Back to Journals » Journal of Pain Research » Volume 11
Both ipsilateral and contralateral localized vibratory stimulations modulated pain-related sensory thresholds on the foot in mice and humans
Authors Doi A , Sakasaki J, Tokunaga C , Sugita F , Kasae S , Nishimura K, Sato Y, Kuratsu T, Hashiguchi S, Shin MC, Yoshimura M
Received 12 January 2018
Accepted for publication 8 June 2018
Published 28 August 2018 Volume 2018:11 Pages 1645—1657
DOI https://doi.org/10.2147/JPR.S162379
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Erica Wegrzyn
Atsushi Doi,1,2,* Juntaro Sakasaki,3,* Chikato Tokunaga,4,* Fumiya Sugita,5,* Syota Kasae,6 Keisuke Nishimura,7 Yushi Sato,8 Takako Kuratsu,9 Sariya Hashiguchi,10 Min-Chul Shin,1,2 Megumu Yoshimura11
1Department of Physical Therapy, Kumamoto Health Science University, Kumamoto, Japan; 2Graduate School of Health Sciences, Kumamoto Health Science University, Kumamoto, Japan; 3Department of Rehabilitation, Tokyo-Wangan Rehabilitation Hospital, Narashino, Japan; 4Department of Rehabilitation, Himeno Hospital, Yame, Japan; 5Department of Rehabilitation, Tamana Central Hospital, Tamana, Japan; 6Department of Rehabilitation, Shimizu Hospital, Kyoto, Japan; 7Department of Rehabilitation, Iizuka Hospital, Iizuka, Japan; 8Department of Rehabilitation, Showa Hospital, Shimonoseki, Japan; 9Department of Rehabilitation, Konan Hospital, Kumamoto, Japan; 10Department of Rehabilitation, Asahino-Sogo Hospital, Kumamoto, Japan; 11Nakamura Hospital, Nogata, Japan
*These authors contributed equally to this work
Purpose: This study was aimed to investigate the effect of localized vibration on sensory thresholds in mice and humans using a novel quantitative method.
Participants and methods: The sensory thresholds of 7-week-old male C57BL/6J mice were measured with four sine-wave electrostimulation frequencies (5, 50, 250, and 2,000 Hz) before and after applying 2-minute vibration to the plantar side of the foot in mice. In human participants (16 males and 16 females; mean age, 21.0±0.8 years), the sensory threshold was measured at 50 Hz before and after applying 2-minute and 5-minute vibrations to the dorsal side of the foot.
Results: Application of a 2-minute vibration at either the ipsilateral or contralateral side modulated the sensory thresholds elicited by a 5- or 50-Hz right electrostimulation in mice. In human participants, application of a 5-minute vibration at either the ipsilateral or contralateral side modulated the sensory threshold elicited by 50-Hz right electrostimulation, but had no effect on local skin temperature. These results suggest that the right side of pain-related Aδ fibers (50 Hz) or C fibers (5 Hz) was modulated by the localized ipsilateral or contralateral side of vibratory stimuli, respectively, in mice and humans.
Conclusion: The ability of contralateral vibration to modify the right sensory thresholds suggests possible involvement of the central nervous system in vibratory modulation.
Keywords: vibration, sensory threshold, electrostimulation, central modulation
Introduction
Sensation can be categorized as either superficial or deep. In superficial sensation, tactile input mediated by Aβ fibers is transmitted to deep-layer neurons in the spinal cord dorsal horn.1 In contrast, superficial or noxious sensation mediated by fast (Aδ fiber) and slow (C fibers) pain fibers synapse with neurons of the substantia gelatinosa in the superficial dorsal horn.1 Aδ or C fiber-mediated pain is involved in numerous chronic pain states that can limit activities of daily living and cause disuse syndrome.
Although many scales for assessing clinical pain, such as the Visual Analog Scale,2 Faces Pain Scale,3 Verbal Numerical Rating Scale,4 Verbal Descriptor Scale,5 and McGill Pain Index,6,7 are available, none of them provide an objective measurement of pain. In contrast, several quantitative scales are used to assess pain in rodents, including the Von Frey filament test,8 tail flick test,9 and hot plate test.10 The Neurometer is used for electrostimulation-induced quantitation of sensory thresholds in both human subjects and rodents.11,12 The benefits of the Neurometer include the ability to represent the sensory threshold as current and the ability to stimulate Aβ, Aδ, and C fibers selectively.13–15 However, the Neurometer is potentially a stand-alone system and cannot alter the stimulation protocol.16 More recently, other specialized electrostimulation-based equipment was developed for quantifying pain, such as PainVision PS-2100 (Nipro Inc., Osaka, Japan) and the STG-4000 series (Multichannel Systems Inc., Reutlingen, Germany). PainVision has been used to quantitatively determine pain intensity as the “degree of pain” calculated from the current production of electrical threshold perception and the current production of a comparable pain sensation.17,18 The STG-4000 series generate stimuli for both current- and voltage-driven stimulations. Any arbitrary analog waveform can be designed as a stimulation signal for every single channel. The programmed stimulation is controlled by the PC-based software.
Numerous treatment strategies have been developed for chronic pain, including medications,19 massage,20 acupuncture,21 stretching,22 physical medicine,23 cognitive-behavioral therapy,24 and alternative therapies.25 In these treatments, especially, the mechanical stimulation (eg, thermal stimulation, pinch, or electrostimulation), which is contained in either the physical medicine or the alternative therapies, has already been reported to have an inhibitory effect on pain.26–28 For example, in 1982, Fitzgerald published a paper on “contralateral” pinch and heat stimulation inducing an inhibitory effect on neuronal activity in the spinal dorsal horn.28 Further, Le Bars et al reported that the activity of convergent dorsal neurons, which receive both low and high threshold afferent inputs, was inhibited by the noxious stimuli applied to various parts of the body.26,27 They proposed this phenomenon as a diffuse noxious inhibitory control (DNIC) hypothesis.26,27
Vibratory stimulation is another form of mechanical stimulation. Previous reports have indicated that whole-body vibration can suppress chronic low back pain,29 knee osteoarthritis,30 and peripheral neuropathy.31,32 Further, it has been reported that local vibratory stimulation also reduces pain in both animal33,34 and human subjects,35,36 increases skin temperature,37 has a positive effect on blood flow at both the exposed and unexposed sides,38 and decreases finger blood flow.39 Moreover, reports have been published on the inhibition of prolonged capsaicin-induced hyperalgesia, caused by daily vibratory stimulation,40 on the suppressive effect of the left vibration for vibrotactile thresholds at the left foot sole.41 It has also been reported that vibration-induced inhibition contains factors of both temporal and spatial summation, which is associated with the amplitude,42 frequency,43,44 threshold,45 duration,45 masking stimulus,46 stimulated mechanoreceptors,46,47 and location and area.48–50
Thus, although numerous studies on the inhibitory effects of noxious mechanical stimulation have already been published, the effects of the contralateral vibratory stimulus for the pain-related sensory threshold have still not been established with measurement of the quantitative sensory threshold. Furthermore, each previous study involved either animal or human experiments, and a comparative inter-species research using both animals and humans has not been reported so far. Therefore, the first purpose of this study was to examine the localized, vibration-induced, bilateral temporal and spatial inhibition of the sensory threshold. The second purpose was to investigate the differences of the response between mouse and human subjects.
Participants and methods
Animals
Male C57BL/6J mice (7 weeks old, 20–23 g, n=26) were purchased from Kyudo, Inc. (Kumamoto, Japan) and housed in an environment with controlled temperature (24°C±1°C) and humidity (55%±10%) on a 12-hour light–dark cycle with ad libitum access to food and water. All animal protocols were approved by the Animal Care Committees of Kumamoto Health Science University (approval no. 14-015) and were conducted in accordance with the National Institute of Health guide for the care and use of laboratory animals (NIH publications No. 80-23, revised 1996).
Vibratory stimuli and sensory threshold measurement in mice
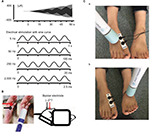
Briefly, a mouse was immobilized in a plastic tube while awake, which was further clamped with an adjusting magnetic base and stand (A-2, Shinwa Rules Co, Sanjyo, Niigata, Japan) and lab clamp (NC-3, Kenis, Osaka, Japan). A vibration device (HB-M01-A, Electric Inc., Tokyo, Japan), which is commercially available at low cost and used for humans, was clamped to the examiner’s hand. The device was then attached to the distal and plantar sides of the right hind paw. The vibration was delivered at the foot sole of either the right (ipsilateral) or left (contralateral) side for 2 minutes (at a frequency of 4,900 times/min; Figure 1).
Sensory thresholds were measured with 5, 50, 250, and 2,000 Hz sine electrostimulation (STG-4002, Multichannel Systems Inc.; Figure 1). The four different electrostimulation frequencies that we used in this study can stimulate Aβ fibers (2,000 Hz), Aδ fibers (50 and 250 Hz), and C fibers (5 Hz).13–15 Therefore, by using these four electrostimulation frequencies, we were able to evaluate the threshold of each of Aβ, Aδ, and C fibers. Under the immobilized condition of the mice, ball-type bipolar electrodes were placed on the plantar side of the right hind paw, and electrostimulation was applied to the plantar side because the knee joint was flexed maximally and the ankle joint was also flexed dorsally under the supine position. Therefore, since the dorsal side of the foot was hidden, we could not stimulate the dorsal side of the hind paw (Figure 1). The electrostimulation-induced withdrawal reflex of the mouse’s hind limb resulted in loss of contact with the electrode. The time from the onset of electrostimulation to the appearance of the withdrawal reflex was measured, and the intensity at which the withdrawal reflex occurred was calculated (μA). Before the vibration was delivered, as control, we measured the sensory thresholds 15 times (five times/set and three sets) for each of the four electrostimulation frequencies (5, 50, 250, and 2,000 Hz). After the vibration was delivered at the foot sole of either the right (ipsilateral) or left (contralateral) side for 2 minutes (at a frequency of 4,900 times/min; Figure 1), the sensory thresholds were remeasured at the point of X-min (X=0, 2, 4, 6, 8, 10, 12, 15) after exposure to the vibration stimulus (Figure 2). We remeasured the sensory thresholds three times for each point, and the averaged threshold was adopted for the value.
Vibratory stimuli and sensory threshold measurement in human participants
A total of 32 healthy participants (16 males, 16 females; mean age, 21.0±0.8 years) were recruited for study participation. First, we investigated the effect of a 2-minute vibration applied to either the ipsilateral or contralateral dorsal foot for the sensory threshold. Then, we examined the effect of a 5-minute vibration applied to either the ipsilateral or contralateral dorsal foot for the sensory threshold. The sensory thresholds of human participants were measured only with 50-Hz electrostimulation based on a program for clinical evaluation (Pain vision, Nipro Inc.) because the equipment producing 5, 50, 250, and 2,000 Hz electrostimulation (STG-4002, Multichannel Systems Inc.), which was used with the mice, was not entirely suitable for use with human participants in this study; therefore, we evaluated the threshold of only Aδ fibers in human participants. Further, although we initially attempted to measure the sensory threshold using the plantar side of the foot, the sensory threshold in each participant fluctuated to a great extent under the control condition before the vibratory stimuli were applied. Therefore, the dorsal side of the foot was selected in human participants. After the dorsal side of the foot was exposed, bipolar electrodes were placed proximal to the metacarpophalangeal joint and on the dorsal side of the right first finger. Then, human participants held the push button, which could release the electrostimulation, in their right hand. After the electrostimulation was administered to the participants, when they experienced maximum pain, they could push the button. Then, the intensity (mA) was automatically measured, and the value was stored in a personal computer.
After we measured the sensory thresholds 15 times (five times/set and three sets) as control condition, a vibration device (YCM-721, Daito Electric Co, Osaka, Japan) was attached to the dorsal side of the foot at either the ipsilateral or contralateral side to provide a vibratory stimulus for 2 minutes or 5 minutes (at a frequency of 5,230 times/min). Thereafter, the sensory thresholds were remeasured at the point of X min (X=0, 2, 4, 6, 8, 10, 12, 15) after exposure to the vibratory stimulus. We remeasured the sensory thresholds three times for each point, and the averaged threshold was adopted for the value (Figures 1 and 2).
Measurement of skin temperature in human participants
Before and after applying the vibratory stimulus on the dorsal foot of either the ipsilateral (five male and four female participants; mean age, 21.0±0.0 years) or contralateral side (seven male and six female participants; mean age, 21.0±1.3 years), we simultaneously measured the skin temperature using a digital thermometer (CT-450WR, CUSTOM, Tokyo, Japan) where the sensory threshold on the dorsal foot was measured. The time of the vibratory stimulus was 5 minutes, and the measurement of the skin temperature was performed before vibration, and at 0 and 15 minutes after the vibration. Then, the skin temperature was normalized.
All the participants provided written informed consent according to the Declaration of Helsinki before the start of the experiments, and all human experiments were approved by the Life Science Committees of Kumamoto Health Science University (approval no. 25-29 and 2016-02).
Statistical analysis
Experimental data are expressed as the mean ± SD. Within-group comparisons (before vibration: control vs 0 minute, after vibration: “0”; before vibration: control vs 15 minutes, after vibration: “15”; Figures 3–8) were performed using Wilcoxon signed rank tests. P<0.05 was the threshold for statistical significance. The average values of all three baseline tests were used for the statistical analysis. Furthermore, in human experiments, the averaged line graphs of either the sensory threshold or the skin temperature are shown. Further, the histograms of the sensory threshold are shown. The histograms present an increase in sensory threshold value (above 100% of the normalized sensory threshold) and a decrease in the sensory threshold value (below 100% of the normalized sensory threshold). Moreover, 100% of the normalized sensory threshold indicates no change in the sensory threshold value. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of R commander designed to add statistical functions frequently used in biostatistics.51
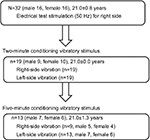  | Figure 3 Flowchart of measurements in human participants. |
Results
Sensory thresholds of four electrostimulation frequencies
The mean sensory thresholds of the right four electrostimulation frequencies were 56.7±11.6, 48.9±14.3, 69.0±19.0, and 345.1±48.7 μA, respectively (Figure 4). The threshold was significantly higher in response to a 2,000-Hz electrostimulation than in response to the other electrostimulation frequencies (P<0.001).
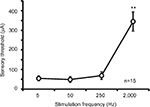  | Figure 4 Averaged values of the sensory thresholds for the right four electrostimulation frequencies in mice (**P<0.001). |
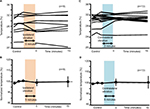
Right sensory thresholds and ipsilateral vibration in mice
Two-minute vibration applied to the ipsilateral plantar sole produced significant increases in the sensory threshold elicited by the right 5-Hz electrostimulation at both 0 minutes (fast response) and 15 minutes (slow response) (Figure 5A, P<0.05). Although ipsilateral 2-minute vibration gradually elevated the averaged value of the sensory threshold at 50-Hz electrostimulation (Figure 5B, 140% as an average value at 15 minutes after stimulation), the sensory threshold did not significantly change (Figure 5B, not significant). Further, ipsilateral 2-minute vibration did not change the sensory threshold by the 250- and 2000-Hz electrostimulation (Figure 5C and 5D, not significant).
Right sensory thresholds and contralateral vibration in mice
Contralateral 2-minute vibration produced significant increases in the sensory threshold of the right hind limb by the 50-Hz electrostimulation, whose modulation was at 15 minutes after vibratory stimulation (slow response; Figure 6B, P<0.05). However, contralateral 2-minute vibration did not change the sensory threshold by the 5-, 250- and 2000-Hz electrostimulation (Figure 6A, C and D, not significant).
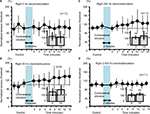
Right sensory thresholds and either side vibration in humans
Neither ipsilateral nor contralateral 2-minute vibration had any effect on the right sensory thresholds in human participants (Figure 7, not significant).
Right sensory thresholds and persistent either side vibration in humans
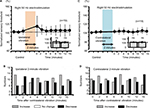
Both ipsilateral and contralateral vibrations for 5 minutes produced significant increase in right sensory thresholds, whose modulation involved a slow response (Figure 8A and C, P<0.05). Furthermore, the right sensory threshold of all the nine subjects increased at 6, 10, and 15 minutes after the right vibration (Figure 8B), and the right sensory threshold of 12 of 13 subjects increased at 10 and 12 minutes after the left vibration (Figure 8D).
Vibration-induced skin temperature in humans
In both ipsilateral and contralateral vibratory stimuli, vibration-induced fluctuation of skin temperature was seen in a few instances. However, there were no significant differences in skin temperature between before and after vibratory stimulation (99.44%±3.76% at 0 minute after the ipsilateral vibration, 100.00%±4.78% at 15 minutes after the ipsilateral vibration, 100.93%±3.49% at 0 minute after the contralateral vibration, 100.70%±5.63% at 15 minutes after the ipsilateral vibration; Figure 9).
Discussion
The present study found that in mice, ipsilateral 2-minute vibration modulated the sensory threshold in response to right 5-Hz electrostimulation, and contralateral 2-minute vibration modulated in response to right 50-Hz electrostimulation. Further, persistent 5-minute vibration, but not 2-minute vibration, of both the ipsilateral and the contralateral sides modulated the sensory threshold in response to right 50-Hz electrostimulation in human subjects, whose modulation was independent of skin temperature. Moreover, except for the modulation of the right sensory threshold caused by the ipsilateral vibratory stimulus in mice, other sensory modulation was observed for 50-Hz electrostmulation in both mice and humans.
In accordance with previous studies, the four different electrostimulation frequencies that we used in this study could stimulate Aβ fibers (2,000 Hz), Aδ fibers (50 and 250 Hz), and C fibers (5 Hz).13–15 However, Koga et al, reported that a 2,000-Hz sine wave mainly activates Aβ fibers (and partially activates Aδ fibers), while a 250-Hz sine wave activates both Aβ and Aδ fibers, and a 5-Hz sine wave stimulates all Aβ fibers, Aδ fibers, and C fibers.52 In experiments on mice, therefore, the ipsilateral vibratory modulation of the sensory threshold in response to right 5-Hz electrostimulation potentially results from C fibers, as the results were not significant at 50 and 250 Hz (Aβ and Aδ fibers), and 2,000 Hz (Aβ fibers; Figure 5). The contralateral vibratory modulation of the sensory threshold in response to right 50-Hz electrostimulation potentially results from Aδ fibers as the results were not significant at 2,000 Hz (Figure 6). In human experiments, both the ipsilateral and contralateral vibratory threshold modulations in response to right 50-Hz electrostimulation derives from Aβ and Aδ fibers, presumably the Aδ fibers.
Cross-species differences in both spatial and temporal summation
For mice, 2-minute vibration may be sufficient to modulate the sensory threshold. However, 2-minute vibration in humans did not affect the threshold (Figure 7). These results suggest that the 2-minute vibration itself did not reach “the threshold” to modulate the sensory threshold in human experiments. Except for the ipsilateral vibratory C fiber modulation and fast response in mice, other sensory modulation was observed for Aδ fibers and slow response in both mice and humans. In mouse experiments, we utilized the vibration device that is used for humans. According to a previous report, the sensory threshold for the vibration decreased with increase of the vibration area,48 suggesting that the intensity of the vibration as a stimulator positively correlates with the area factor. Therefore, the ipsilateral vibratory modulation in mice may have involved both slow and fast responses because the vibratory stimulus for humans had a strong intensity for mice. Although ipsilateral 50-Hz vibration-induced threshold modulation of the sensory threshold did not change significantly, the averaged value of the sensory threshold was gradually elevated (Figure 5B, 140% as slow response), suggesting that ipsilateral vibratory modulation in mice may affect both C and Aδ fibers because of the ipsilateral strong vibration. Either ipsilateral or contralateral persistent (5-minute) vibration in humans modulated the sensory thresholds (Figure 8), suggesting that temporal summation may have compensated for the limited spatial (area) summation, and the persistent vibratory stimulus finally reached “the threshold” to modulate the sensory threshold.
We cannot fully explain the relationship among the foot location of the sensory threshold, the vibratory modulation, and the inter-species differences because there is no research on the comparison of the sensory threshold between the dorsal and plantar sides of the foot. However, the dorsal side of the hand appears to be more sensitive to two-point discrimination than the volar side of the hand,53 suggesting that the dorsal side of the foot is also more sensitive to sensation than the plantar side of the foot. If this is true, the 2-minute experiments in humans should have a more drastic effect than that in mice. However, the results were the opposite (Figures 5–7). A few studies have examined age-dependent changes in sensation, such as in warm, cold, and vibration.54,55 In this study, although the age of the mice was less than that of the humans, we do not believe that age is the main factor for the difference in the response, since the age itself cannot be simply compared between these two species.
Cutaneous mechanoreceptors and stimulus frequency in mice
The peak sensitivity of the Pacinian corpuscle-mediated system is at approximately a 250-Hz vibration.56,57 The non-Pacinian, Meissner’s corpuscles-mediated, rapid-adapting system appears to be activated at up to a 50-Hz vibration.56,57 Although we cannot be certain, either 50- or 250-Hz sine waves may also activate different types of cutaneous mechanoreceptors. Therefore, a persistent (5-minute) 50-Hz electrostimulation may strongly activate the specific cutaneous mechanoreceptor system to modulate the sensory threshold resulting from the Aδ fibers.
Vibratory stimulus and skin temperature in humans
A previous study reported that local vibratory stimulation increases skin temperature.37 In that study, Oliveri et al used 100-Hz vibration and a 15-minute stimulus to measure skin temperature at the vibrated small spot, directly.37 We believe that there are two reasons why the 5-minute vibration in our study did not increase skin temperature. One involves the timing of the stimulation. The other involves the location at which the skin temperature was measured. If we had measured the skin temperature at the vibrated small spot, the increase of temperature would have perhaps been detectable. Another study reported that increase in skin temperature reduced the vibration threshold on the forearm.58 Therefore, if the vibration increased the skin temperature of the surrounding area, the sensory threshold may have been reduced. The opposite may be true for the elevation of the sensory threshold.
Contralateral vibratory modulation and central mechanisms
Two alternative regions were potentially responsible for contralateral vibration-induced changes in the right sensory threshold. First is the brainstem descending inhibitory system,59,60 and the second is the cortical region.61,62 Descending serotonergic projections originate in the raphe nuclei,59 while the noradrenergic system constitutes A5, A6, and A7 cell groups.60 These descending inhibitory systems project to spinal cord dorsal horn neurons via the dorsolateral funiculus.60,63,64 The vibrotactile stimulation-induced medial lemniscus (ML) ascending pathway terminates in the nucleus gracilis, nucleus cuneatus, posterior lateral nucleus of the thalamus, and parabrachial nuclei (PV).65,66 Further, some Aβ fibers, which are activated by vibrotactile stimulation, terminate in a deep layer of the dorsal horn to relay ascending information via the anterior spinothalamic (ST) pathway.67 A previous study reported that the ST ascending pathway targets not only the thalamus but also the caudal ventrolateral medulla (VLM), lateral PV, and periaqueductal gray matter (PAG).68,69 For example, PAG neurons project to the A5, A6, and A7 cells to modulate nociception.70 The PAG and VLM form synapses with the raphe nuclei,71,72 and the raphe neurons project to the A7.73,74 Thus, vibrotactile stimulation may have activated both the ML and ST ascending pathways and indirectly affected the raphe nuclei and A5, A6, and A7 cells, which are components of the serotonergic and noradrenergic descending inhibitory systems.
As for the second alternative region, we propose cortical modulation. Pain stimuli activate the contralateral thalamus,75–77 primary somatosensory cortex (SI),61 contralateral anterior cingulate cortex,61 and bilateral secondary somatosensory cortex (SII).61,62 In one study, focal pain sensation changed regional cerebral blood flow (rCBF) in the contralateral SI, contralateral SII, contralateral insula, and others.78 The ST ascending pathway sends more fibers to the primary motor cortex (M1) and SI than to the pre-motor cortex or somatosensory association cortex.79,80 Torquati et al81 reported that activation of the bilateral posterior SII was associated with pain stimulation. In contrast, vibrotactile stimulation activated the bilateral thalamus, contralateral SI, bilateral SII, and others.82 Vibrotactile stimulation also changed rCBF in the contralateral SI and SII.78 The ML ascending pathway sends more fibers to the premotor cortex and M1 than to the SI or SII.79,80 Moreover, the bilateral anterior SII has been associated with somatosensory stimulation.81 Thus, both pain sensation and vibrotactile stimulation overlap in the activation of the contralateral thalamus, contralateral SI, and the bilateral SII.
Our study has the following limitations. “The central mechanisms” in response to the contralateral vibratory stimulation which we proposed in this study remain a hypothesis. Therefore, in animal experiments, using whole animal live imaging or electrophysiological techniques, such as in vivo cortical, spinal imaging, or in vivo intracellular recording under an anesthetized condition, and in human experiments using electroencephalography or functional magnetic resonance imaging, it is necessary to demonstrate the involvement of the central nervous system in the contralateral vibratory stimulation-induced sensory modulation.
Clinical implication
DNIC represents inhibitory modulation, usually performed by a “pain inhibits pain” test paradigm.83 Additionally, DNIC is a technique for reducing pain, which is inhibited by the noxious stimuli applied to various parts of the body.26,27 In fact, the DNIC technique has been used in clinical medicine.84 In this study, we used vibration as a conditioning stimulus instead of using noxious stimuli. Our vibratory conditioning stimulus, which is applied to the contralateral side of body, potentially shares commonalities with the DNIC. Further, the mechanisms of DNIC are thought of as “central mechanisms,” which may be in line with our “central hypothesis” for the contralateral vibratory modulation. Therefore, our animal and human study, which used precise and quantitative evaluation may be useful for the elucidation of the “DNIC and central mechanisms”.
Conclusion
In conclusion, we used a novel quantitative method to show that local bilateral vibration elevates the right sensory thresholds in mice and in human subjects. The contralateral vibratory modulation of the right sensory threshold suggests the involvement of the central nervous system.
Disclosure
The authors report no conflicts of interest in this work.
References
Abraira VE, Ginty DD. The sensory neurons of touch. Neuron. 2013;79(4):618–639. | ||
Stauffer ME, Taylor SD, Watson DJ, Peloso PM, Morrison A. Definition of nonresponse to analgesic treatment of arthritic pain: an analytical literature review of the smallest detectable difference, the minimal detectable change, and the minimal clinically important difference on the pain visual analog scale. Int J Inflam. 2011;2011:231926. | ||
Lee JY. Measurement of Trigeminal Neuralgia Pain: Penn Facial Pain Scale. Neurosurg Clin N Am. 2016;27(3):327–336. | ||
Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain. 2010;11(2):109–118. | ||
Wysham NG, Miriovsky BJ, Currow DC, et al. Practical Dyspnea Assessment: Relationship Between the 0-10 Numerical Rating Scale and the Four-Level Categorical Verbal Descriptor Scale of Dyspnea Intensity. J Pain Symptom Manage. 2015;50(4):480–487. | ||
Main CJ. Pain assessment in context: a state of the science review of the McGill pain questionnaire 40 years on. Pain. 2016;157(7):1387–1399. | ||
Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2. Pain. 2009;144(1-2):35–42. | ||
Bradman MJ, Ferrini F, Salio C, Merighi A. Practical mechanical threshold estimation in rodents using von Frey hairs/Semmes-Weinstein monofilaments: Towards a rational method. J Neurosci Methods. 2015;255:92–103. | ||
Hole K, Tjølsen A. The tail-flick and formalin tests in rodents: changes in skin temperature as a confounding factor. Pain. 1993;53(3):247–254. | ||
Vilela FC, Vieira JS, Giusti-Paiva A, Silva MLD. Experiencing early life maternal separation increases pain sensitivity in adult offspring. Int J Dev Neurosci. 2017;62:8–14. | ||
Tsui BC, Shakespeare TJ, Leung DH, Tsui JH, Corry GN. Reproducibility of current perception threshold with the Neurometer(®) vs the Stimpod NMS450 peripheral nerve stimulator in healthy volunteers: an observational study. Can J Anaesth. 2013;60(8):753–760. | ||
Kiso T, Nagakura Y, Toya T, et al. Neurometer measurement of current stimulus threshold in rats. J Pharmacol Exp Ther. 2001;297(1):352–356. | ||
Masson EA, Veves A, Fernando D, Boulton AJ. Current perception thresholds: a new, quick, and reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia. 1989;32(10):724–728. | ||
Tay B, Wallace MS, Irving G. Quantitative assessment of differential sensory blockade after lumbar epidural lidocaine. Anesth Analg. 1997;84(5):1071–1075. | ||
Liu S, Kopacz DJ, Carpenter RL. Quantitative assessment of differential sensory nerve block after lidocaine spinal anesthesia. Anesthesiology. 1995;82(1):60–63. | ||
Oh D, Yun T, Kim J, et al. The Measurement of the Sensory Recovery Period in Zygoma and Blow-Out Fractures with Neurometer Current Perception Threshold. Arch Plast Surg. 2016;43(5):411–417. | ||
Ohtori S, Kawaguchi H, Takebayashi T, et al. PainVision Apparatus Is Effective for Assessing Low Back Pain. Asian Spine J. 2014;8(6):793–798. | ||
Wang D, Zhang K, Han S, Yu L. PainVision® Apparatus for Assessment of Efficacy of Pulsed Radiofrequency Combined with Pharmacological Therapy in the Treatment of Postherpetic Neuralgia and Correlations with Measurements. Biomed Res Int. 2017;2017:5670219. | ||
Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. | ||
Gok Metin Z, ArikanDonmez A, Izgu N, Ozdemir L, Arslan IE. Aromatherapy Massage for Neuropathic Pain and Quality of Life in Diabetic Patients. J Nurs Scholarsh. 2017;49(4):379–388. | ||
Cabýoglu MT, Ergene N, Tan U. The mechanism of acupuncture and clinical applications. Int J Neurosci. 2006;116(2):115–125. | ||
Mata Diz JB, de Souza JR, Leopoldino AA, Oliveira VC. Exercise, especially combined stretching and strengthening exercise, reduces myofascial pain: a systematic review. J Physiother. 2017;63(1):17–22. | ||
Espí-López GV, Arnal-Gómez A, Balasch-Bernat M, Inglés M. Effectiveness of Manual Therapy Combined With Physical Therapy in Treatment of Patellofemoral Pain Syndrome: Systematic Review. J Chiropr Med. 2017;16(2):139–146. | ||
Knoer lR, Lavoie Smith EM, Weisberg J. Chronic Pain and Cognitive Behavioral Therapy: An Integrative Review. West J Nurs Res. 2016;38(5):596–628. | ||
Singh H, Bhushan S, Arora R, Singh Buttar H, Arora S, Singh B. Alternative treatment strategies for neuropathic pain: Role of Indian medicinal plants and compounds of plant origin-A review. Biomed Pharmacother. 2017;92:634–650. | ||
Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6(3):283–304. | ||
Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain. 1979;6(3):305–327. | ||
Fitzgerald M. The contralateral input to the dorsal horn of the spinal cord in the decerebrate spinal rat. Brain Res. 1982;236(2):275–287. | ||
Wang XQ, Pi YL, Chen PJ, et al. Whole body vibration exercise for chronic low back pain: study protocol for a single-blind randomized controlled trial. Trials. 2014;15:104. | ||
Anwer S, Alghadir A, Zafar H, Al-Eisa E. Effect of whole body vibration training on quadriceps muscle strength in individuals with knee osteoarthritis: a systematic review and meta-analysis. Physiotherapy. 2016;102(2):145–151. | ||
Kessler NJ, Hong J. Whole body vibration therapy for painful diabetic peripheral neuropathy: a pilot study. J Bodyw Mov Ther. 2013;17(4):518–522. | ||
Hong J, Barnes M, Kessler N. Case study: use of vibration therapy in the treatment of diabetic peripheral small fiber neuropathy. J Bodyw Mov Ther. 2013;17(2):235–238. | ||
Lundeberg T, Abrahamsson P, Bondesson L, Haker E. Vibratory stimulation compared to placebo in alleviation of pain. Scand J Rehabil Med. 1987;19(4):153–158. | ||
Lundeberg T, Nordemar R, Ottoson D. Pain alleviation by vibratory stimulation. Pain. 1984;20(1):25–44. | ||
Yarnitsky D, Kunin M, Brik R, Sprecher E. Vibration reduces thermal pain in adjacent dermatomes. Pain. 1997;69(1-2):75–77. | ||
Kakigi R, Shibasaki H. Mechanisms of pain relief by vibration and movement. J Neurol Neurosurg Psychiatry. 1992;55(4):282–286. | ||
Oliveri DJ, Lynn K, Hong CZ. Increased skin temperature after vibratory stimulation. Am J Phys Med Rehabil. 1989;68(2):81–85. | ||
Furuta M, Sakakibara H, Miyao M, Kondo T, Yamada S. Effect of vibration frequency on finger blood flow. Int Arch Occup Environ Health. 1991;63(3):221–224. | ||
Ye Y, Griffin MJ. Reductions in finger blood flow induced by 125-Hz vibration: effect of area of contact with vibration. Eur J Appl Physiol. 2013;113(4):1017–1026. | ||
Kim HK, Schattschneider J, Lee I, Chung K, Baron R, Chung JM. Prolonged maintenance of capsaicin-induced hyperalgesia by brief daily vibration stimuli. Pain. 2007;129(1-2):93–101. | ||
Gu C, Griffin MJ. Vibrotactile thresholds at the sole of the foot: Effect of vibration frequency and contact location. Somatosens Mot Res. 2011;28(3-4):86–93. | ||
Gescheider GA, Zwislocki JJ, Rasmussen A. Effects of stimulus duration on the amplitude difference limen for vibrotaction. J Acoust Soc Am. 1996;100(4 Pt 1):2312–2319. | ||
Green BG. Vibrotactile temporal summation: effect of frequency. Sens Processes. 1976;1(2):138–149. | ||
Hämäläinen H, Pertovaara A, Soininen K, Järvilehto T. Is there low frequency vibrotactile temporal summation? Scand J Psychol. 1981;22(3):203–206. | ||
van Doren CL. Temporal summation by Pacinian corpuscles precludes entrainment at the detection threshold. J Acoust Soc Am. 1985;77(6):2188–2189. | ||
Gescheider GA, Hoffman KE, Harrison MA, Travis ML, Bolanowski SJ. The effects of masking on vibrotactile temporal summation in the detection of sinusoidal and noise signals. J Acoust Soc Am. 1994;95(2):1006–1016. | ||
Lamoré PJ, Keemink CJ. Evidence for different types of mechanoreceptors from measurements of the psychophysical threshold for vibrations under different stimulation conditions. J Acoust Soc Am. 1988;83(6):2339–2351. | ||
Gu C, Griffin MJ. Spatial summation of vibrotactile sensations at the foot. Med Eng Phys. 2013;35(8):1221–1227. | ||
Morioka M, Griffin MJ. Thresholds for the perception of hand-transmitted vibration: dependence on contact area and contact location. Somatosens Mot Res. 2005;22(4):281–297. | ||
Whitehouse DJ, Morioka M, Griffin MJ. Effect of contact location on vibrotactile thresholds at the fingertip. Somatosens Mot Res. 2006;23(1-2):73–81. | ||
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. | ||
Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1:13. | ||
Gellis M, Pool R. Two-point discrimination distances in the normal hand and forearm: application to various methods of fingertip reconstruction. Plast Reconstr Surg. 1977;59(1):57–63. | ||
Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115(3):410–418. | ||
Verrillo RT. Age related changes in the sensitivity to vibration. J Gerontol. 1980;35(2):185–193. | ||
Bensmaïa SJ, Leung YY, Hsiao SS, Johnson KO. Vibratory adaptation of cutaneous mechanoreceptive afferents. J Neurophysiol. 2005;94(5):3023–3036. | ||
Makous JC, Friedman RM, Vierck CJ. A critical band filter in touch. J Neurosci. 1995;15(4):2808–2818. | ||
Verrillo RT, Bolanowski SJ. The effects of skin temperature on the psychophysical responses to vibration on glabrous and hairy skin. J Acoust Soc Am. 1986;80(2):528–532. | ||
Bowker RM, Westlund KN, Sullivan MC, Coulter JD. Organization of descending serotonergic projections to the spinal cord. Prog Brain Res. 1982;57:239–265. | ||
Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263(1):15–31. | ||
Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991;251(4999):1355–1358. | ||
Simões C, Hari R. Relationship between responses to contra- and ipsilateral stimuli in the human second somatosensory cortex SII. Neuroimage. 1999;10(4):408–416. | ||
Westlund KN, Coulter JD. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry. Brain Res. 1980;2(3):235–264. | ||
Westlund KN, Carlton SM, Zhang D, Willis WD. Direct catecholaminergic innervation of primate spinothalamic tract neurons. J Comp Neurol. 1990;299(2):178–186. | ||
Massopust LC, Hauge DH, Ferneding JC, Doubek WG, Taylor JJ. Projection systems and terminal localization of dorsal column afferents: an autoradiographic and horseradish peroxidase study in the rat. J Comp Neurol. 1985;237(4):533–544. | ||
Wild JM. Avian somatosensory system: II. Ascending projections of the dorsal column and external cuneate nuclei in the pigeon. J Comp Neurol. 1989;287(1):1–18. | ||
Abraira VE, Kuehn ED, Chirila AM, et al. The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell. 2017;168(1-2):295–310. | ||
Todd AJ, Mcgill MM, Shehab SA. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur J Neurosci. 2000;12(2):689–700. | ||
Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11(12):823–836. | ||
Bajic D, Proudfit HK. Projections of neurons in the periaqueductal gray to pontine and medullary catecholamine cell groups involved in the modulation of nociception. J Comp Neurol. 1999;405(3):359–379. | ||
Snowball RK, Dampney RA, Lumb BM. Responses of neurones in the medullary raphe nuclei to inputs from visceral nociceptors and the ventrolateral periaqueductal grey in the rat. Exp Physiol. 1997;82(3):485–500. | ||
Zagon A. Innervation of serotonergic medullary raphe neurons from cells of the rostral ventrolateral medulla in rats. Neuroscience. 1993;55(3):849–867. | ||
Clark FM, Proudfit HK. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res. 1991;547(2):279–288. | ||
Clark FM, Proudfit HK. The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res. 1991;538(2):231–245. | ||
Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372(6508):770–773. | ||
Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci. 1994;14(11 Pt 2):6779–6795. | ||
Gingold SI, Greenspan JD, Apkarian AV. Anatomic evidence of nociceptive inputs to primary somatosensory cortex: relationship between spinothalamic terminals and thalamocortical cells in squirrel monkeys. J Comp Neurol. 1991;308(3):467–490. | ||
Coghill RC, Talbot JD, Evans AC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14(7):4095–4108. | ||
Jang SH, Seo JP. Differences of the medial lemniscus and spinothalamic tract according to the cortical termination areas: A diffusion tensor tractography study. Somatosens Mot Res. 2015;32(2):67–71. | ||
Jang SH, Kwon YH, Lee MY, Lee DY, Hong JH. Termination differences in the primary sensorimotor cortex between the medial lemniscus and spinothalamic pathways in the human brain. Neurosci Lett. 2012;516(1):50–53. | ||
Torquati K, Pizzella V, Babiloni C, et al. Nociceptive and non-nociceptive sub-regions in the human secondary somatosensory cortex: an MEG study using fMRI constraints. Neuroimage. 2005;26(1):48–56. | ||
Golaszewski SM, Siedentopf CM, Koppelstaetter F, et al. Human brain structures related to plantar vibrotactile stimulation: a functional magnetic resonance imaging study. Neuroimage. 2006;29(3):923–929. | ||
Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–615. | ||
Staud R, Robinson ME, Vierck CJ, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101(1-2):167–174. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.