Introduction
Lung cancer is the most common cause of death from malignant tumors worldwide, and non-small cell lung cancer (NSCLC) accounts for approximately 80%‐85% of all lung cancer cases.1,2 Brain metastases (BM) are a frequent neurological complication of NSCLC and have historically been associated with a poorer prognosis. BM are estimated to occur in approximately 30–54% of patients with NSCLC after treatment.3,4 As shown in a recent multicenter clinical study on a group of patients with NSCLC with BM, the median survival time was 7 months with the best supportive care.5–8 Surgical resection and radiotherapy are the current local methods utilized in the treatment of BM in NSCLC, but evidence on how to utilize these local methods precisely is lacking. The lack of a consistent and reliable prognostic marker has prompted us to look for a better tool.
Molecular tests of metabolic processes in tumor tissue have provided new prognostic markers, which are starting to be incorporated into clinical management strategies.9,10 Novel noninvasive biochemical biomarkers of tumor prognosis would improve tumor characterization and would have the advantage of being available for cases where radiotherapy was not performed. The utility of advanced magnetic resonance imaging (MRI) techniques in diagnosis and evaluation of treatment response has been validated.11 This study explores to what extent magnetic resonance spectroscopy (MRS) can play a role in NSCLC patients with BM and analyzes clinical studies with particular reference to prognostic value.
Since the 1980s, MRS has been applied clinically for the examination of tumor metabolic processes,12 and this area of research continues to provide new insights into tumors.13,14 Clinical MRS enables the noninvasive evaluation of the biochemical composition of tumor tissues, which is used to examine metabolic alterations in cancers and measures the concentration of variety of biomolecules from a volume of interest.15 MRS is widely used to study the biology of tumor metabolism and has also been shown to effectively estimate treatment efficacy in tumors.16–18 Therefore, it is possible to investigate the metabolic features of BM that can be predictive for patient survival. However, thus far, no previous study has investigated MRS metabolite parameters for their prognostic potential significance in NSCLC with BM. Taking into account the characteristics of tumor metabolism, MRS may further improve outcome prediction. This information would be valuable to further identify high-risk patients for close surveillance and provide clinical benefit by identifying nonresponding patients for alternate therapy. In this study, we aimed to evaluate the predictive roles of MRS metabolite parameters as an imaging predictor of survival in NSCLC patients with BM.
Methods
Patient Selection and Study Design
We reviewed a total of 68 NSCLC patients with BM at Qingdao Center Hospital between March 2015 and September 2017. The patient flow chart is shown in Figure 1. BM were detected by enhanced MRI. Extracranial disease had been resected or stably controlled, and all patients had ≤3 BM. All NSCLC patients with BM signed a consent form to undergo MRS. MRI and MRS imaging were performed before treatment. The patient selection criteria were as follows: (i) surgery, percutaneous lung puncture biopsy or bronchoscopic biopsy pathological diagnosis of NSCLC; (ii) no contraindications to MRI; (iii) all initial Eastern Cooperative Oncology Group (ECOG) scores were ≤2; (iv) no neurosurgery, stroke, history of primary brain tumor; and (v) no other medical pathologies of the nervous system. Patient characteristics, including gender, age, ECOG score, smoking status, histology, primary T stage, primary N stage, primary AJCC stage, and MRS metabolic parameters were collected from electronic medical records. This retrospective cohort study was approved by the institutional review board of Qingdao Center Hospital institutions. All patients were provided with written informed consent. The study was performed in accordance with the International Conference on Harmonisation Guidelines on Good Clinical Practice and the Declaration of Helsinki.
 |
Figure 1 Flow diagram of the patient selection process. |
1H-MRS Metabolic Characteristics of BM
All patients underwent MRI (T1, T2-weighted and dynamic contrast-enhanced images) and single-voxel 1H-MRS using 3.0 T clinical scanner magnetic resonance machine (Philips Healthcare, Andover, MA, USA) prior to treatment. The point-resolved spectroscopic (PRESS) method was used for multivoxel MRS sequence scanning (TR:2000 ms, TE:144 ms, FOV: 230 mm×230 mm, slice thickness: 10 mm, scan time: 350 seconds, voxel size: 1×1×1mm3). The multivoxel MRS imaging was acquired in two different tissues: tumor tissue and normal tissue, and avoids influencing factors the scalp, such as necrosis, skull, bleeding, and blood vessels. The scan program performed water suppression scanning and voxel shimming. (Figure 2) A commercially available imaging workstation was used for postprocessing of 1H-MRS metabolite data. The MRI and 1H-MRS of patients were interpreted by two experienced radiologists who were aware of the tumor location and draw the MRS regions of interest. The center of the voxel grid is located in the maximum area of the lesion cross-section. The relative metabolite intensities of the signals from choline (Cho), N-acetyl-aspartate (NAA), and creatine (Cr) in the tumor voxels were analyzed. The metabolite ratios examined included Cho/NAA, Cho/Cr, and NAA/Cr.
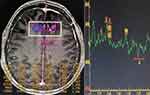 |
Figure 2 Data acquired at 3.0 T with short echo time elliptical excitation chemical shift imaging. |
Response Evaluation and Follow-Up
Tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST)1.1.19 According to the RECIST guidelines, complete response (CR) was defined as total regression of all assessable lesions; partial response (PR) was defined as a decrease of at least 30% in the sum of the longest dimensions of the target lesions; progressive disease (PD) was defined as more than a 20% increase in primary tumor volume or appearance of new lesions; the remaining patients which did not meet the criteria of PD or PR were categorized as stable disease (SD). Follow-up protocol was performed at regular intervals: 3 monthly for the first 3 years, 6 monthly during the following year and annually thereafter. The MRI/MRS was performed during the follow-up and the patients received laboratory tests, physical examination, computed tomography or PET-CT for evaluation of primary and distant metastasis. Besides, we performed the imaging examination at every follow-up. During the follow-up, all patients did not receive any systematic treatment. If the patients have recurrence or metastasis, we conducted local or systemic treatment. The endpoints overall survival (OS) and progression-free survival (PFS), were defined as the time from the start of radiotherapy to the date of death and tumor progression, respectively. Follow-up was conducted on 68 patients until November 2018. The median follow-up time was 16 months (range: 8–36 months).
Statistical Analysis
SPSS 23.0 software (SPSS Inc., Chicago, IL, USA) was used in all statistical analyses. Associations of the tumor response to radiotherapy with 1H-MRS-detected metabolic characteristics of BM were evaluated by the chi-square test with continuity correction. The sensitivity and specificity were calculated using a receiver operating characteristic (ROC) analysis. To test our research hypothesis, univariate Cox regression was performed on the data of each individual measured metabolite. Categorical variables with P<0.05 in univariate Cox regression analysis were included in a multivariate Cox regression model. Differences were considered significant when P<0.05; all P values presented are two-sided.
Results
Patient Characteristics
Table 1 presents the clinical characteristics of 68 enrolled patients, 34 women (50%) and 34 men (50%), and all received stereotactic radiotherapy (48–60Gy/6-8 fraction, median: 8Gy). The average age of patients in this study was 56 years (range, 48–71 years). In the majority of patients, the histological type of NSCLC was adenocarcinoma. A total of 36 (52.9%) patients had a prior history of smoking. There were 29 patients of intracranial progression, 57 patients with any progression, 55 deaths at the time of the final follow-up. The median survival time of the included patients was 19.5 months.
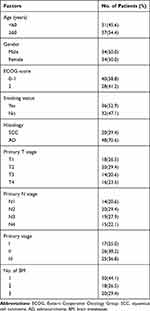 |
Table 1 Baseline Clinical Characteristics of 68 Patients with BM from NSCLC |
Analysis of 1H-MRS Metabolites
The patients had a median Cho of 2.05 (range, 0.93–3.90), NAA of 0.78 (range, 0.23–1.71), Cr of 1.27 (range, 0.45–2.10), Cho/Cr of 1.65 (range, 0.72–5.55), Cho/NAA of 2.48 (range, 0.88–13.13) and NAA/Cr of 0.63 (range, 0.18–1.91). All of the parameters that are calculated from 1H-MRS are summarized in Table 2. We attempted to establish the optimal cutoff value for 1H-MRS metabolite parameters in our study population through ROC curve analysis. Cho/Cr was found to have the largest area under the curve (AUC=0.723; 95% confidence interval [CI], 0.594–0.851; P=0.002). (Figure 3) The AUCs were 0.524 (P=0.739), 0.550 (P=0.481), 0.415 (P=0.233), 0.543 (P=0.545) and 0.600 (P=0.159) for NAA, Cho, Cr, Cho/NAA and NAA/Cr, respectively. The optimal cutoff value for the NAA, Cho, Cr, Cho/NAA, Cho/Cr and NAA/Cr were 0.50, 1.50, 0.50, 3.61, 1.46 and 0.99, respectively. Based on the 1H-MRS metabolite parameter cutoff values, the patients were stratified into two groups.
 |
Table 2 1H-MRS-Detected Metabolic Characteristics of Brain Metastases for NSCLC Patients |
 |
Figure 3 ROC curve of Cho/Cr for recurrence prediction. |
Prognostic Value of 1H-MRS Metabolite Parameters and Clinicopathological Factors
The results of the univariate analyses of clinicopathological factors are included in Table 3. Univariate analysis demonstrated that age (HR, 2.182; 95% CI, 1.067–4.460; P=0.032) is correlated with OS, and we found no significant association between OS and clinicopathological characteristics, including gender, primary T stage, primary N stage, primary stage, histology, ECOG score, and smoking status (all P>0.05). Age (HR, 2.530; 95% CI, 1.221–5.242; P=0.013) and ECOG score (HR, 2.140; 95% CI, 1.103–4.155; P=0.025) were significantly associated with PFS (Figure 4). Table 4 shows the findings from univariate analyses of the 1H-MRS metabolite parameters. In the univariate analyses, Cho/Cr (HR, 3.424; 95% CI, 1.578–7.432; P=0.002) predicted OS, and Cho/Cr (HR, 5.167; 95% CI, 1.997–13.364; P=0.001) and NAA/Cr (HR, 2.686; 95% CI, 1.341–5.380; P=0.005) predicted PFS. We did not find significant correlations between OS and the following metabolite parameters: Cho, Cr, NAA, NAA/Cr, and Cho/NAA (all P>0.05).
 |
Table 3 A Summary of the Univariate Cox Regression Model Based on Baseline Characteristics |
 |
Table 4 A Summary of the Univariate Cox Regression Model Based on 1H-MRS Parameters |
 |
Figure 4 Survival curves for overall survival (A) and progression-free survival (B) in Cho/Cr≤1.46 and Cho/Cr>1.46 group in non-small cell lung cancer patients with brain metastases. |
Multivariate Analysis of Independent Prognostic Indicators
As shown in Table 5, age, ECOG score, NAA/Cr and Cho/Cr were included in the multivariate analyses. The results showed that Cho/Cr was significantly related to OS (HR, 2.956; 95% CI, 1.315–6.645; P=0.009) and PFS (HR, 3.925; 95% CI, 1.489–10.348; P=0.006). Age could not be a prognostic factor for OS (P=0.235) and PFS (P=0.135). Therefore, multivariate analysis demonstrated that Cho/Cr is considered an independent prognostic indicator for OS and PFS.
 |
Table 5 Multivarite Analysis of OS and PFS in 68 NSCLC Patients with BM |
Discussion
Metabolic alterations of tumor tissues are associated with patient prognosis. The analysis of metabolic feature composition can offer an early approach to assess the association between metabolic parameter and outcomes. This study showed that the Cho/Cr levels of tumors, detected noninvasively by 1H-MRS at diagnosis, predict survival in a cohort of NSCLC patients with BM, and Cho/Cr was validated as the only independent factor for OS and PFS in the multivariate analysis. Gender, primary T stage, primary N stage, primary stage, histology, ECOG score, and smoking status had no significant effect on survival.
Metabolic alterations are one of the core features of tumor cells and are considered to be an important indicator of tumor diagnosis and evaluation of tumor biological behavior. In tumors, metabolites can be monitored before treatment by MRS, and MRS shows great promise as an imaging technique for identifying benign and malignant tumors in the brain.20,21 In recent years, studies have reported that MRS metabolic parameters can reflect the biochemical composition of brain tumors and can provide valuable prognostic information in gliomas. More comprehensive descriptions of the molecular basis for the known biochemical changes in metabolites in tumors are available. The typical MRS-detected metabolic profile of brain metastases includes reduction in Cr and NAA, an increase in Cho and appearance of Lip peaks.16,22,23 Cho reflects the turnover of the cell membrane during normal cell proliferation.24,25 A greater decrease in Cho may be related to less tumor proliferation, and thus better prognosis.26 The findings of Maheshwari et al24 prove that the Cho signal detected by MRS is strongly correlated with phosphocholine and free Cho, while phosphatidylcholine in intact cell membranes is not. Therefore, we speculate that the Cho biological signal may reflect cell proliferation and cell death. Therefore, a smaller decrease in Cho may actually reflect an increase in tumor cell death, which could account for the improved clinical outcomes. In a study by Tedeschi et al,27 all progressive cases showed a Cho signal increase between studies of more than 45%, whereas all stable cases showed an elevation of less than 35%, no change, or even a decreased signal. Wald et al28 found that a reduction in Cho levels indicates the transformation of tumors to necrotic tissue by MRS imaging. Menard et al29 conducted a histopathologic study of diagnostic validity to evaluate the value of Cho levels in a malignant prostate gland, and the results confirmed that Cho levels were correlated with tumor recurrence. In a study of malignant breast tumors, Mackinnon et al30 found that the Cho peak detected by MRS could distinguish between benign and malignant breast tumors with relatively high sensitivity and specificity. However, our results demonstrated that the Cho levels of BM are not an independent prognostic factor (P=0.459 for OS and P=0.242 for PFS). These discrepancies may reflect the heterogeneity of patients in each clinical study.
In addition to the Cho results, another important finding is that patients with higher Cho/Cr had worse prognosis, which is consistent with the expected high rates of proliferation in aggressive tumors. Matsusue et al31 revealed that Cho/Cr (P<0.01) has the potential to improve the overall diagnostic accuracy in distinguishing glioma progression. Fink et al32 found that Cho/Cr (AUC=0.913, P=0.002) appear to outperform DWI for distinguishing glioma recurrence. A study of eighty-five cancer patients with brain metastases demonstrated that a decrease in the Cho/Cr ratio likely reflects an inhibition of proliferative activity and early apoptotic cell death (P<0.001).33 Despite the positive trends observed, recent work of Liu et al34 showed that Cho/Cr >2 is not significantly correlated with mortality from brain metastases in lung cancer patients after radiotherapy. Can MRS accurately evaluate survival and tumor progression after radiotherapy for BM in NSCLC patients? The results of the Kaplan-Meier analysis and Log rank test of our study revealed that patients with elevated Cho/Cr values (Cho/Cr>1.46) exhibited a poorer prognosis than those with Cho/Cr≤1.46 (OS, P=0.002; PFS, P=0.001). In our work, multivariate analysis performed using the characteristics selected in the univariate analysis revealed that Cho/Cr was significantly correlated with mortality (P=0.009) and tumor progression (P=0.006). Combined with previous research, these data indicate that MRS metabolic parameters can play an important role in cancer prognosis.
Our research has several limitations, including the limited number of enrolled patients from a single-center retrospective study, which can be associated with inherent selectivity bias. In addition, although we used statistical methods accurately, the variable cutoff values of NAA, Cho, Cr, Cho/Cr, Cho/NAA and NAA/Cr are limitations to this study. Since the cutoff values in this and other previous studies analyzing MRS metabolic parameters are not consistent, another large-scale study or prospective study is required to confirm accurate cutoff values. Therefore, the results need to be further validated in larger cohorts and prospective studies.
After stereotactic radiotherapy for BM, the patients with elevated Cho/Cr values had poorer survival, but increased NAA, Cho, Cr, Cho/NAA and NAA/Cr did not serve as reliable indicators of BM progression.
Acknowledgments
This study was supported by Grant (2020117) from the The Gusu Health Talent Program, a grant from the National Key research, Suzhou Cancer Clinical Medical Center (Szzx201506) and Develop Program of China (No.2018YFC1313200).
Disclosure
The authors report no conflicts of interest in this work.
References
1. National Collaborating Centre for Cancer (UK). The diagnosis and treatment of lung cancer (Update). Cardiff. 2011.
2. Robnett TJ, Machtay M, Stevenson JP, et al. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol. 2001;19(5):1344–1349. doi:10.1200/JCO.2001.19.5.1344
3. Carolan H, Sun AY, Bezjak A, et al. Does the incidence and outcome of brain metastases in locally advanced non-small cell lung cancer justify prophylactic cranial irradiation or early detection? Lung Cancer. 2005;49(1):109–115. doi:10.1016/j.lungcan.2004.12.004
4. Park HS, Decker RH, Wilson LD, Yu JB. Prophylactic cranial irradiation for patients with locally advanced non-small-cell lung cancer at high risk for brain metastases. Clin Lung Cancer. 2015;16(4):292–297. doi:10.1016/j.cllc.2014.11.005
5. Burel-Vandenbos F, Ambrosetti D, Coutts M, Pedeutour F. EGFR mutation status in brain metastases of non-small cell lung carcinoma. J Neurooncol. 2013;111(1):1–10. doi:10.1007/s11060-012-0990-5
6. Chen AM, Jahan TM, Jablons DM, et al. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: clinical implications for the subsequent management of the brain. Cancer. 2007;109(8):1668–1675. doi:10.1002/cncr.22565
7. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. doi:10.1016/S0360-3016(96)00619-0
8. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi:10.1200/JCO.2011.38.0527
9. Cho YJ, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430.
10. Johnson R, Wright KD, Gilbertson RJ. Molecular profiling of pediatric brain tumors: insight into biology and treatment. Curr Oncol Rep. 2009;11(1):68–72. doi:10.1007/s11912-009-0011-9
11. Tong E, McCullagh KL, Iv M. Advanced imaging of brain metastases: from augmenting visualization and improving diagnosis to evaluating treatment response. Front Neurol. 2020;11:270. doi:10.3389/fneur.2020.00270
12. Evanochko WT, Ng TC, Glickson JD. Application of in vivo NMR spectroscopy to cancer. Magn Reson Med. 1984;1(4):508–534. doi:10.1002/mrm.1910010410
13. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033.
14. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309
15. Sorensen AG. Magnetic resonance as a cancer imaging biomarker. J Clin Oncol. 2006;24(20):3274–3281. doi:10.1200/JCO.2006.06.6597
16. Chernov MF, Hayashi M, Izawa M, et al. Proton magnetic resonance spectroscopy (MRS) of metastatic brain tumors: variations of metabolic profile. Int J Clin Oncol. 2006;11(5):375–384. doi:10.1007/s10147-006-0589-y
17. Chernov MF, Ono Y, Kubo O, Hori T. Comparison of 1H-MRS-detected metabolic characteristics in single metastatic brain tumors of different origin. Brain Tumor Pathol. 2006;23(1):35–40. doi:10.1007/s10014-006-0198-5
18. Sijens PE, van Dijk P, Oudkerk M. Correlation between choline level and Gd-DTPA enhancement in patients with brain metastases of mammary carcinoma. Magn Reson Med. 1994;32(5):549–555. doi:10.1002/mrm.1910320502
19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
20. Liimatainen T, Hakumaki JM, Kauppinen RA, Ala-Korpela M. Monitoring of gliomas in vivo by diffusion MRI and (1) H MRS during gene therapy-induced apoptosis: interrelationships between water diffusion and mobile lipids. NMR Biomed. 2009;22(3):272–279. doi:10.1002/nbm.1320
21. Lotumolo A, Caivano R, Rabasco P, et al. Comparison between magnetic resonance spectroscopy and diffusion weighted imaging in the evaluation of gliomas response after treatment. Eur J Radiol. 2015;84(12):2597–2604. doi:10.1016/j.ejrad.2015.09.005
22. Ishimaru H, Morikawa M, Iwanaga S, et al. Differentiation between high-grade glioma and metastatic brain tumor using single-voxel proton MR spectroscopy. Eur Radiol. 2001;11(9):1784–1791. doi:10.1007/s003300000814
23. Moller-Hartmann W, Herminghaus S, Krings T, et al. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002;44(5):371–381. doi:10.1007/s00234-001-0760-0
24. Maheshwari SR, Fatterpekar GM, Castillo M, Mukherji SK. Proton MR spectroscopy of the brain. Semin Ultrasound CT MR. 2000;21(6):434–451. doi:10.1016/S0887-2171(00)90036-2
25. Miller BL, Chang L, Booth R, et al. In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci. 1996;58(22):1929–1935. doi:10.1016/0024-3205(96)00182-8
26. Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59(1):80–84.
27. Tedeschi G, Lundbom N, Raman R, et al. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg. 1997;87(4):516–524. doi:10.3171/jns.1997.87.4.0516
28. Wald LL, Nelson SJ, Day MR, et al. Serial proton magnetic resonance spectroscopy imaging of glioblastoma multiforme after brachytherapy. J Neurosurg. 1997;87(4):525–534. doi:10.3171/jns.1997.87.4.0525
29. Menard C, Smith IC, Somorjai RL, et al. Magnetic resonance spectroscopy of the malignant prostate gland after radiotherapy: a histopathologic study of diagnostic validity. Int J Radiat Oncol Biol Phys. 2001;50(2):317–323. doi:10.1016/S0360-3016(01)01480-8
30. Mackinnon WB, Barry PA, Malycha PL, et al. Fine-needle biopsy specimens of benign breast lesions distinguished from invasive cancer ex vivo with proton MR spectroscopy. Radiology. 1997;204(3):661–666. doi:10.1148/radiology.204.3.9280241
31. Matsusue E, Fink JR, Rockhill JK, et al. Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology. 2010;52(4):297–306. doi:10.1007/s00234-009-0613-9
32. Fink JR, Carr RB, Matsusue E, et al. Comparison of 3 Tesla proton MR spectroscopy, MR perfusion and MR diffusion for distinguishing glioma recurrence from posttreatment effects. J Magn Reson Imaging. 2012;35(1):56–63. doi:10.1002/jmri.22801
33. Chernov MF, Hayashi M, Izawa M, et al. Early metabolic changes in metastatic brain tumors after gamma knife radiosurgery: 1H-MRS study. Brain Tumor Pathol. 2004;21(2):63–67. doi:10.1007/BF02484512
34. Liu Y, Liu X, Xu L, et al. Magnetic resonance imaging evaluation of treatment efficacy and prognosis for brain metastases in lung cancer patients after radiotherapy: a preliminary study. Thorac Cancer. 2018;9(7):865–873. doi:10.1111/1759-7714.12763
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
